Generation of 1-(trifluoromethyl)isoquinolines via a copper-catalyzed reaction of isoquinoline-N-oxide with Togni reagent†
Congbin
Fan‡
ab,
Junjie
Song‡
a,
Guanyinsheng
Qiu
b,
Gang
Liu
*a and
Jie
Wu
*b
aJiangxi Key Laboratory of Organic Chemistry, Jiangxi Science & Technology Normal University, Nanchang 330013, China. E-mail: liugang0926@163.com
bDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn; Fax: +86 21 6564 1740; Tel: +86 21 6510 2412
First published on 15th July 2014
Abstract
Isoquinoline-N-oxides react with Togni reagent catalyzed by copper(II) triflate, leading to 1-(trifluoromethyl)isoquinolines in good yields. The reaction proceeds smoothly under mild conditions with high efficiency.
1. Introduction
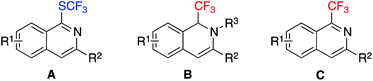
In the past decade, we have been interested in the generation of natural-product-like compounds with privileged scaffolds using the strategy of diversity-oriented synthesis.1 Among the libraries of small molecules we have synthesized,2 several hits of isoquinolines for the inhibition of cervical carcinoma were discovered in the subsequent biological evaluation. Prompted by this result and with an expectation of finding more active compounds, the accessibility of diverse isoquinolines is of high demand.Recently, the synthesis of fluorinated molecules has attracted much attention within the chemical community. Different methods have been reported for the incorporation of fluoro-containing groups into organic compounds.3,4 We are also interested in this field for the construction of fluorinated heterocycles.5,6 It would be desirable to provide facile routes for fluorinated isoquinolines.6,7 Xu and Liu have developed a silver-catalyzed oxidative aminofluorination of alkynes for the synthesis of 4-fluoroisoquinolines.7a 1-((Trifluoromethyl)thio)isoquinolines A (Fig. 1) can be accessed through a silver(I)-catalyzed reaction of 2-alkynylbenzaldoxime with silver (trifluoromethyl)thiolate in the presence of p-methoxybenzenesulfonyl chloride.6a We have also described an efficient route to 1-(trifluoromethyl)-1,2-dihydroisoquinolines B (Fig. 1) under mild conditions via a silver(I)-catalyzed reaction of 2-alkynylaryl aldimine with trimethyl(trifluoromethyl)silane.6b In order to expand the diversity of fluorinated isoquinolines, we envisioned that 1-(trifluoromethyl)isoquinolines C (Fig. 1) could be prepared as well, which would be beneficial for our specific biological assays. Therefore, we initiated a program for the method development of the construction of 1-(trifluoromethyl)isoquinolines.
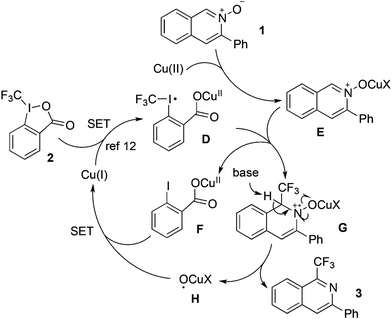
The importance of the trifluoromethyl group in many drugs or drug candidates has been recognized.8 Higher solubility and lipophilicity have been observed for trifluoromethyl-substituted compounds, leading to better membrane permeability and increased bioavailability. Therefore, methods for the trifluoromethylation process have been developed.9 On the other hand, as mentioned above, we have built up a library of isoquinolines over the past few years. Our strategy focused on tandem reactions using 2-alkynylbenzaldoximes as the starting material.10 The key intermediate of isoquinoline-N-oxide has been identified during the transformation.11 In the meantime, the Togni reagent has been used widely for introduction of the trifluoromethyl group into small molecules.12 We conceived that 1-(trifluoromethyl)isoquinolines would be constructed through reaction of isoquinoline-N-oxide with Togni reagent.13 The proposed synthetic route is presented in Scheme 1.
We hypothesized that a SET (single electron transfer) process would occur to give intermediate D in the presence of Togni reagent and copper catalyst based on the result disclosed by Bouyssi and Baudoin.12a In the meantime, complex E would be formed when isoquinoline-N-oxide was treated with copper salt. Thus, after the addition of trifluoromethyl radical to isoquinoline-N-oxide, intermediate G would be afforded with the formation of intermediate F. The subsequent transformation would produce the expected 1-(trifluoromethyl)isoquinoline 3 and radical H. Therefore, we started to investigate the feasibility of this transformation.
2. Results and discussion
Initially, a model reaction of isoquinoline-N-oxide 1a and Togni reagent 2 was explored (Table 1). In the beginning, the reaction was performed in the presence of CuCl (10 mol%) and DMAP (2.0 equiv.) in MeCN at 40 °C. To our delight, the desired product 3a was obtained in 26% yield (Table 1, entry 1). This result encouraged us to carry out the further screening of other metal catalysts. The yield could not be improved when the catalyst was changed to CuBr or CuI (Table 1, entries 2 and 3). A lower yield was observed when FeCl2 was used as a replacement (Table 1, entry 4). Only a trace amount of product was detected when CuSCN was employed in the transformation (Table 1, entry 5). Further examination of copper salts revealed (Table 1, entries 6–9) that the reaction worked efficiently in the presence of copper(II) triflate, giving rise to the corresponding product 3a in 51% yield (Table 1, entry 9). A control experiment indicated that no reaction occurred in the absence of metal catalyst (Table 1, entry 10). Further, a survey of bases demonstrated that no better result was obtained when other bases were utilized in the reaction process (Table 1, entries 11–16). The reaction performed in various solvents was then investigated (Table 1 entries 17–21), leading to the expected product 3a in lower yields. The yield could not be improved when the reaction temperature was changed to 60 or 80 °C (Table 1 entries 22 and 23). The yield was dramatically decreased when the reaction took place at room temperature (data not shown in Table 1). Gratifyingly, the reaction afforded the corresponding product 3a in 66% yield when the amount of DMAP was increased to 3.0 equiv. (Table 1, entry 24).| Entry | [M] | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: isoquinoline-N-oxide 1a (0.5 mmol), Togni reagent 2 (0.6 mmol), metal catalyst (10 mol%), base (2.0 equiv.), solvent (2.0 mL), 40 °C. b Isolated yield based on isoquinoline-N-oxide 1a. c The reaction occurred at 60 °C. d The reaction was performed at 80 °C. e In the presence of 3.0 equiv. of DMAP. | ||||
| 1 | CuCl | DMAP | MeCN | 26 |
| 2 | CuBr | DMAP | MeCN | 20 |
| 3 | CuI | DMAP | MeCN | 18 |
| 4 | FeCl2 | DMAP | MeCN | 15 |
| 5 | CuSCN | DMAP | MeCN | Trace |
| 6 | CuBr2 | DMAP | MeCN | 11 |
| 7 | CuCl2 | DMAP | MeCN | 14 |
| 8 | Cu(OAc)2 | DMAP | MeCN | 27 |
| 9 | Cu(OTf)2 | DMAP | MeCN | 51 |
| 10 | — | DMAP | MeCN | nr |
| 11 | Cu(OTf)2 | Cs2CO3 | MeCN | 45 |
| 12 | Cu(OTf)2 | NaOH | MeCN | 42 |
| 13 | Cu(OTf)2 | t-BuOK | MeCN | 24 |
| 14 | Cu(OTf)2 | DBU | MeCN | 39 |
| 15 | Cu(OTf)2 | Et3N | MeCN | 35 |
| 16 | Cu(OTf)2 | K2CO3 | MeCN | 46 |
| 17 | Cu(OTf)2 | DMAP | DCE | 23 |
| 18 | Cu(OTf)2 | DMAP | Toluene | 16 |
| 19 | Cu(OTf)2 | DMAP | DMF | 22 |
| 20 | Cu(OTf)2 | DMAP | THF | 15 |
| 21 | Cu(OTf)2 | DMAP | 1,4-Dioxane | 13 |
| 22c | Cu(OTf)2 | DMAP | MeCN | 45 |
| 23d | Cu(OTf)2 | DMAP | MeCN | 49 |
| 24e | Cu(OTf)2 | DMAP | MeCN | 66 |
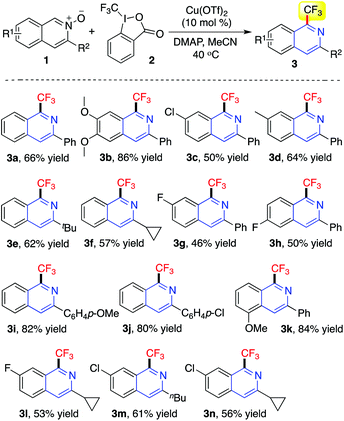
The scope of this copper(II)-catalyzed reaction of isoquinoline-N-oxides 1 with Togni reagent 2 was then explored under the above optimized conditions (10 mol% of Cu(OTf)2, DMAP, MeCN, 40 °C). The results are summarized in Table 2. All the reactions worked well to afford the expected products 3 in moderate to good yields. Reactions of isoquinoline-N-oxides 1 with an electron-donating group attached on the aromatic ring gave rise to the corresponding products in higher yields, compared with the results obtained from isoquinoline-N-oxides 1 with an electron-withdrawing group attached to the aromatic ring. For example, the methoxy-substituted compound 3b was produced in 86% yield, whereas the chloro-substituted product 3c was generated in 50% yield. Additionally, the reactions of isoquinoline-N-oxides 1 with different groups at the R2 position were examined. As expected, all transformations proceeded smoothly under the standard conditions, leading to the desired 1-(trifluoromethyl)isoquinolines in reasonable yields. During the process, not only aromatic groups but also alkyl groups were all compatible.
| a Isolated yield based on isoquinoline-N-oxide 1. |
|---|
|
3. Conclusion
In conclusion, we have described a copper(II)-catalyzed reaction of isoquinoline-N-oxides with Togni reagent, leading to 1-(trifluoromethyl)isoquinolines in good yields. The trifluoromethyl group could be easily introduced at the 1-position of isoquinoline under mild conditions. The reaction scope has been demonstrated. Currently, incorporation of the trifluoromethyl group into other heterocycles is ongoing in our laboratory.4. Experimental section
The general experimental procedure for the synthesis of 1-(trifluoromethyl)isoquinolines via a copper-catalyzed reaction of isoquinoline-N-oxide with Togni reagent is as follows: Isoquinoline N-oxide 1 (0.2 mmol) was added to a solution of 1-trifluoromethyl-1,2-benziodoxol-3(1H)-one (Togni reagent) (0.24 mmol), DMAP (0.6 mmol), and Cu(OTf)2 (0.02 mmol) in CH3CN (2.0 mL) under N2 atmosphere. The mixture was stirred at 40 °C for 8–12 h. After completion of the reaction as indicated by TLC, the solvent was evaporated and the residue was purified by column chromatography on silica gel to provide the product 3.3-Phenyl-1-(trifluoromethyl)isoquinoline 3a
1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 8.8 Hz, 1H), 7.92 (s, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.75 (dd, J = 6.4, 2.8 Hz, 2H), 7.72–7.65 (m, 1H), 7.60 (t, J = 7.5 Hz, 1H), 7.53–7.44 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 152.9, 148.0, 131.7, 130.5, 129.9, 129.8, 128.6, 128.4 128.2, 127.9, 127.5, 125.8 (q, 1JCF = 286.1 Hz), 122.2; 19F NMR (378 MHz, CDCl3) δ −62.05; HRMS (ESI) calcd for C16H11F3N: 274.0838 (M + H+), found: 274.0832.6,7-Dimethoxy-3-phenyl-1-(trifluoromethyl)isoquinoline 3b
1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 7.3 Hz, 2H), 7.33 (t, J = 7.4 Hz, 2H), 7.29–7.24 (m, 1H), 6.74 (d, J = 10.9 Hz, 2H), 6.41 (s, 1H), 3.91 (s, 3H), 3.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 154.2, 152.4, 149.5, 148.3, 143.3, 130.9, 128.8, 128.1, 127.4, 127.0, 125.3 (q, 1JCF = 283.8 Hz), 124.1, 119.0, 110.6, 107.7, 56.4, 55.8; 19F NMR (378 MHz, CDCl3) δ −63.95; HRMS (ESI) calcd for C18H15F3NO2: 334.1049 (M + H+), found: 334.1066.7-Chloro-3-phenyl-1-(trifluoromethyl)isoquinoline 3c
1H NMR (400 MHz, CDCl3) δ 8.29 (s, 1H), 7.89 (s, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.73 (dd, J = 6.5, 2.9 Hz, 2H), 7.55 (d, J = 8.7 Hz, 1H), 7.52–7.46 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 153.0, 149.3, 137.1, 131.3, 130.5, 130.0, 129.8, 129.3, 129.2, 128.7, 128.3, 127.6, 125.3 (q, 1JCF = 283.3 Hz), 125.7, 121.2; 19F NMR (378 MHz, CDCl3) δ −62.32; HRMS (ESI) calcd for C16H10ClF3N: 308.0448 (M + H+), found: 308.0432.7-Methyl-3-phenyl-1-(trifluoromethyl)isoquinoline 3d
1H NMR (400 MHz, CDCl3) δ 8.08 (s, 1H), 7.87 (s, 1H), 7.78–7.68 (m, 3H), 7.46 (m, 4H), 2.57 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 151.5, 147.2, 141.1, 131.9, 130.5, 129.9, 129.6, 128.2, 127.7, 127.1, 126.7, 125.2 (q, 1JCF = 284.8 Hz), 121.2, 22.6; 19F NMR (378 MHz, CDCl3) δ −61.77; HRMS (ESI) calcd for C17H13F3N: 288.0995 (M + H+), found: 288.1004.3-(tert-Butyl)-1-(trifluoromethyl)isoquinoline 3e
1H NMR (400 MHz, CDCl3) δ 8.21 (t, J = 10.4 Hz, 1H), 7.82–7.72 (m, 2H), 7.62–7.56 (m, 1H), 7.52 (t, J = 7.3 Hz, 1H), 1.58 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 156.1, 151.6, 133.4, 129.9, 127.8, 126.6, 126.4, 125.4 (q, 1JCF = 286.1 Hz), 124.1, 121.5, 30.2, 27.8; 19F NMR (378 MHz, CDCl3) δ −62.28; HRMS (ESI) calcd for C14H15F3N: 254.1151 (M + H+), found: 254.1124.3-Cyclopropyl-1-(trifluoromethyl)isoquinoline 3f
1H NMR (400 MHz, CDCl3) δ 8.25 (d, J = 8.7 Hz, 1H), 7.71 (d, J = 7.9 Hz, 1H), 7.64–7.58 (m, 1H), 7.55 (d, J = 14.7 Hz, 1H), 7.45 (s, 1H), 2.63–2.69 (m, 1H), 1.17–1.28 (m, 2H), 0.79–0.87 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 151.7, 150.3, 132.7, 129.8, 128.4, 128.0, 127.3, 127.2, 125.3 (q, 1JCF = 282.3 Hz) 122.1, 15.4, 7.8; 19F NMR (378 MHz, CDCl3) δ −62.13; HRMS (ESI) calcd for C13H11F3N: 238.0838 (M + H+), found: 238.0837.7-Fluoro-3-phenyl-1-(trifluoromethyl)isoquinoline 3g
1H NMR (400 MHz, CDCl3) δ 7.93 (dd, J = 10.6, 8.7 Hz, 2H), 7.83 (dd, J = 8.9, 5.8 Hz, 1H), 7.72 (dd, J = 6.6, 3.0 Hz, 2H), 7.53–7.45 (m, 3H), 7.41–7.34 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 163.3 (d, 1JCF = 250.4 Hz), 152.5, 148.8, 131.4, 130.6, 130.5, 129.9, 129.8, 128.3, 127.2, 125.2 (q, 1JCF = 278.3 Hz), 118.7, 107.2; 19F NMR (378 MHz, CDCl3) δ −62.54, −105.61; HRMS (ESI) calcd for C16H10F4N: 292.0744 (M + H+), found: 292.0748.6-Fluoro-3-phenyl-1-(trifluoromethyl)isoquinoline 3h
1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 10.3, 5.0 Hz, 1H), 7.86 (s, 1H), 7.78–7.70 (m, 2H), 7.53–7.42 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 161.5 (d, 1JCF = 252.3 Hz), 151.2, 149.1, 131.3, 130.1, 129.9, 128.3, 126.6, 126.5, 124.8 (q, 1JCF = 273.3 Hz), 120.9, 111.8; 19F NMR (378 MHz, CDCl3) δ −62.08, −109.13; HRMS (ESI) calcd for C16H10F4N: 292.0744 (M + H+), found: 292.0752.3-(4-Methoxyphenyl)-1-(trifluoromethyl)isoquinoline 3i
1H NMR (400 MHz, CDCl3) δ 9.31 (s, 1H), 8.08 (d, J = 8.7 Hz, 2H), 8.02–7.94 (d, J = 8.2 Hz, 2H), 7.68 (t, J = 7.3 Hz, 1H), 7.58–7.53 (m, 1H), 7.04 (d, J = 8.7 Hz, 2H), 3.89 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 152.3, 148.4, 131.4, 130.4, 128.2, 127.6, 127.5, 126.8, 126.7, 124.4 (q, 1JCF = 275.4 Hz), 115.4, 114.2, 55.4; 19F NMR (378 MHz, CDCl3) δ −62.07; HRMS (ESI) calcd for C17H13F3NO: 304.0944 (M + H+), found: 304.0941.3-(4-Chlorophenyl)-1-(trifluoromethyl)isoquinoline 3j
1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 8.8 Hz, 1H), 7.91 (s, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.75–7.66 (m, 3H), 7.62 (t, J = 7.4 Hz, 1H), 7.46 (d, J = 8.5 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 151.1, 146.9, 136.0, 131.2, 130.7, 130.1, 130.0, 128.8, 128.5, 127.9, 127.4, 127.1, 125.3 (q, 1JCF = 288.3 Hz), 122.3; 19F NMR (378 MHz, CDCl3) δ −62.08; HRMS (ESI) calcd for C16H10ClF3N: 308.0448 (M + H+), found: 308.0456.5-Methoxy-3-phenyl-1-(trifluoromethyl)isoquinoline 3k
1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 7.1 Hz, 2H), 7.33 (t, J = 7.7 Hz, 2H), 7.23–7.28 (m, 1H), 7.19 (t, J = 7.9 Hz, 1H), 6.84 (t, J = 7.6 Hz, 2H), 6.78 (s, 1H), 4.93 (t, J = 8.4 Hz, 1H), 3.87 (s, 3H), 1.00 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 155.2, 148.6, 138.1, 133.0, 131.1, 128.9, 128.8, 128.5, 127.0, 125.8 (q, 1JCF = 287.5 Hz), 125.6, 116.4, 114.1, 55.8; 19F NMR (378 MHz, CDCl3) δ −63.55; HRMS (ESI) calcd for C17H13F3NO: 304.0944 (M + H+), found: 304.0938.3-Cyclopropyl-7-fluoro-1-(trifluoromethyl)isoquinoline 3l
1H NMR (400 MHz, CDCl3) δ 8.84 (s, 1H), 8.02 (d, J = 7.8 Hz, 1H), 7.82 (d, J = 7.9 Hz, 1H), 7.60 (s, 1H), 2.04 (m, 1H), 1.23–1.27 (m, 2H), 0.83–0.88 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 162.1 (d, 1JCF = 252.6 Hz), 152.7, 148.5, 133.0, 130.9, 128.9, 128.0, 126.0, 125.1 (q, 1JCF = 276.7 Hz), 106.9, 14.2, 8.9; 19F NMR (378 MHz, CDCl3) δ −62.17, −107.01; HRMS (ESI) calcd for C13H10F4N: 256.0744 (M + H+), found: 256.0739.3-Butyl-7-chloro-1-(trifluoromethyl)isoquinoline 3m
1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 11.6 Hz, 1H), 7.77 (dd, J = 8.9, 5.9 Hz, 1H), 7.71 (s, 1H), 7.34 (t, J = 8.3 Hz, 1H), 3.03–2.94 (m, 2H), 1.75 (dt, J = 15.4, 7.6 Hz, 2H), 1.49 (dt, J = 14.5, 7.3 Hz, 2H), 0.99 (t, J = 7.3 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 151.1, 150.4, 133.0, 129.9, 129.8, 127.3, 127.2, 125.3, 125.2 (q, 1JCF = 286.9 Hz), 124.8, 36.8, 28.5, 22.5, 13.8; 19F NMR (378 MHz, CDCl3) δ −62.64; HRMS (ESI) calcd for C14H14ClF3N: 288.0761 (M + H+), found: 288.0772.7-Chloro-3-cyclopropyl-1-(trifluoromethyl)isoquinoline 3n
1H NMR (400 MHz, CDCl3) δ 7.26–7.04 (m, 4H), 2.04 (s, 1H), 1.19–1.29 (m, 2H), 1.00–0.90 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 150.4, 150.2, 133.8, 132.8, 128.9, 125.2, 125.1, 125.0 (q, 1JCF = 280.6 Hz), 124.7, 28.5, 19.1, 8.1; 19F NMR (378 MHz, CDCl3) δ −62.09; HRMS (ESI) calcd for C13H10ClF3N: 272.0448 (M + H+), found: 272.0469.Acknowledgements
Financial support from the National Natural Science Foundation of China (no. 21032007, 21372046) is gratefully acknowledged.Notes and references
- (a) D. P. Walsh and Y.-T. Chang, Chem. Rev., 2006, 106, 2476 CrossRef CAS PubMed; (b) P. Arya, D. T. H. Chou and M.-G. Baek, Angew. Chem., Int. Ed., 2001, 40, 339 CrossRef CAS; (c) S. L. Schreiber, Science, 2000, 287, 1964 CrossRef CAS; (d) M. D. Burke and S. L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46 CrossRef PubMed; (e) S. L. Schreiber, Nature, 2009, 457, 153 CrossRef CAS PubMed; (f) D. S. Tan, Nat. Chem. Biol., 2005, 1, 74 CrossRef CAS PubMed; (g) C. Cordier, D. Morton, S. Murrison, A. Nelson and C. O'Leary-Steele, Nat. Prod. Rep., 2008, 25, 719 RSC; (h) W. R. J. D. Galloway, A. I. Llobet and D. R. Spring, Nat. Commun., 2010, 1, 80 Search PubMed; (i) S. Oh and S. B. Park, Chem. Commun., 2011, 47, 12754 RSC.
- For reviews, see: (a) Y. Luo, X. Pan, X. Yu and J. Wu, Chem. Soc. Rev., 2014, 43, 834 RSC; (b) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (c) H. Wang, Y. Kuang and J. Wu, Asian J. Org. Chem., 2012, 1, 302 CrossRef CAS.
- (a) Biomedicinal Aspects of Fluorine Chemistry, ed. R. Filler and Y. Kobayashi, Elsevier, Amsterdam, 1982 Search PubMed; (b) Fluorine in Bioorganic Chemistry, ed. J. T. Welch and S. Eswarakrishman, Wiley, New York, 1991 Search PubMed.
- For some reviews on the synthesis of fluorinated heterocycles, see: (a) V. A. Petrov, in Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications, John Wiley & Sons, Inc., Hoboken, NJ, 2009 Search PubMed; (b) E. V. Nosova, G. N. Lipunova, V. N. Charushin and O. N. Chupakhin, J. Fluorine Chem., 2010, 131, 1267 CrossRef CAS PubMed; (c) S. Zhu, Y. Wang, W. Peng, L. Song and G. Jin, Curr. Org. Chem., 2002, 6, 1057 CrossRef CAS; (d) M. J. Silvester, Aldrichimica Acta, 1991, 24, 31 CAS; For some selective examples, see: (e) P. Kwiatkowski, T. D. Beeson, J. C. Conrad and D. W. C. MacMillan, J. Am. Chem. Soc., 2011, 133, 1738 CrossRef CAS PubMed; (f) Y. Kishi, H. Nagura, S. Inagi and T. Fuchigami, Chem. Commun., 2008, 3876 RSC; (g) S. Fustero, S. Catalan, M. Sanchez-Rosello, A. Simon-Fuentes and C. del Pozo, Org. Lett., 2010, 12, 3484 CrossRef CAS PubMed.
- (a) J. Sheng, S. Li and J. Wu, Chem. Commun., 2014, 50, 578 RSC; (b) Q. Xiao, J. Sheng, Z. Chen and J. Wu, Chem. Commun., 2013, 49, 8647 RSC; (c) J. Sheng, C. Fan and J. Wu, Chem. Commun., 2014, 50, 5494 RSC.
- (a) Q. Xiao, J. Sheng, Q. Ding and J. Wu, Eur. J. Org. Chem., 2014, 217 CrossRef CAS; (b) X. Wang, G. Qiu, L. Zhang and J. Wu, Tetrahedron Lett., 2014, 55, 962 CrossRef CAS PubMed.
- (a) T. Xu and G. Liu, Org. Lett., 2012, 14, 5416 CrossRef CAS PubMed; (b) C. Si and A. G. Myers, Angew. Chem., Int. Ed., 2011, 50, 10409 CrossRef CAS PubMed; (c) Q. Liu, Y. Wu, P. Chen and G. Liu, Org. Lett., 2013, 15, 6210 CrossRef CAS PubMed.
- (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC.
- For reviews, see: (a) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (b) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS PubMed; (c) S. Roy, B. T. Gregg, G. W. Gribble, V.-D. Le and S. Roy, Tetrahedron, 2011, 67, 2161 CrossRef CAS PubMed; (d) T. Liu and Q. Shen, Eur. J. Org. Chem., 2012, 6679 CrossRef CAS; (e) Y. Ye and M. S. Sanford, Synlett, 2012, 2005 CAS; (f) X.-F. Wu, H. Neumann and M. Beller, Chem. – Asian J., 2012, 7, 1744 CrossRef CAS PubMed; (g) A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950 CrossRef CAS PubMed; (h) P. Chen and G. Liu, Synthesis, 2013, 2919 CAS.
- For selected examples, see: (a) J. Sheng, C. Fan, Y. Ding, X. Fan and J. Wu, Chem. Commun., 2014, 50, 4188 RSC; (b) G. Liu, H. Liu, S. Pu and J. Wu, RSC Adv., 2013, 3, 10666 RSC; (c) Q. Xiao, J. Sheng, Q. Ding and J. Wu, Eur. J. Org. Chem., 2014, 217 CrossRef CAS; (d) Q. Xiao, S. Ye and J. Wu, Org. Lett., 2012, 14, 3430 CrossRef CAS PubMed.
- (a) Z. Huo, H. Tomeba and Y. Yamamoto, Tetrahedron Lett., 2008, 49, 5531 CrossRef CAS PubMed; (b) H.-S. Yeom, J.-E. Lee and S. Shin, Angew. Chem., Int. Ed., 2008, 47, 7040 CrossRef CAS PubMed; (c) H.-S. Yeom, S. Kim and S. Shin, Synlett, 2008, 924 CAS; (d) Q. Ding and J. Wu, Adv. Synth. Catal., 2008, 350, 1850 CrossRef CAS.
- (a) E. Pair, N. Monteiro, D. Bouyssi and O. Baudoin, Angew. Chem., Int. Ed., 2013, 52, 5346 CrossRef CAS PubMed; (b) Y. Li and A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8221 CrossRef CAS PubMed; (c) B. Zhang, C. Mück-Lichtenfeld, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2013, 52, 10792 CrossRef CAS PubMed; (d) P. Gao, X.-B. Yan, T. Tao, F. Yang, T. He, X.-R. Song, X.-Y. Liu and Y.-M. Liang, Chem. – Eur. J., 2013, 19, 14420 CrossRef CAS PubMed; (e) X. Wang, Y. Ye, S. Zhang, J. Feng, Y. Xu, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2011, 133, 16410 CrossRef CAS PubMed; (f) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 12462 CrossRef CAS PubMed; (g) A. T. Parsons, T. D. Senecal and S. L. Buchwald, Angew. Chem., Int. Ed., 2012, 51, 2947 CrossRef CAS PubMed; (h) J. Xu, Y. Fu, D.-F. Luo, Y.-Y. Jiang, B. Xiao, Z.-J. Liu, T.-J. Gong and L. Liu, J. Am. Chem. Soc., 2011, 133, 15300 CrossRef CAS PubMed; (i) S. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O'Duill, K. Wheelhouse, G. Rassias, M. Médebielle and V. Gouverneur, J. Am. Chem. Soc., 2013, 135, 2505 CrossRef CAS PubMed; (j) X. Wang, Y. Ye, G. Ji, Y. Xu, S. Zhang, J. Feng, Y. Zhang and J. Wang, Org. Lett., 2013, 15, 3730 CrossRef CAS PubMed; (k) Z. Fang, Y. Ning, P. Mi, P. Liao and X. Bi, Org. Lett., 2014, 16, 1522 CrossRef CAS PubMed.
- (a) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (b) J. P. Brand, D. F. Gonzalez, S. Nicolai and J. Waser, Chem. Commun., 2011, 47, 102 RSC; (c) P. Eisenberger, S. Gischig and A. Togni, Chem. – Eur. J., 2006, 12, 2579 CrossRef CAS PubMed; (d) K. Niedermann, J. M. Welch, R. Koller, J. Cvengros, N. Santschi, P. Battaglia and A. Togni, Tetrahedron, 2010, 66, 5753 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, 1H and 13C NMR spectra of compounds 3. See DOI: 10.1039/c4qo00173g |
| ‡ C. Fan and J. Song contributed equally. |
| This journal is © the Partner Organisations 2014 |