Imino exchange reaction in a dearomatization strategy: synthesis of N-acyl diarylamines and phenothiazines from two anilines†
Li
Zhang
a,
Huiqing
Wang
b,
Bo
Yang
*b and
Renhua
Fan
*a
aDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: rhfan@fudan.edu.cn; Fax: +86-21-6510-2412; Tel: +86-21-6564-2019
bDepartment of Urology, Changhai Hospital, 168 Changhai Road, Shanghai 200433, China. E-mail: yangbochanghai@126.com
First published on 18th August 2014
Abstract
N-Sulfonyl cyclohexadienimines generated from an iodine(III)-induced oxidative dearomatization of N-sulfonyl protected para-substituted anilines are ready to undergo an imino exchange reaction with another aniline, which provides an alternative way to access N-acyl diarylamines and phenothiazines.
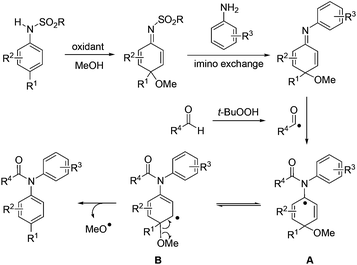
As a result of the inherent reactivity stored within the aromatic systems, the dearomatization strategy has been intensively explored and utilized as a powerful tool in organic synthesis.1 The oxidative dearomatization of para-substituted anilines forms cyclohexadienimines, and these dearomatized products have proven to be highly reactive intermediates for various 1,4-additions.2,3 However, some of our recent studies revealed that a 1,2-imino exchange reaction of N-sulfonyl cyclohexadienimines might be preferred over the 1,4-addition when another aniline was used as a nucleophile. This reaction offers strategic opportunities for preparing a variety of diarylamine derivatives4 from two anilines. Herein, we wish to present our success in applying this strategy in the synthesis of N-acyl diarylamines and phenothiazines.
We conceived that the aromatization of the imino exchanged product (N-aryl cyclohexadienimine) might occur via a single-electron-transfer way triggered by a free radical. As shown in Scheme 1, the addition of an in situ generated aldehydic radical5 to the nitrogen–carbon double bond of N-aryl cyclohexadienimine affords a radical intermediate A.6 After an isomerisation and a β-scission, N-acyl diarylamine is formed.
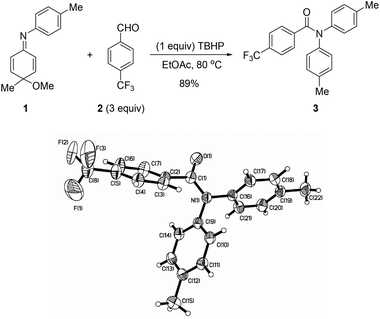
We initiated our investigation on the reaction of N-aryl cyclohexadienimine 1 with 4-(trifluoromethyl)benzaldehyde 2 and TBHP. Among the reaction solvents and temperatures examined, ethyl acetate proved to be the best reaction medium at 80 °C. The best ratio of N-aryl cyclohexadienimine, 4-methoxybenzaldehyde, and TBHP was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, leading to N-acyl diarylamine 3 in 89% yield. The structure of compound 3 was confirmed by its single-crystal diffraction analysis (Scheme 2).7
1, leading to N-acyl diarylamine 3 in 89% yield. The structure of compound 3 was confirmed by its single-crystal diffraction analysis (Scheme 2).7
With the optimized reaction conditions established, the synthesis of N-acyl diarylamines from anilines and aldehydes was investigated (Table 1). The PhI(OAc)2-induced oxidative dearomatization and the Bi(OTf)3-catalyzed 1,2-imino exchange reaction were conducted in methanol in one pot. After a simple workup process, the crude mixture was treated with aldehydes and TBHP. For most cases, the reaction proceeded smoothly and afforded N-acyl diarylamines in moderate to good yields. With respect to aldehydes, arylaldehydes bearing a range of substituents were suitable reaction partners. The reaction of butyraldehyde did not give rise to the desired product. A variety of anilines were suitable substrates for this three-step synthesis. The imino exchange reaction with 4-amino-pyridine was complex.
| Entry | Ar1 | Ar2 | R4 | Producta (%) |
|---|---|---|---|---|
| a Reported yields are of the isolated products. | ||||
| 1 | 4-MeC6H4 | 4-MeC6H4 | 4-CF3C6H4 | 3 (82) |
| 2 | 4-MeC6H4 | 4-MeC6H4 | 4-FC6H4 | 4 (80) |
| 3 | 4-MeC6H4 | 4-MeC6H4 | 4-CNC6H4 | 5 (83) |
| 4 | 4-MeC6H4 | 4-MeC6H4 | 4-NO2C6H4 | 6 (82) |
| 5 | 4-MeC6H4 | 4-MeC6H4 | 4-ClC6H4 | 7 (86) |
| 6 | 4-MeC6H4 | 4-MeC6H4 | 4-MeOC6H4 | 8 (79) |
| 7 | 4-MeC6H4 | 4-MeC6H4 | C6H5 | 9 (85) |
| 8 | 4-MeC6H4 | 4-MeC6H4 | nPr | 10 (0) |
| 9 | 4-EtC6H4 | 4-MeC6H4 | 4-MeOC6H4 | 11 (75) |
| 10 | 4-nBuC6H4 | 4-MeC6H4 | 4-MeOC6H4 | 12 (80) |
| 11 | 4-iPrC6H4 | 4-MeC6H4 | 4-MeOC6H4 | 13 (76) |
| 12 | 4-MeC6H4 | C6H5 | 4-MeOC6H4 | 14 (66) |
| 13 | 4-MeC6H4 | 3,5-(Me)2C6H3 | 4-MeOC6H4 | 15 (62) |
| 14 | 4-MeC6H4 | 3,4-(Me)2C6H3 | 4-MeOC6H4 | 16 (77) |
| 15 | 4-MeC6H4 | 4-MeOC6H4 | 4-MeOC6H4 | 17 (81) |
| 16 | 4-MeC6H4 | 4-ClC6H4 | 4-MeOC6H4 | 18 (85) |
| 17 | 4-MeC6H4 | 4-FC6H4 | 4-MeOC6H4 | 19 (76) |
| 18 | 4-MeC6H4 | 4-Pyridyl | 4-MeOC6H4 | 20 (0) |
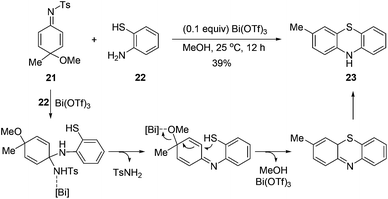
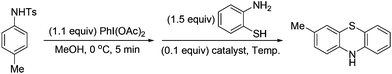
Interestingly, under the standard conditions, an unexpected product, phenothiazine 23, was isolated in 39% yield from the imino exchange reaction with 2-aminobenzenethiol. It is proposed that this product was formed from an intramolecular trapping of the imino exchange intermediate by the thiol group (Scheme 3). Phenothiazines constitute a valuable class of compounds.8 They are widely used as analgesic,9 anti-inflammatory,10 antiplatelet,11 and multiple drug resistance reverting agents.12 Because of the importance of these compounds, recently, the groups of Jørgensen,13 Ma,14 and Zeng15 have developed transition-metal catalyzed coupling reactions to prepare phenothiazines from a variety of aryl halides.
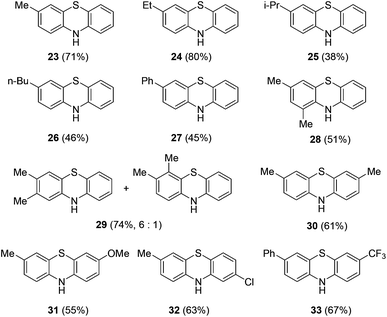
To develop an alternative way to access phenothiazines from anilines, various metal salts were examined to promote the formation of phenothiazine 23 in a one-pot reaction (Table 2). Copper(I) iodide proved to be the best Lewis acid to promote the imino exchange and the intramolecular trapping reaction. In the presence of 0.1 equiv. of copper(I) iodide, the reaction at reflux gave rise to phenothiazines 23 in 71% yield (Table 2, entry 15). In the absence of the added catalysts, the one-pot reaction still produced compound 23 in 37% yield. But the formation of compound 23 was not observed when N-Ts cyclohexadienimine 21 was used in the absence of a catalyst. It is supposed that the formation of phenothiazine might also be promoted by acetic acid metabolized from PhI(OAc)2 in dearomatization reaction. Under the optimized conditions, a range of para-substituted anilines reacted with 2-aminobenzenethiols to afford the corresponding phenothiazines in moderate to good yields (Scheme 4). When 3,4-dimethylbenzenamine was used, the attack by the thiol group at the less hindered position was preferred.
| Entry | Catalyst | Temperature (°C) | 23 (%) |
|---|---|---|---|
| a Reported yields are of the isolated products. | |||
| 1 | Bi(OTf)3 (0.1 equiv.) | 0–25 | 37 |
| 2 | Pd(OAc)2 (0.1 equiv.) | 0–25 | 28 |
| 3 | AgOTf (0.1 equiv.) | 0–25 | 26 |
| 4 | Sc(OTf)3 (0.1 equiv.) | 0–25 | 27 |
| 5 | FeCl3 (0.1 equiv.) | 0–25 | 25 |
| 6 | Yb(OTf)3 (0.1 equiv.) | 0–25 | 25 |
| 7 | Zn(OTf)2 (0.1 equiv.) | 0–25 | 34 |
| 8 | Cu(OTf)2 (0.1 equiv.) | 0–25 | 43 |
| 9 | Cu(OAc)2 (0.1 equiv.) | 0–25 | 33 |
| 10 | CuCl2 (0.1 equiv.) | 0–25 | 36 |
| 11 | CuBr2 (0.1 equiv.) | 0–25 | 37 |
| 12 | CuCl (0.1 equiv.) | 0–25 | 41 |
| 13 | CuBr (0.1 equiv.) | 0–25 | 42 |
| 14 | CuI (0.1 equiv.) | 0–25 | 48 |
| 15 | CuI (0.1 equiv.) | 0–65 | 71 |
| 16 | CuI (0.05 equiv.) | 0–65 | 57 |
| 17 | CuI (0.2 equiv.) | 0–65 | 67 |
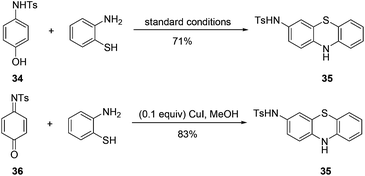
It is noteworthy that when N-Ts protected 4-aminophenol 34 was used as the substrate, the reaction afforded phenothiazin-3-amine 35 as the product. N-Tosyl quinine monosulfonimide 36 isolated from the corresponding oxidative dearomatization reaction could be converted to compound 35 (Scheme 5).
In conclusion, we have developed an alternative method to prepare N-acyl diarylamines and phenothiazines from anilines using a dearomatization strategy. The key step in this strategy is the imino exchange reaction. Work is currently ongoing to explore its reaction mechanism and possible synthetic applications, and these results will be reported in due course.
Financial support from the National Natural Science Foundation of China (no. 21332009), Specialized Research Fund for the Doctoral Program of Higher Education (no. 20120071110009), and Shanghai Science and Technology Committee (13431900103) is gratefully acknowledged.
Notes and references
- Reviews on dearomatization, see: (a) A. R. Pape, K. P. Kaliappan and E. P. Kündig, Chem. Rev., 2000, 100, 2917 CrossRef CAS PubMed; (b) P. L. Smith, M. D. Chordia and W. D. Harman, Tetrahedron, 2001, 57, 8203 CrossRef CAS; (c) D. Magdziak, S. J. Meek and T. R. R. Pettus, Chem. Rev., 2004, 104, 1383 CrossRef CAS PubMed; (d) F. López Ortiz, M. J. Iglesias, I. Fernández, C. M. Andújar Sánchez and G. R. Gómez, Chem. Rev., 2007, 107, 1580 CrossRef PubMed; (e) S. Quideau, L. Pouységu and D. Deffieux, Synlett, 2008, 467 CrossRef CAS PubMed; (f) L. Pouységu, D. Deffieux and S. Quideau, Tetrahedron, 2010, 66, 2235 CrossRef PubMed; (g) S. P. Roche and J. A. Porco Jr., Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS PubMed; (h) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 12662 CrossRef PubMed; (i) Q. Ding, Y. Ye and R. Fan, Synthesis, 2013, 1 Search PubMed.
- (a) S. C. Banfield, D. B. England and M. A. Kerr, Org. Lett., 2001, 3, 3325 CrossRef CAS PubMed; (b) S. K. Jackson, S. C. Banfield and M. A. Kerr, Org. Lett., 2005, 7, 1215 CrossRef CAS PubMed; (c) S. Quideau, L. Pouységu, A. Ozanne and J. Gagnepain, Molecules, 2005, 10, 201 CrossRef CAS; (d) A. Jean, J. Cantat, D. Bérard, D. Bouchu and S. Canesi, Org. Lett., 2007, 9, 2553 CrossRef CAS PubMed; (e) C. Sabot, D. Bérard and S. Canesi, Org. Lett., 2008, 10, 4629 CrossRef CAS PubMed; (f) M.-A. Giroux, K. C. Guérard, M.-A. Beaulieu, C. Sabot and S. Canesi, Eur. J. Org. Chem., 2009, 3871 CrossRef CAS PubMed.
- (a) L. Wang and R. Fan, Org. Lett., 2012, 14, 3596 CrossRef CAS PubMed; (b) L. Zhang, W. Wang and R. Fan, Org. Lett., 2013, 15, 2018 CrossRef CAS PubMed; (c) M. Yang, J. Tang and R. Fan, Org. Lett., 2013, 15, 3464 CrossRef CAS PubMed; (d) C. Zheng, J. Chen and R. Fan, Org. Lett., 2014, 16, 816 CrossRef CAS PubMed; (e) X. Feng, H. Wang, B. Yang and R. Fan, Org. Lett., 2014, 16, 3600 CrossRef CAS PubMed.
- (a) A. Ricci, Modern Amination Methods, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) R. N. Salvatore, C. H. Yoon and K. W. Jung, Tetrahedron, 2001, 57, 7785 CrossRef CAS; (c) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27 RSC.
- M.-B. Zhou, R.-J. Song, X.-H. Ouyang, Y. Liu, W.-T. Wei, G.-B. Deng and J.-H. Li, Chem. Sci., 2013, 4, 2690 RSC , and references cited therein.
- S. H. Kyne, C. H. Schiesser and H. Matsubara, J. Org. Chem., 2008, 73, 427 CrossRef CAS PubMed , and references cited therein.
- Crystallographic data for compound 3 (CCDC 1014922).
- (a) M. Paulino, F. Iribarne, F. Dubin, S. Aguilera-Morales, O. Tapia and A. O. Stoppani, Mini-Rev. Med. Chem., 2005, 5, 499 CrossRef CAS; (b) M. J. Ohlow and B. Moosmann, Drug Discovery Today, 2011, 16, 119 CrossRef CAS PubMed; (c) K. Pluta, B. Morak-M1odawska and M. Jeleń, Eur. J. Med. Chem., 2011, 46, 3179 CrossRef CAS PubMed; (d) M. K. Kathiravan, A. B. Salake, A. S. Chothe, P. B. Dudhe, R. P. Watode, M. S. Mukta and S. Gadhwe, Bioorg. Med. Chem. Lett., 2012, 20, 5678 CrossRef CAS PubMed.
- A. Rajasekaran and P. P. Thampi, Eur. J. Med. Chem., 2004, 39, 273 CrossRef CAS PubMed.
- J. Unkovic, A. Barbier, M. Combes and P. Vic, Arch. Toxicol., Suppl., 1988, 12, 16 CAS.
- G. A. Silva, L. M. M. Costa, F. C. F. Brito, A. L. P. Miranda, E. J. Barreiro and C. A. M. Fraga, Bioorg. Med. Chem. Lett., 2004, 12, 3149 CrossRef CAS PubMed.
- (a) Y. C. Mayur, S. Jagadeesh and K. N. Thimmaiah, Mini-Rev. Med. Chem., 2006, 6, 1383 CrossRef CAS; (b) A. Bisi, M. Meli, S. Gobbi, A. Rampa, M. Tolomeo and L. Dusonchet, Bioorg. Med. Chem., 2008, 16, 6474 CrossRef CAS PubMed.
- T. Dahl, C. W. Tornøe, B. Bang-Anderson, P. Nielsen and M. Jørgensen, Angew. Chem., Int. Ed., 2008, 47, 1726 CrossRef CAS PubMed.
- D. W. Ma, Q. Geng, H. Zhang and Y. W. Jiang, Angew. Chem., Int. Ed., 2010, 49, 1291 CrossRef CAS PubMed.
- C. Dai, X. F. Sun, X. Z. Tu, L. Wu, D. Zhan and Q. L. Zeng, Chem. Commun., 2012, 48, 5367 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, copies of 1H NMR and 13C NMR of new compounds. CCDC 1014922. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00201f |
| This journal is © the Partner Organisations 2014 |