Zinc hydroxystannate: a promising solid acid–base bifunctional catalyst†
Swetha Sandesh,
Ganapati V. Shanbhag* and
A. B. Halgeri
Materials science division, Poornaprajna Institute of Scientific Research, (PPISR), Bidalur Post, Devanahalli, Bangalore-562110, India. E-mail: shanbhag@poornaprajna.org; Fax: +91 2361 1836; Tel: +91 2740 8552
First published on 14th November 2013
Abstract
Zinc hydroxystannate is shown to be a highly active and selective solid bifunctional catalyst for carbonylation of glycerol with urea. It has strong basicity with Lewis acidic Zn, low hygroscopicity and good thermal stability which makes it a potential catalyst for heterogeneous acid–base catalysed reactions.
Glycerol carbonate (GlyC) is an industrially important product having wide applications in various fields like the pharmaceutical, polymer and paint industries. Synthesis of GlyC from glycerol, a byproduct of biodiesel synthesis, and urea, a cheap and abundant chemical has made this reaction more attractive. Many research groups are working on designing solid base catalyst for the synthesis of GlyC from carbonylation of glycerol with urea or alkyl carbonates. The heterogeneous catalysts, such as lanthanum oxide,1 zinc hydrotalcite2 and recently Fujita et al.,3 reported homogeneous Zn species as catalysts for this reaction. However, these catalysts showed activity with moderate yields under energy intensive vacuum conditions. Lopez and his co workers4 reported an expensive Au–Pd/MgO catalyst, where flow of nitrogen was used to remove byproduct, ammonia. Due to a highly viscous nature of glycerol, various solvents were used to get high GlyC yield with metallic or organometallic sulphates as catalysts.5
In the present work, zinc hydroxystannate (ZnSn(OH)6) has been reported for the first time as a solid bifunctional catalyst and has been applied for the synthesis of GlyC from glycerol and urea. Crystal Structure of ZHS along ‘c’ direction in the unit cell is represented in Fig. 1. It has a perovskite type crystal structure with metal atoms octahedrally coordinated with corner sharing hydroxyl groups to form Sn(OH)6 and Zn(OH)6 octahedra.6 Synthesis of zinc hydroxystannate material was first reported by Basciano et al.,7 and it was also synthesized as controlled nanocubes for nanodevice applications.8 Metal hydroxystannates, in general, have been applied as gas sensors,9 thermal decomposition material10 and also studied as photocatalyst by many researchers,11,12 but have not been used so far as heterogeneous catalyst for organic transformations.
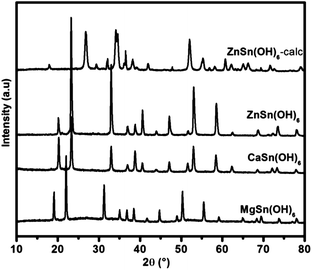
ZnSn(OH)6 (hereafter, ZHS) was prepared by chemical co-precipitation method using zinc chloride (ZnCl2) and tin chloride (SnCl4·5H2O) with NaOH as a co-precipitant. The precipitate was stirred for 2 h at room temperature, filtered, washed repeatedly with distilled water till free from chloride and dried in an oven at 120 °C for 6 h and then activated at 170 °C for 1 h under static air. Same method was followed to synthesize other MSn(OH)6 (M = Mg, Ca) using different MCl2.6 To get more insight on its properties, ZHS was converted into its oxide by calcining at 600 °C for 5 h (named as ZHS-calc). The XRD patterns confirmed the formation of the above compounds (Fig. 2). MgO, CaO, ZnO, SnO2 were procured commercially and activated at 800 °C. Mg/Al hydrotalcite (HTc Mg/Al) and Zn/Al hydrotalcite (HTc Zn/Al) were synthesized as per literature method.13
ZHS was characterised by using various techniques like XRD, FTIR, TGA, SEM, N2 sorption and basicity measurement by Hammett indicators. All the diffraction peaks in the XRD pattern could be indexed to cubic ZHS with a lattice parameter a = 7.80 A°. It is also confirmed that ZHS crystallizes with space group Pn![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) according to the standard XRD data file source (ICDS = #27767). For ZHS catalyst, the hkl values were indexed and the major diffraction peaks at 2θ values of 19.6, 22.6, 32.5, 36.2, 38.4, 40.1, 46.7, 52.4, 57.8 are ascribed to (011), (111), (112), (122), (103), (113), (213), (313), and (233) crystal planes of cubic ZHS respectively (Fig. 2). XRD pattern of all other MSn(OH)6 catalysts were matched with ICSD patterns.
according to the standard XRD data file source (ICDS = #27767). For ZHS catalyst, the hkl values were indexed and the major diffraction peaks at 2θ values of 19.6, 22.6, 32.5, 36.2, 38.4, 40.1, 46.7, 52.4, 57.8 are ascribed to (011), (111), (112), (122), (103), (113), (213), (313), and (233) crystal planes of cubic ZHS respectively (Fig. 2). XRD pattern of all other MSn(OH)6 catalysts were matched with ICSD patterns.
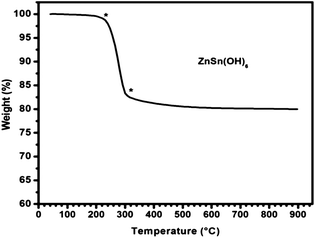
The thermal stability of the ZHS catalyst was determined by TGA studies (Fig. 3). Interestingly, there was only 0.5% weight loss up to 250 °C which indicates the presence of very low amount of water of hydration in ZHS. This property of low hygroscopicity is very useful for any base catalysts, where it is not necessary to maintain moisture free conditions for carrying out reactions. Hydrophobic environment on the catalyst surface of bifunctional catalyst plays an important role to achieve high activity for acid–base catalysed reactions.14 Also the material has reasonably good thermal stability of ∼250 °C and can be used for the reaction below this temperature. The weight loss in ZHS started after 250 °C with a rapid loss of hydroxyl groups which led to the formation of oxide.
SEM and BET surface area by N2 sorption measurement were performed to determine the morphology of the ZHS catalyst. It exhibited uniform cuboid shape with 2 μm particle size as shown in Fig. 4 and the N2 adsorption measurement showed very low BET surface area of 7 m2 g−1.
The qualitative estimation of basicity in synthesized catalysts were performed using Hammett indicators. The procedure was followed as described by J. M. Fraile et al.15 using neutral red (pKBH+ 6.8–8.0), phenolphthalein (pKBH+ 8.0–9.6), nile blue (pKBH+ 10.1–11.1) and tropaeolin-0 (pKBH+ 11.1–12.7) indicators. The change in colour from nile blue to pink in ZHS and other MSn(OH)6 indicated the strength of basicity in the range pKBH+ = 10.1–11.1 (ESI Table S1†). The amount of basicity present in all the catalysts was measured by titration method. ZHS contained basicity of 33 μmol g−1, higher than other solid base catalysts such as CaO, MgO, HTc MgAl and HTc ZnAl (Table 1). No leachable basicity was observed for all the above solid catalysts.
| Catalyst | Basicity (indicator method) | Glycerol conversion (mol%) | GlyC selectivity (mol%) |
|---|---|---|---|
| Total (μmol g−1) a = phenolphthalein/b = nile blue | |||
a Reaction conditions: glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea; 1 urea; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; catalyst weight = 5 wt% temperature = 165 °C, time = 5 h. Basicity error limit = ±1 μmol g−1. Recycle catalyst: ZHS. 1; catalyst weight = 5 wt% temperature = 165 °C, time = 5 h. Basicity error limit = ±1 μmol g−1. Recycle catalyst: ZHS. |
|||
| Blank | — | 0.1 | 99.9 |
| ZHS | 33b | 98 | 99.6 |
| ZHS-calc | 20a/3b | 64 | 99.2 |
| CaSn(OH)6 | 7a/22b | 90.5 | 92 |
| MgSn(OH)6 | 23a/11b | 86 | 98.8 |
| ZnO | 10a | 30.6 | 99.2 |
| SnO2 | No colour change | 30.2 | 99.4 |
| Sn(OH)4 | 3a | 46.8 | 99.4 |
| MgO | 13a | 12.6 | 99 |
| CaO | 18a | 20.2 | 99.6 |
| HTc (Mg/Al) | 26a | 10 | 99 |
| HTc (Zn/Al) | 18a | 65 | 99.5 |
| Recycle 1 | — | 97.8 | 99.6 |
| Recycle 2 | — | 96.4 | 99.1 |
| Recycle 3 | — | 95.2 | 99.5 |
The results of GlyC synthesis from glycerol and urea through carbonylation reaction using various catalysts are represented in Table 1. ZHS catalyst showed the maximum glycerol conversion of 98% with almost 100% GlyC selectivity. Similarly, CaSn(OH)6 and MgSn(OH)6 showed high glycerol conversion of 90.5 and 86% respectively. The calcination of ZHS to 600 °C decreased the basicity from 33 to 23 μmol g−1 and hence conversion decreased from 98 to 64%. Mild acidic ZnO and SnO2, and basic oxides like MgO and CaO showed low activity for glycerol carbonylation. The higher activity of ZHS (98%) compared with ZnAl HTc (65%) could be due to difference in their basicities. The results show that in addition to the strong basicity, Lewis acidic Zn in the catalyst may also act as a promoter for carbonylation of glycerol with urea. This is in agreement with other reports that Lewis acidic Zn helps in improving the yield of GlyC.‡,2,3
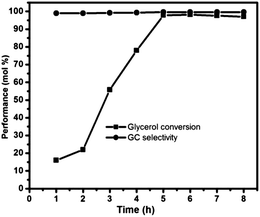
Effect of reaction time was studied for carbonylation reaction of glycerol with urea under the reaction condition shown in Table 1. The conversion was 17% for 1 h and increased with the increase in reaction time and reached 98% for 5 h and remained constant thereafter (Fig. 5).
For leaching test, the reaction was carried out with ZHS for two hour under reaction conditions shown in Table 1. The reaction was then stopped and the reaction mixture was centrifuged to separate the catalyst (glycerol conversion = 23%). The reaction was then continued without the catalyst for another 3 hours.
There was no increase in glycerol conversion after removing the catalyst which indicated the absence of leached active sites and the reaction was truely heterogeneous. It is further confirmed by atomic absorption spectrometer analysis of reaction mixture for the presence of Zn after separating the catalyst. The measurement showed the absence of Zn in the reaction mixture (detection limit = 0.01 ppm). Hence it was confirmed that there was no leaching of active sites during the carbonylation reaction with ZHS.
Reusability of ZHS catalyst was carried out for 3 successive runs by separating catalyst from the reaction media followed by washing with acetone and dried at 120 °C for 2 h. Only marginal decrease in conversion by 2.8% after 3 catalyst recycles was observed as shown in Table 1. XRD patterns of both fresh and recycled catalysts indicated that there was no change in phase purity after three recycles (ESI Fig. S2†).
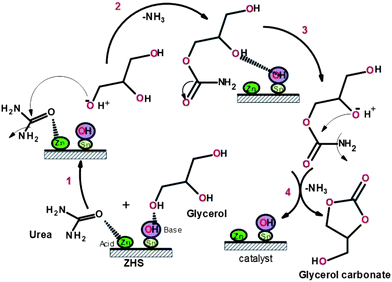
Based on the above results, a plausible reaction mechanism is proposed for ZHS catalyzed carbonylation of glycerol with urea. Scheme 1 shows that basic site in the ZHS activates the OH group of glycerol by removing proton whereas Lewis acid Zn activates the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of urea.
O) of urea.
The carbonylation product is formed by the removal of NH3 in the 2nd step. This intermediate product undergoes cyclization by losing another molecule of NH3 to form GlyC. Since the reaction is reversible, the extraction of NH3 is essential to get high conversion of glycerol to GlyC and hence nitrogen was bubbled to remove NH3 during the reaction.
Further work on the general applicability of metal hydroxystannate as bifunctional or basic catalyst for other organic transformations is under progress. The initial screening of the catalyst showed good activity and selectivity for the transesterification of glycerol with dimethyl carbonate (ESI Table S2†).
In conclusion, ZHS is a novel, bifunctional solid catalyst for the carbonylation of glycerol with urea. It has strong basicity and good thermal stability. Lewis acidic zinc present in the catalyst facilitates the reaction to give a high product yield. Although it is a double metal hydroxide, it possesses low hygroscopicity which makes it a potential catalyst for other base catalyzed organic transformations. It showed excellent glycerol conversion of 98% with almost 100% glycerol carbonate selectivity and also showed good reusability.
Swetha S acknowledges CSIR, New Delhi for providing Senior Research Fellowship and also thankful to Manipal University for permitting this research as a part of the Ph.D programme.
Notes and references
- L. Wang, Y. Ma, Y. Wang, S. Liu and Y. Deng, Catal. Commun., 2011, 12, 1458 CrossRef CAS PubMed.
- M. J. Climent, A. Corma, P. D. Frutos, S. Iborra, M. Noy, A. Velty and P. Concepcion, J. Catal., 2010, 269, 140 CrossRef CAS PubMed.
- S. Fujita, Y. Yamanishi and M. Arai, J. Catal., 2013, 297, 137 CrossRef CAS PubMed.
- H. A. Rahim, Q. He, J. A. Lopez-Sanchez, C. Hammond, N. Dimitratos, M. Sankar, A. F. Carley, C. J. Kiely, D. W. Knighta and G. J. Hutchings, Catal. Sci. Technol., 2012, 2, 1914 Search PubMed.
- J.-L. Dubois, et al., US pat. 0245513 A1, 2011.
- M. Wang, X. Cao, Y. Huang, C. Guo, L. Huang, S. Yinb and T. Sato, CrystEngComm, 2012, 14, 2950 RSC.
- L. C. Basciano, R. C. Peterson and P. Roeder, Can. Mineral., 1998, 36, 1203 CAS.
- Z. Qin, Y. Huang, Q. Wang, J. Qi, X. Xinga and Y. Zhang, CrystEngComm, 2010, 12, 4156 RSC.
- L. Han, J. Liu, Z. Wang, K. Zhang, H. Luo, B. Xu, X. Zou, X. Zheng, B. Ye and X. Yu, CrystEngComm, 2012, 14, 3380 RSC.
- G. Wrobel, M. Piech, S. Dardona, Y. Ding and P. Gao, Cryst. Growth Des., 2009, 9, 4457 Search PubMed.
- Y. Chen, D. Li, M. He, Y. Hu, Y. Ruan, Y. Lin, J. Hu, Y. Zheng and Y. Shao, Appl. Catal., B, 2012, 113, 134 CrossRef PubMed.
- L. Wang, K. Tang, Z. Liu, D. Wang, J. Sheng and W. Cheng, J. Mater. Chem., 2011, 21, 4352 RSC.
- W. T. Reichle, J. Catal., 1985, 94, 547 CrossRef CAS.
- M. Sasidharan, S. Fujita, M. Ohashi, Y. Goto, K. Nakashima and S. Inagaki, Chem. Commun., 2011, 47, 10422 RSC.
- J. M. Fraile, N. Garcia, J. A. Mayoral, E. Pires and L. Roldan, Appl. Catal., A, 2009, 364, 87 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1. Basicity measurement of all catalyst by Hammett indicator method. Fig. S2. FT-IR spectra of ZHS catalyst. Fig. S3. XRD pattern of recycled catalyst. See DOI: 10.1039/c3ra44370a |
‡ Experimental procedure: carbonylation reaction of glycerol and urea was performed using 2 necked round bottom flask with glycerol to urea mol ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 wt% catalyst with respect to total reactant weight. N2 was bubbled to remove NH3 formed during the reaction. The mixture was stirred vigorously at 165 °C under atmospheric pressure. Obtained product was analysed using GC-FID with Stabilwax column and confirmed through GCMS analysis. 1, 5 wt% catalyst with respect to total reactant weight. N2 was bubbled to remove NH3 formed during the reaction. The mixture was stirred vigorously at 165 °C under atmospheric pressure. Obtained product was analysed using GC-FID with Stabilwax column and confirmed through GCMS analysis.Basicity measurements: the basicity was determined quantitatively by titration with 0.01 M benzoic acid. In a typical procedure, the catalyst was suspended (0.15 g) in a toluene solution of nile blue/phenolphthalein (2 ml, 0.1 mg ml−1) and stirred for 30 min, and then titrated with a toluene solution of benzoic acid (0.01 M) to determine the total basicity. The indicator changed colour from colourless to pink with respect to phenolphthalein and blue to pink in case of nile blue. The colour returned to colourless (for phenolphthalein) or blue (for nile blue) when the surface was neutralized with benzoic acid. For leachable basicity measurement, catalyst (0.5 g) was shaken in water (50 ml) for 1 h at room temperature, filtered and a methanol solution of nile blue/phenolphthalein (5 ml, 0.1 mg ml−1) was added to the filtrate. It was titrated with methanolic solution of benzoic acid (0.01 M) to determine the leachable basicity. |
| This journal is © The Royal Society of Chemistry 2014 |