Selective formation of C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) N bonds via C(sp3)–H activation of isochroman in the presence of DTBP†
N bonds via C(sp3)–H activation of isochroman in the presence of DTBP†
J.
Feng
,
M. F.
Lv
,
G. P.
Lu
and
C.
Cai
*
Chemical Engineering College, Nanjing University of Science & Technology, 200 Xiaolingwei, Nanjing 210094, P. R. China. E-mail: c.cai@ njust.edu.cn; Fax: +86-25-84315030
First published on 9th December 2014
Abstract
An approach for the synthesis of isochroman derivatives via direct C(sp3)–H bond and N–H bond coupling is described. The C–N (amine or amide) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (imidate) products can be selectively achieved by controlling the amount of oxidants. Moreover, this metal- and base-free protocol is simple, easy-to-handle, and atom-economical which may have potential application in drug synthesis.
N (imidate) products can be selectively achieved by controlling the amount of oxidants. Moreover, this metal- and base-free protocol is simple, easy-to-handle, and atom-economical which may have potential application in drug synthesis.
Over the past few decades, significant progress has been made in direct C–H functionalization reactions.1 In particular, C–H bond activation reaction such as cross-dehydrogenative coupling (CDC) reaction is of immense importance because of its atom-economical and the environmental sustainability. CDC protocols have been successfully employed to access a diverse array of C–C and C–heteroatom bonds, by functionalizing C–H bonds of all types (sp, sp2, sp3).2 Among them, the functionalization of an inert C(sp3)–H bond has received special attention in recent years.3 The existing reports in this area mainly focus on the C(sp3)–H bond functionalization of cycloalkanes or benzylic arenes,4 chelating group assisted C(sp3)–H bond functionalization5 and the functionalization of the C(sp3)–H bond adjacent to heteroatoms.6
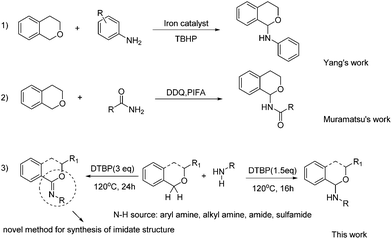
Isochroman derivatives are a class of important compounds which exhibit various potential bioactivities7 and serve as important building blocks in synthetic chemistry and drug design.8 Typically, isochroman derivatives have been prepared by different methods such as reduction of the corresponding esters,9 intramolecular or intermolecular cyclization reactions10 and decorations of isochroman.11 Recently guided by the powerful C–H activation technology, direct C–H bond functionalization of isochroman derivatives have appeared, and the direct C–C bond formations at the C(1) position of isochroman have been widely studied.12 However, only a few methods for the catalytic carbon–heteroatom formation, especially the C–N formation, were reported (Scheme 1, eqn (1)). In 2013, Yang and co-workers reported the iron-catalyzed direct C(sp3)–H amination reactions of isochroman derivatives.13 Subsequently, Muramatsu and co-workers succeeded in the amidation of isochroman utilizing DDQ and PIFA as oxidants (Scheme 1 eqn (2)).14
While acknowledging the pioneering work in this area, some issues such as the use of toxic reagents, limited substrate scopes (only two examples were reported in Muramatsu's work; the substrates of Yang's work were limited to aryl amines) and complex experimental procedures (separation of the iron mud from the products etc.) still need to be addressed. Moreover, the reactions under metal-free conditions are more appreciable in the nitrogen-containing pharmaceutical synthesis. In this regard, studies on the C–N coupling of isochroman derivatives and amines are still the cherished target.
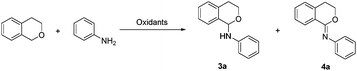
During our previous research, DTBP was found to be a suitable oxidant to promote the C–N formation between isochroman and aniline. Surprisingly, isochroman-1-imine was also detected by variation of reaction conditions. Screening of the references revealed that there lacks an efficient method for synthesis of the imidate structure,15 especially isochroman-1-imine derivatives. Herein, we wish to report a straightforward, convenient and selective CDC protocol for the sp3 C–H amination, amidation and further imidization of isochroman under metal-free conditions (Scheme 1 eqn (3)).
Initially, the coupling of isochroman and aniline was selected to optimize the reaction conditions. As shown in Table 1, the oxidant was crucial for this reaction. The reaction could not occur without an oxidant (entry 1), while the yield of 3a was increased to 82% in the presence of 1.5 equivalents of DTBP (entry 2). Other oxidants or oxidation systems were then screened, and the results revealed that DTBP was still the best choice (entries 2–7). Next, the reaction temperature was examined, and 120 °C was found to be the most appropriate temperature (entries 2, 10 and 11).
| Entry | Oxidants | Time (h) | Temp. (°C) | Yield of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
Yield of 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (%) b (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aniline (1.3 mmol), isochroman (1 mmol), oxidant (1.5 mmol), neat. b The yield was determined by LC. | |||||
| 1 | 16 | 120 | 0 | 0 | |
| 2 | DTBP (1.5 eq.) | 16 | 120 | 82 | Trace |
| 3 | TBHP (1.5 eq.) | 16 | 120 | 7 | 0 |
| 4 | BPO (1.5 eq.) | 16 | 120 | 0 | 0 |
| 5 | DDQ (2 eq.) | 16 | 120 | 10 | 0 |
| 6 | FeCl2 (0.1 eq.)/TBHP (2 eq.) | 16 | 120 | 55 | 0 |
| 7 | CuBr (0.1 eq.)/TBHP (2 eq.) | 16 | 120 | 26 | 0 |
| 8 | DTBP (1.5 eq.) | 4 | 120 | 22 | 0 |
| 9 | DTBP (1.5 eq.) | 24 | 120 | 79 | 8 |
| 10 | DTBP (1.5 eq.) | 16 | 100 | 43 | 0 |
| 11 | DTBP (1.5 eq.) | 16 | 140 | 62 | 8 |
| 12 | DTBP (2 eq.) | 16 | 120 | 77 | 11 |
| 13 | DTBP (3 eq.) | 16 | 120 | 28 | 62 |
| 14 | DTBP (3 eq.) | 24 | 120 | 8 | 76 |
| 15 | DTBP (5 eq.) | 24 | 120 | 7 | 76 |
| 16 | DTBP (3 eq.) | 36 | 120 | 5 | 77 |
Accompanied by the C–N coupling product 3a, a trace amount of imidate product 4a was also observed at 120 °C for 16 h (entry 2). Encouraged by the formation of unexpected product 4a, the oxidant equivalents and reaction time were then checked, respectively, to seek the possibility for selective synthesis of product 3a or 4a. To our delight, a moderate yield of product 4a was obtained when DTBP was added to 3 equivalents (entry 13). Further extension of the reaction time afforded the product 4a in a satisfactory yield (entry 14). However, adding more DTBP (4 equivalents) or prolonging the reaction time to 36 h did not give any significant improvement (entries 15 and 16).
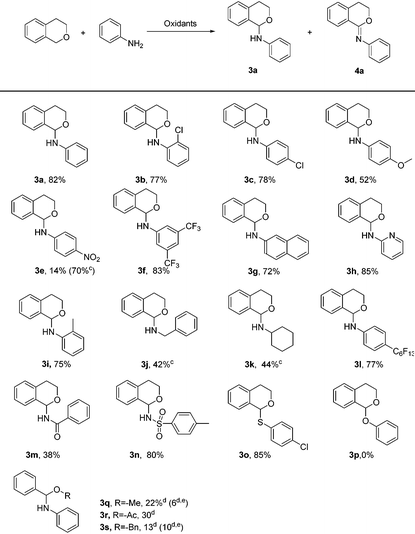
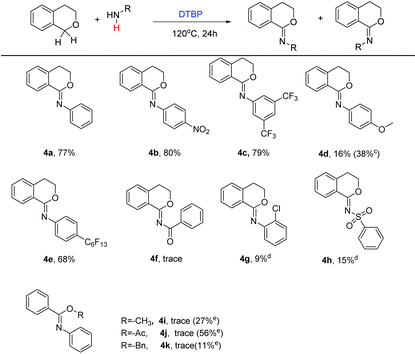
With the optimized conditions in hand, a series of aromatic amines, aliphatic amines and amides were chosen to establish the scope and generality of the C–N couplings (Table 2). As can be seen from Table 2, the present methodology was applicable to diverse aryl amines (3a–3i). The position of the substituent such as chloro had no significant influence on this reaction with comparable results obtained (3bvs.3c). The electronic effect played an important role, aryl amines bearing electron-withdrawing substituents coupled with isochroman smoothly to afford the corresponding products in better yields than those with electron-donating groups. As for 4-nitroaniline, the C–N product 3e and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N product 4b existed simultaneously even at the starting of the coupling. Product 4b could be obtained in the yield of 70%, compared to 14% of product 3e, in 16 hours. It is worth noting that 1-naphthylamine and 2-aminopyridine were well tolerated under the reaction conditions, giving the corresponding products in 72% and 85% yield, respectively (3g, 3h). This protocol was also suitable to aniline bearing a fluorous tail, which may have potential use in fluorine chemistry. Benzyl amine and alkyl amine were also examined. Unfortunately, along with the target products in moderate yields, some byproducts were also formed which could hardly be separated (3j, 3k). These byproducts may come from the C–H functionalization of the corresponding amine substrates 3. Examples using amides as the substrates were also investigated. Benzenesulfonamide underwent the coupling smoothly with 80% yield of product (3n), while benzoic amide was less reactive (3m). Next, linear benzylic ether was tried to replace isochroman. However, the reaction did not show significant selectivity, producing N-(methoxy(phenyl)methyl) aniline, methyl-N-phenyl benzimidate and N-phenylbenzamide as a mixture (3q–3r).
N product 4b existed simultaneously even at the starting of the coupling. Product 4b could be obtained in the yield of 70%, compared to 14% of product 3e, in 16 hours. It is worth noting that 1-naphthylamine and 2-aminopyridine were well tolerated under the reaction conditions, giving the corresponding products in 72% and 85% yield, respectively (3g, 3h). This protocol was also suitable to aniline bearing a fluorous tail, which may have potential use in fluorine chemistry. Benzyl amine and alkyl amine were also examined. Unfortunately, along with the target products in moderate yields, some byproducts were also formed which could hardly be separated (3j, 3k). These byproducts may come from the C–H functionalization of the corresponding amine substrates 3. Examples using amides as the substrates were also investigated. Benzenesulfonamide underwent the coupling smoothly with 80% yield of product (3n), while benzoic amide was less reactive (3m). Next, linear benzylic ether was tried to replace isochroman. However, the reaction did not show significant selectivity, producing N-(methoxy(phenyl)methyl) aniline, methyl-N-phenyl benzimidate and N-phenylbenzamide as a mixture (3q–3r).
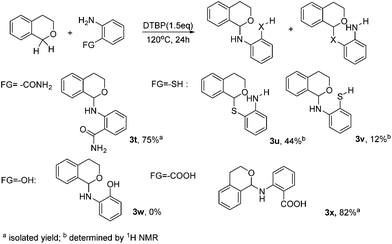
To further broaden the scope of our methodology, ortho-bifunctionalized substrates were then applied for the C–N coupling (Scheme 2). Interestingly, 2-aminobenzamide afforded 3t as the only product. Likewise, 2-aminobenzoic acid afforded 3x in the yield of 82%. However, two main products were obtained with 2-aminobenzenethiol. The ratio of the C–S coupling product and C–N coupling product is nearly 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 2, 3u, 3v). 2-Aminophenol failed in this reaction may be due to the instability of the substrate. From the above results, it may be briefly concluded that, in this DTBP mediated CDC coupling, the reactivity order is –SH > –NH2 > –CONH2, –COOH.
1 (Scheme 2, 3u, 3v). 2-Aminophenol failed in this reaction may be due to the instability of the substrate. From the above results, it may be briefly concluded that, in this DTBP mediated CDC coupling, the reactivity order is –SH > –NH2 > –CONH2, –COOH.
As mentioned above, the unexpected N-phenylisochroman-1-imine could be obtained under relatively harsher conditions. To explore the generality for the formation of imidate compounds, some representative amine or amide substrates were screened (Table 3). Aryl amines were found to be suitable for this transformation. Particularly, anilines substituted with electron-withdrawing groups such as –CF3, –NO2 smoothly coupled with isochroman to deliver the imidate in good yields (4b, 4c). In contrast, the substrate bearing a methoxyl group was less reactive with N-(4-methoxyphenyl)isochroman-1-imine and N-(4-methoxyphenyl) isochroman-1-amine obtained as a mixture in the yield of 16% and 38%, respectively (4d). The fluorous tail substituted aniline was also applied to the optimized conditions, giving the product 4e in 68% yield. In regard to the benzamide and sulfamide, only a trace of the product was formed due to its low reactivity (4f, 4h). Linear benzylic ether (or ester) was also tried; N-phenylbenzamide instead of the desired imidate product was produced in moderate yields. Comparatively, benzyl acetate showed higher yields.
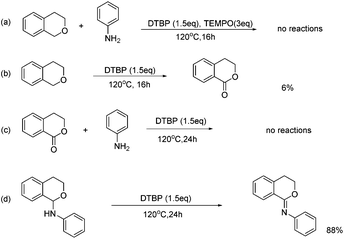
A series of controlled experiments were then carried out to investigate the details of the mechanism for the C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N formation protocols (Scheme 3). Excess TEMPO was initially added as a radical inhibitor. As expected, no desired product was observed, indicating that the present reaction may follow a radical mechanism. Then we further tried to clarify the unexpected C
N formation protocols (Scheme 3). Excess TEMPO was initially added as a radical inhibitor. As expected, no desired product was observed, indicating that the present reaction may follow a radical mechanism. Then we further tried to clarify the unexpected C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N formation process. A small amount of isochroman-1-one could be observed in the presence of DTBP without adding any coupling partners. It is possible that aniline reacted with isochroman-1-one to afford N-phenylisochroman-1-imine. However, the negative result of the reaction between isochroman-1-one and aniline excluded the possibility. In the course of the C
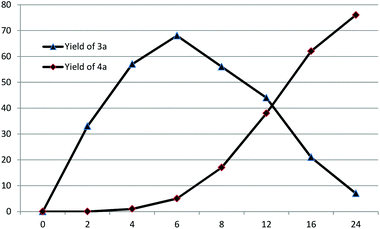
N formation process. A small amount of isochroman-1-one could be observed in the presence of DTBP without adding any coupling partners. It is possible that aniline reacted with isochroman-1-one to afford N-phenylisochroman-1-imine. However, the negative result of the reaction between isochroman-1-one and aniline excluded the possibility. In the course of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N formation process, N-phenylisochroman-1-amine may work as the intermediate and subsequent tracking of the reaction process have proved this point (Fig. 1). In the reaction process, the C–N and C
N formation process, N-phenylisochroman-1-amine may work as the intermediate and subsequent tracking of the reaction process have proved this point (Fig. 1). In the reaction process, the C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds were formed successively by variation of time. Remarkably, the C–N formation and C
N bonds were formed successively by variation of time. Remarkably, the C–N formation and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N formation could be selectively achieved because of the reactivity difference in the first C–H functionalization process and the subsequent dehydrogenation process.
N formation could be selectively achieved because of the reactivity difference in the first C–H functionalization process and the subsequent dehydrogenation process.
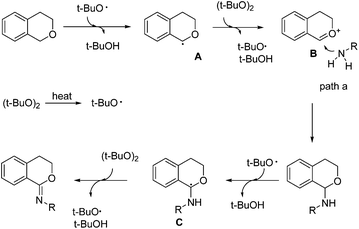
Based on the above experiments and the reported literature,3e a plausible reaction mechanism was proposed as shown in Scheme 4. DTBP decomposed into the tert-butoxyl radical under heating. A hydrogen abstraction of the C–H bond adjacent to an oxygen atom produced intermediate A, which was further oxidized to intermediate B. Aniline then coupled with B giving the C–N formation product 3a. When excess DTBP existed, the C–H bond of product 3a could be further activated. Similarly, the hydrogen abstraction of the C–H bond adjacent to an oxygen atom produced intermediate C, which could finally lead to imidate product 4a.
Conclusions
In summary, an unprecedented C–N formation via the direct oxidative cross-coupling of isochroman with amines (or amides) under metal-free conditions has been developed. This protocol can also afford the unexpected C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N formation products in the excess amount of an oxidant. Moreover, simple, metal- and base-free conditions, and atom-economy make this protocol more environmentally friendly.
N formation products in the excess amount of an oxidant. Moreover, simple, metal- and base-free conditions, and atom-economy make this protocol more environmentally friendly.
Notes and references
- For selected reviews or books on transition-metal-catalyzed C–H functionalization, see: R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242 RSC; J.-Q. Yu and Z.-J. Shi, C-H Activation, Springer, Berlin, Germany, 2010 CrossRef CAS PubMed; C. L. Sun, B. J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; A. N. Campbell and S. S. Stahl, Acc. Chem. Res., 2012, 45, 851 CrossRef PubMed; S. I. Kozhushkov and L. Ackermann, Chem. Sci., 2013, 4, 886 RSC.
- For selected reviews on C(sp)–H or C(sp2)–H bond functionalization via CDC protocols, see: C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; W. Shi, C. Liu and A.-W. Lei, Chem. Soc. Rev., 2011, 40, 2761 RSC; C. Liu, H. Zhang, W. Shi and A.-W. Lei, Chem. Rev., 2011, 111, 1780 CrossRef PubMed; K. M. Engle, T. S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef PubMed; X.-W. Guo, Z.-P. Li and Z.-J. Li, Process Chem., 2010, 22, 1434 Search PubMed.
- For selected reviews on C(sp3)–H bond functionalization involving CDC protocols, see: L. McMurray, F. O. Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885 RSC; M. Uyanik and K. Ishihara, ChemCatChem, 2012, 4, 177 CrossRef CAS; J. L. Roizen, M. E. Harvey and J. D. Bois, Acc. Chem. Res., 2012, 45, 911 CrossRef PubMed; S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef PubMed; B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC.
- For selected references, see: X. Y. Guo and C.-J. Li, Org. Lett., 2011, 13, 4977 CrossRef CAS PubMed; A. P. Antonchick and L. Burgmann, Angew. Chem., Int. Ed., 2013, 52, 3267 CrossRef PubMed; T. Hoshikawa and M. Inoue, Chem. Sci., 2013, 4, 3118 RSC; Z. J. Li, Y. Zhang, L. Z. Zhang and Z. Q. Liu, Org. Lett., 2014, 16, 382 CrossRef PubMed; Y. F. Zhu and Y. Y. Wei, Chem. Sci., 2014, 5, 2379 RSC; Q. Michaudel, D. Thevenet and P. S. Baran, J. Am. Chem. Soc., 2012, 134, 2547 CrossRef PubMed; B. L. Tran, B. J. Li, M. Driess and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 2555 CrossRef PubMed; B. Du, B. Jin and P. Sun, Org. Lett., 2014, 16, 3032 CrossRef PubMed; F. Jia and Z.-P. Li, Org. Chem. Front., 2014, 1, 194 RSC.
- For selected references, see: Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2013, 135, 5308 CrossRef CAS PubMed; S.-J. Lou, D.-Q. Xu, D.-F. Shen, Y.-F. Wang, Y.-K. Liu and Z.-Y. Xu, Chem. Commun., 2012, 48, 11993 RSC; C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef PubMed; G. Majji, S. Guin, A. Gogoi, S. K. Rout and B. K. Patel, Chem. Commun., 2013, 49, 3031 RSC; Y. Wu, P. Y. Choy, F. Mao and F. Y. Kwong, Chem. Commun., 2013, 49, 689 RSC; D. L. Priebbenow and C. Bolm, Org. Lett., 2014, 16, 1650 CrossRef PubMed.
- For selected examples, see: S. Conejero, M. Paneque, M. L. Poveda, L. L. Santos and E. Carmona, Acc. Chem. Res., 2010, 43, 572 CrossRef CAS PubMed; X. Liu, B. Sun, Z. Xie, X. Qin, L. Liu and H. X. Lou, J. Org. Chem., 2013, 78, 3104 CrossRef PubMed; S. Pan, J. Liu, H. Li, Z. Wang, X. Guo and Z. Li, Org. Lett., 2010, 12, 1932 CrossRef PubMed; Z. Xie, Y. Cai, H. Hu, C. Lin, J. Jiang, Z. Chen, L. Wang and Y. Pan, Org. Lett., 2013, 15, 4600 CrossRef PubMed; D. Liu, C. Liu, H. Li and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 4453 CrossRef PubMed; H. Richter and O. G. Mancheño, Eur. J. Org. Chem., 2010, 4460 Search PubMed; K. B. Raju, N. K. Bejjanki and K. Nagaiah, RSC Adv., 2014, 4, 50795 RSC.
- P. Martin and P. Consroe, Science, 1976, 194, 965 Search PubMed; M. J. de Groot, A. A. Alex and B. C. Jones, J. Med. Chem., 2002, 45, 1983 CrossRef CAS PubMed; S. Yamaori, M. Kushihara, I. Yamamoto and K. Watanabe, Biochem. Pharmacol., 2010, 79, 1691 CrossRef PubMed.
- For selected references, see: F. Lehmann, A. Pettersen, E. A. Currier, V. Sherbukhin, R. Olsson, U. Hacksell and K. Luthman, J. Med. Chem., 2006, 49, 2232 CrossRef CAS PubMed; C. A. Maier and B. Wünsch, J. Med. Chem., 2002, 45, 438 CrossRef PubMed.
- For representative references see: N. Sakai, T. Moriya and T. Konakahara, J. Org. Chem., 2007, 72, 5920 CrossRef CAS PubMed.
- For representative references see: G. B. Jones, J. M. Wright, G. Hynd, J. K. Wyatt, P. M. Warner, R. S. Huber, A. Li, M. W. Kilgore, R. P. Sticca and R. S. Pollenz, J. Org. Chem., 2002, 67, 5727 CrossRef CAS PubMed; L. Pehlivan, E. Métay, D. Delbrayelle, G. Mignani and M. Lemaire, Eur. J. Org. Chem., 2012, 4689 CrossRef.
- Y. Yu, W. Yang, F. Rominger and A. S. K. Hashmi, Angew. Chem., 2013, 125, 7735 ( Angew. Chem., Int. Ed. , 2013 , 52 , 7586 ) CrossRef.
- For selected C–C or C–S bond formations at the C(1) position of isochroman examples, see: Z. Li, R. Yu and H. Li, Angew. Chem., Int. Ed., 2008, 47, 7497 CrossRef CAS PubMed; C.-X. Song, G.-X. Cai, T. R. Farrell, Z.-P. Jiang, H. Li, L.-B. Gan and Z.-J. Shi, Chem. Commun., 2009, 6002 RSC; C. A. Correia and C.-J. Li, Heterocycles, 2010, 82, 555 CrossRef; H. Richter, R. Rohlmann and O. G. Mancheño, Chem. – Eur. J., 2011, 17, 11622 CrossRef PubMed; A. Pinter and M. Klussmann, Adv. Synth. Catal., 2012, 354, 701 CrossRef; X. Liu, B. Sun, Z. Xie, X. Qin, L. Liu and H. Lou, J. Org. Chem., 2013, 78, 3104 CrossRef PubMed; Z. Meng, S. Sun, H. Yuan, H. Lou and L. Liu, Angew. Chem., Int. Ed., 2014, 53, 543 CrossRef PubMed; S. J. Park, J. R. Price and M. H. Todd, J. Org. Chem., 2012, 77, 949 CrossRef PubMed; W. Muramatsu, K. Nakano and C.-J. Li, Org. Lett., 2013, 15, 3650 CrossRef PubMed; K. Qvortrup, D. A. Rankic and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 626 CrossRef PubMed; C. Shen, P. F. Zhang, Q. Sun, S. Q. Bai, T. S. Andy Hor and X. G. Liu, Chem. Soc. Rev., 2015, 44, 291 RSC.
- D. Chen, F. Pan, J. Gao and J. Yang, Synlett, 2013, 2085 CAS.
- W. Muramatsu and K. Nakano, Org. Lett., 2014, 16, 2042 CrossRef CAS PubMed.
- R. Abhijit, K. Versha and A. P. Bhaduri, Synth. Commun., 2006, 36, 715–727 CrossRef CAS; V. F. Burdukovskii and D. M. Mognonov, Russ. Chem. Bull., 2008, 57, 1247 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures; spectral data for all reported compounds. See DOI: 10.1039/c4qo00293h |
| This journal is © the Partner Organisations 2015 |