Niobium doped hexagonal mesoporous silica (HMS-X) catalyst for vapor phase Beckmann rearrangement reaction†
Abstract
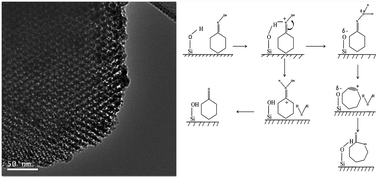
The synthesis of ε-caprolactam, a demanding monomer, through a heterogeneous catalytic pathway has remained a key area of research in the last decade. The Beckmann rearrangement reaction in the vapor phase using a solid acid catalyst was found to be very effective for producing ε-caprolactam. It is observed that niobium incorporated into mesoporous silica serves as a good catalyst for the Beckmann rearrangement reaction. Recently developed mesoporous silica, having an ordered honeycomb structure, is very useful as it can lead to effective diffusion of reactants and products for several reactions. In this study, Nb-doped mesoporous HMS-X nanocomposite materials with different Nb loadings were prepared by a one step hydrothermal synthesis and characterized by BET surface area and porosity measurements, wide and small angle XRD, SEM, HR-TEM, elemental mapping, FTIR, 29Si-NMR and NH3-TPD techniques. The activity of the Nb–HMS-X catalyst was evaluated for the vapour phase Beckmann rearrangement reaction. The catalyst characterization study shows that Nb is highly dispersed on the HMS-X matrix at lower Nb loadings. At higher Nb loadings it is present in the extra-framework position as revealed from XRD, HR-TEM and 29Si-NMR studies. The NH3-TPD result shows the presence of acidic sites on the catalyst surface, which are active sites for the Beckmann rearrangement reaction. Using the Nb–HMS-X catalyst (Si/Nb = 13) under vapour-phase reaction conditions [temperature = 350 °C, weight hourly space velocity (WHSV) = 15 h−1, cyclohexanone oxime in benzene, cyclohexanone oxime : benzene weight ratio of 1 : 11] gave 100% cyclohexanone oxime conversion with 100% ε-caprolactam selectivity, with a space time yield of 1.4–1.6 × 10−3 mol h−1 gcat−1. The catalyst was highly recyclable up to 9 times without significant loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...