Synthesis and application of a new triphosphorus ligand for regioselective linear hydroformylation: a potential way for the stepwise replacement of PPh3 for industrial use†
Caiyou
Chen
a,
Pan
Li
b,
Zhoumi
Hu
a,
Heng
Wang
a,
Huaisu
Zhu
a,
Xinquan
Hu
b,
Yan
Wang
a,
Hui
Lv
*a and
Xumu
Zhang
*a
aCollege of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei 430072, P. R. China
bCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, P. R. China. E-mail: xumu@whu.edu.cn; lvhui@iccas.ac.cn
First published on 15th July 2014
Abstract
A new triphosphorus ligand, Tribi, was developed for the regioselective linear hydroformylation of terminal and internal olefins. It was shown to have excellent catalytic activity and regioselectivity. More importantly, the synthesis of the new triphosphorus ligand was scaled-up (>140 g scale) for practical industrial use. An effective method was also developed for the stepwise replacement of PPh3 by Tribi for potential industrial use.
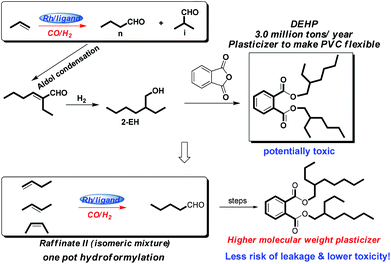
Discovered by Otto Roelen in 1938,1 hydroformylation is now the most commonly used industrial homogeneous reaction in the world, converting readily available inexpensive chemical feedstock into aldehydes which serve as versatile intermediates and building blocks for the generation of numerous chemical products.2 It has been estimated that over 10 million tons of oxo products are produced annually worldwide via the hydroformylation process and this number is still steadily increasing.3 Among all the commercial hydroformylation processes, the most important one is the production of n-butyraldehyde with a worldwide consumption of over 50% of all aldehydes by weight.3 Largely produced by big companies like BASF, Dow Chemical, Shell, Oxea, Evonick, Eastman and others, n-butyraldehyde is mainly used to manufacture the standard plasticizer4 bis(2-ethylhexyl) phthalate (DEHP) which is used in polyvinyl chloride (PVC), with an annual production of over 3 million tons (Fig. 1). However, DEHP has the potential disadvantage of leakage leading to toxicity. To overcome this inherent problem, an isomeric mixture of butenes has been employed as a substrate,5 resulting in the production of a higher molecular weight plasticizer with less risk of leakage and lower toxicity (Fig. 1). But in the latter process, it is particularly challenging to ensure high regioselectivity when the isomeric mixture of butenes is subjected to hydroformylation.
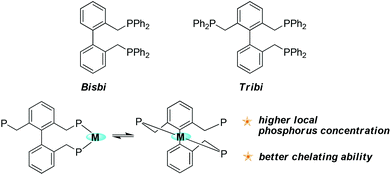
In order to achieve high regioselectivity, several generations of catalysts have been developed for industrial use.6 Due to their superior reactivity, chemo- and regioselectivity compared with the corresponding cobalt catalysts, rhodium precursors with various bisphosphorus ligands have been used as the third generation catalysts playing a dominant role in industry and gaining intensive attention since first being discovered by Wilkinson in 1965.7 For the rhodium-catalyzed hydroformylation processes, numerous new bisphosphorus ligands have been developed and patented.8–15 A good example of such a ligand is the Bisbi ligand (Fig. 2) which has been developed and used in industry for more than 20 years since its first discovery by Eastman in 1987.8 Recently, our group successfully developed conceptually new tetraphosphorus ligands and unprecedented regioselectivity was achieved.16 Even though bisphosphorus ligands and tetraphosphorus ligands have been intensively investigated for industrial use, it seems that very rare examples of triphosphorus ligands have been reported for highly regioselective linear hydroformylation.17 Further development of triphosphorus ligands for practical industrial use is highly desirable. Herein, we report the scale-up synthesis (>140 g scale) and application of a new triphosphorus ligand which we have named Tribi (Fig. 2) for potential industrial use. We envision that the two identical coordination modes will ensure a higher local phosphine concentration at the rhodium center and provide better chelating ability (Fig. 2), resulting in the production of more selective catalytic species compared with those obtained with the corresponding Bisbi ligand. As a result, better regioselectivity is expected.
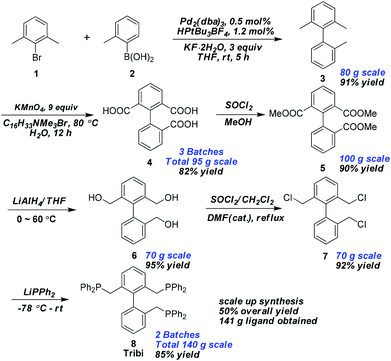
One of the key factors of a ligand for practical use in industry is that the ligand synthesis has to be efficient and can be scaled-up, which remains a challenging problem for many widely used ligands. Herein we have developed a very efficient route for the scale-up synthesis of the new Tribi ligand from readily available starting materials (Fig. 3). Ligand skeleton 3 was easily obtained by utilizing a simple Suzuki coupling method.18 The following newly developed method smoothly converted 3 to acid 4, which is very challenging and unprecedented for the oxidation of three methyl groups in two different aryl rings in a single step utilizing the traditional oxidant KMnO4. The key to the success was the addition of the surfactant C16H33NMe3Br. Acid 4 was subsequently esterified and reduced with lithium aluminium hydride to give alcohol 6 in high yield. The desired Tribi ligand, 8, was obtained by chlorination of alcohol 6 followed by treatment with lithium diphenylphosphine generated in situ in very high overall yield (50%). For comparison, the Bisbi ligand (Fig. 2) was synthesized simultaneously following known procedures.8a
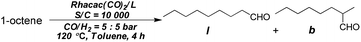
Hydroformylation using the two ligands was then examined. Screening of the reaction conditions for the Tribi ligand was initiated using 1-octene as the substrate. The ligand/metal ratio played a substantial role in determining the regioselectivity. The linear/branched (l/b) ratio was dramatically increased from 3.7 to 40.6 with an increase of the ligand/Rh ratio (Table 1, entries 1–5). The ligand/Rh ratio was fixed at 4 and the catalyst loading was investigated. When the catalyst loading was lowered from S/C = 2000 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, the regioselectivity was maintained and the percentage of the isomerized alkene decreased slightly (Table 1, entry 6). A longer reaction time (4 h) was required to increase the value of the turnover number (TON; Table 1, entry 7). The reaction temperature was then examined. To our surprise, the regioselectivity increased when the temperature was increased from 100 to 120 °C while a further increase of the reaction temperature to 140 °C led to decreased regioselectivity (Table 1, entries 8–9). The syngas pressure was found to be important for determination of the regioselectivity. The best results were obtained with a pressure of 5
000, the regioselectivity was maintained and the percentage of the isomerized alkene decreased slightly (Table 1, entry 6). A longer reaction time (4 h) was required to increase the value of the turnover number (TON; Table 1, entry 7). The reaction temperature was then examined. To our surprise, the regioselectivity increased when the temperature was increased from 100 to 120 °C while a further increase of the reaction temperature to 140 °C led to decreased regioselectivity (Table 1, entries 8–9). The syngas pressure was found to be important for determination of the regioselectivity. The best results were obtained with a pressure of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 bar (Table 1, entries 10–11).
5 bar (Table 1, entries 10–11).
| Entry | L/Rh | S/C | Temp.b (°C) | CO/H2 (bar) | Time (h) | Iso.c (%) | l/bd (%) | Lineare (%) | TONf |
|---|---|---|---|---|---|---|---|---|---|
| a Tribi as ligand, toluene as solvent, decane as internal standard, all results were repeated three times, best L/Rh ratio, S/C, temperature and syngas pressure were highlighted by bold or italic text. b Oil bath temperature. c Percentage of the isomerized alkene. d Linear/branched ratio, determined by GC analysis. e Percentage of linear aldehyde. f Turnover number, determined on the basis of the alkene conversion by GC analysis. | |||||||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2000 | 100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1 | 5.2 | 3.7 | 78.5 | 2.0 × 103 |
| 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2000 | 100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1 | 5.1 | 36.4 | 97.3 | 1.8 × 103 |
| 3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2000 | 100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1 | 5.0 | 38.3 | 97.5 | 1.7 × 103 |
| 4 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
2000 | 100 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 10 10
|
1 | 5.0 | 39.3 | 97.5 | 1.7 × 103 |
| 5 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2000 | 100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1 | 4.7 | 40.6 | 97.6 | 1.6 × 103 |
| 6 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
100 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1 | 3.4 | 41.1 | 97.6 | 4.6 × 103 |
| 7 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
100 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 10 10
|
4 | 6.3 | 35.8 | 97.3 | 9.9 × 103 |
| 8 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 000
|
120 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 10 10
|
4 | 6.9 | 42.5 | 97.7 | 9.9 × 103 |
| 9 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
140 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
4 | 6.4 | 34.4 | 97.2 | 9.9 × 103 |
| 10 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
120 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
4 | 5.5 | 22.9 | 95.8 | 9.4 × 103 |
| 11 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_i_char_2009.gif) 000 000
|
120 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5 5
|
4 | 8.7 | 67.8 | 98.6 | 9.9 × 10 3 |
In order to compare the regioselectivity of the two ligands (Fig. 2), Bisbi was also employed in the hydroformylation of 1-octene and 1-hexene using different reaction temperatures. As summarized in Table 2, it is clear that in all of the cases Tribi afforded better regioselectivity than Bisbi. These results correspond to the hypothesis we made that the two identical coordination modes of Tribi result in a higher local phosphine concentration at the rhodium center and affords better chelating ability, forming more selective catalytic species. A lower reaction temperature resulted in better regioselectivity of both ligands (Table 2, entries 1–2, 5–6). Increasing the reaction temperature resulted in decreased regioselectivity (Table 2, entries 3–4, 7–8). It should be noted that, at high temperature the regioselectivity of the Tribi ligand remained high (Table 2, entries 3, 7), whereas for the Bisbi ligand, high temperature led to a distinct drop of the regioselectivity (Table 2, entries 4, 8). These results indicate that when the two ligands are employed in the hydroformylation process at high temperature, the Tribi ligand affords better results than the Bisbi ligand, this is very important for practical use and enables the aldehyde products to be easily separated by distillation at high temperature.
| Entry | Substrate | L | T (°C) | Iso.c (%) | l/bd (%) | Lineare (%) | TONf |
|---|---|---|---|---|---|---|---|
a S/C = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, [Rh] = 0.2 mM, toluene as solvent, decane as internal standard, ligand/metal ratio = 4, CO/H2 = 5 000, [Rh] = 0.2 mM, toluene as solvent, decane as internal standard, ligand/metal ratio = 4, CO/H2 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 bar, reaction time = 4 h, all results were repeated three times.
b Oil bath temperature.
c Percentage of the isomerized alkene.
d Linear/branched ratio, determined by GC analysis.
e Percentage of linear aldehyde.
f Turn over number, determined on the basis of the alkene conversion by GC analysis. 5 bar, reaction time = 4 h, all results were repeated three times.
b Oil bath temperature.
c Percentage of the isomerized alkene.
d Linear/branched ratio, determined by GC analysis.
e Percentage of linear aldehyde.
f Turn over number, determined on the basis of the alkene conversion by GC analysis.
|
|||||||
| 1 | 1-Octene | Tribi | 120 | 8.7 | 67.8 | 98.6 | 9.9 × 103 |
| 2 | 1-Octene | Bisbi | 120 | 11.1 | 43.7 | 97.8 | 9.9 × 103 |
| 3 | 1-Octene | Tribi | 140 | 8.9 | 51.2 | 98.1 | 9.9 × 103 |
| 4 | 1-Octene | Bisbi | 140 | 14.8 | 34.1 | 97.2 | 9.9 × 103 |
| 5 | 1-Hexene | Tribi | 120 | 6.9 | 66.8 | 98.5 | 9.9 × 103 |
| 6 | 1-Hexene | Bisbi | 120 | 8.1 | 47.5 | 97.9 | 9.9 × 103 |
| 7 | 1-Hexene | Tribi | 140 | 7.1 | 44.1 | 97.8 | 9.9 × 103 |
| 8 | 1-Hexene | Bisbi | 140 | 14.2 | 30.9 | 96.9 | 9.9 × 103 |
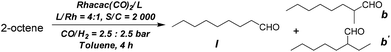
Hydroformylation of internal olefins was also conducted using 2-octene as a representative substrate and using Tribi and Bisbi for further comparison. As internal alkenes are relatively less reactive for hydroformylation, the S/C ratio was changed to 2000. In order to ensure a relatively high regioselectivity, the syngas pressure was fixed at 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 bar. As shown in Table 3, apart from the linear aldehyde and the normal branched aldehyde b, 2-ethylheptanal was also formed as the third major aldehyde product b′. In all of the cases, the Tribi ligand was shown to have better regioselectivity (Table 3, entries 1, 3) than the Bisbi ligand (Table 3, entries 2, 4).
2.5 bar. As shown in Table 3, apart from the linear aldehyde and the normal branched aldehyde b, 2-ethylheptanal was also formed as the third major aldehyde product b′. In all of the cases, the Tribi ligand was shown to have better regioselectivity (Table 3, entries 1, 3) than the Bisbi ligand (Table 3, entries 2, 4).
| Entry | L | T (°C) | l/b/b′c (%) | Lineard (%) | TONe |
|---|---|---|---|---|---|
a S/C = 2000, [Rh] = 1.0 mM, toluene as solvent, decane as internal standard, ligand/metal ratio = 4, CO/H2 = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 bar, reaction time = 4 h, all results were repeated three times.
b Oil bath temperature.
c Linear/branched ratio, determined by GC analysis.
d Percentage of linear aldehyde of all the aldehydes determined.
e Turn over number, determined on the basis of the alkene conversion by GC analysis. 2.5 bar, reaction time = 4 h, all results were repeated three times.
b Oil bath temperature.
c Linear/branched ratio, determined by GC analysis.
d Percentage of linear aldehyde of all the aldehydes determined.
e Turn over number, determined on the basis of the alkene conversion by GC analysis.
|
|||||
| 1 | Tribi | 120 | 10.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45 0.45 |
87.4 | 1.4 × 103 |
| 2 | Bisbi | 120 | 8.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 0.50 |
84.5 | 1.4 × 103 |
| 3 | Tribi | 140 | 9.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.46 0.46 |
86.9 | 1.7 × 103 |
| 4 | Bisbi | 140 | 7.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.46 0.46 |
82.9 | 1.6 × 103 |
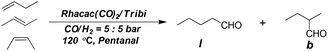
Butene mixture is a cheaply available C4 feedstock usually generated from Crack-C4 from naphtha steam cracking.19 As mentioned in Fig. 1, hydroformylation of the so called Raffinate II which contains 1-butene, cis/trans-2-butene, and the isomeric butanes can afford the highly desired pentanal which upon further transformation gives the new low toxicity plasticizer. However, hydroformylation of butenes is rarely reported academically probably due to problems in operation and challenges in ensuring high regioselectivity.20 Herein, we report the hydroformylation of butenes utilizing Tribi ligand for practical industrial use. 1-Butene, cis/trans-2-butene and butene mixture were independently hydroformylated at a scale of 150 g (Table 4). In order to mimic industrial hydroformylation conditions, the syngas pressure was fixed at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 bar and the S/C ratio was set as 4000 using pentanal as the solvent. High regioselectivity and TOFs were obtained in all cases. Unsurprisingly, a much higher turn over frequency (TOF) was obtained for 1-butene than for 2-butenes and the regioselectivity obtained was the highest (Table 4, entry 1). cis-2-Butene exhibited a larger reaction rate while the regioselectivity obtained was slightly lower compared with trans-2-butene (Table 4, entries 2–3). The butene mixture was also employed for the direct conversion to the highly desired pentanal. Good regioselectivity together with a high TOF was obtained (Table 4, entry 4) thereby demonstrating the great potential usage of the new Tribi ligand in industry.
5 bar and the S/C ratio was set as 4000 using pentanal as the solvent. High regioselectivity and TOFs were obtained in all cases. Unsurprisingly, a much higher turn over frequency (TOF) was obtained for 1-butene than for 2-butenes and the regioselectivity obtained was the highest (Table 4, entry 1). cis-2-Butene exhibited a larger reaction rate while the regioselectivity obtained was slightly lower compared with trans-2-butene (Table 4, entries 2–3). The butene mixture was also employed for the direct conversion to the highly desired pentanal. Good regioselectivity together with a high TOF was obtained (Table 4, entry 4) thereby demonstrating the great potential usage of the new Tribi ligand in industry.
| Entry | Substrate | Time (h) | Conv.c (%) | l/bd (%) | Lineare (%) | TOFf (h−1) |
|---|---|---|---|---|---|---|
a S/C = 4000, pentanal as solvent, ligand/metal ratio = 5, CO/H2 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 bar, temperature = 120 °C, substrates were employed in 150 g scale.
b
trans-2-Butene/cis-2-butene/1-butene = 0.35 5 bar, temperature = 120 °C, substrates were employed in 150 g scale.
b
trans-2-Butene/cis-2-butene/1-butene = 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25.
c Conversion of butenes, determined by the consumption of CO volume.
d Linear/branched ratio, determined by GC analysis.
e Percentage of linear aldehyde of all the aldehydes determined.
f Turn over frequency, determined by the largest CO consumption rate. 0.25.
c Conversion of butenes, determined by the consumption of CO volume.
d Linear/branched ratio, determined by GC analysis.
e Percentage of linear aldehyde of all the aldehydes determined.
f Turn over frequency, determined by the largest CO consumption rate.
|
||||||
| 1 | 1-Butene | 3.5 | 81 | 29.9 | 96.8 | 3.0 × 103 |
| 2 | cis-2-Butene | 4.5 | 80 | 15.2 | 93.8 | 2.3 × 103 |
| 3 | trans-2-Butene | 5 | 79 | 19.3 | 95.1 | 1.2 × 103 |
| 4 | Butene mixtureb | 4.5 | 81 | 21.2 | 95.5 | 1.9 × 103 |
In fact, many industrial hydroformylation processes still use PPh3 as the ligand which results in rather poor regioselectivity. As shown in Table 5, a very low l/b ratio of 1.9 was obtained with PPh3 as the only ligand (Table 5, entry 1). Considering the high price of Rhacac(CO)2 which should not be removed from the reaction system, it would be of a lot of benefit if PPh3 were to be replaced by the stepwise addition of a more efficient ligand which could considerably improve the regioselectivity of the Rhacac(CO)2–PPh3 catalytic system. According to this hypothesis, different equivalents of the Tribi ligand relative to rhodium were added stepwise in order to improve the regioselectivity in the hydroformylation of 1-octene. To our delight we found that the addition of the Tribi ligand effectively improved the regioselectivity from 1.9 to 56.5 (l/b ratio, Table 5, entries 1, 6). Increasing the Tribi/Rh ratio led to a steady improvement of the regioselectivity (Table 5, entries 2–6). The trend of the improvement of the regioselectivity is clearly depicted in Fig. 4 (see ESI†). The highest l/b ratio was 56.5 utilizing the Tribi/Rh ratio of 10 (Table 5, entry 6), and this l/b ratio was close to that obtained when only Tribi was employed as the ligand (Table 5, entry 7). These results indicate that the Tribi ligand can form a much more stable coordinating complex with rhodium compared with PPh3. As a result, the Tribi ligand can effectively replace the PPh3 ligand to form much more selective catalytic species with rhodium and to afford much better regioselectivity. The results shown in Table 5 will be very useful for the stepwise replacement of PPh3 for practical industrial use.
| Entry | PPh3/Tribi/Rh | Iso.b (%) | l/bc (%) | Lineard (%) | TONe |
|---|---|---|---|---|---|
a S/C = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, [Rh] = 0.2 mM, toluene as solvent, decane as internal standard, CO/H2 = 5 000, [Rh] = 0.2 mM, toluene as solvent, decane as internal standard, CO/H2 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 bar, temperature = 120 °C (oil bath), reaction time = 4 h, all results were repeated three times.
b Percentage of the isomerized alkene.
c Linear/branched ratio, determined by GC analysis.
d Percentage of linear aldehyde.
e Turn over number, determined on the basis of the alkene conversion by GC analysis. 5 bar, temperature = 120 °C (oil bath), reaction time = 4 h, all results were repeated three times.
b Percentage of the isomerized alkene.
c Linear/branched ratio, determined by GC analysis.
d Percentage of linear aldehyde.
e Turn over number, determined on the basis of the alkene conversion by GC analysis.
|
|||||
| 1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7 | 1.9 | 66.1 | 9.7 × 103 |
| 2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.7 | 3.0 | 75.0 | 9.6 × 103 |
| 3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.1 | 17.5 | 94.6 | 9.5 × 103 |
| 4 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15.1 | 38.0 | 97.4 | 9.5 × 103 |
| 5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20.9 | 52.9 | 98.1 | 9.5 × 103 |
| 6 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
22.0 | 56.5 | 98.3 | 9.5 × 103 |
| 7 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8.7 | 67.8 | 98.6 | 9.9 × 103 |
Conclusions
In conclusion, a new triphosphorus ligand was developed for the regioselective linear hydroformylation of terminal and internal olefins. Importantly, the synthesis of the Tribi ligand was very efficient and can be scaled-up for potential practical industrial use. The newly developed Tribi ligand exhibited better regioselectivity than the Bisbi ligand. Moreover, a butene mixture was also smoothly hydroformylated with high regioselectivity and TOF thus demonstrating the potential for the considerable practical usage of the new Tribi ligand in industry. At last, an effective method has been developed for the stepwise replacement of PPh3 for potential industrial use.We are grateful for the financial support by the grant from Wuhan University (203273463), “111” Project of the Ministry of Education of China and the National Natural Science Foundation of China (grant no. 21372179).
Notes and references
- O. Roelen, (Chemische Verwertungsgesellschaft Oberhausen m.b.H.), German Patent, DE 849548, 1938/1952 Search PubMed; O. Roelen, U.S. Patent, 2327066, 1943 Search PubMed; Chem. Abstr. 1944 38 3631 Search PubMed.
- J. Pospech, I. Fleischer, R. Franke, S. Buchholz and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 2852 CrossRef CAS PubMed.
- R. Franke, D. Selent and A. Börner, Chem. Rev., 2012, 112, 5675 CrossRef CAS PubMed.
- Hydrocarbon processing; 1980; Section 2, pp. 93–102.
- K.-D. Wiese, G. Protzmann, J. Koch and W. Büschken, (Oxeno Olefinchemie GmbH), German Patent, DE 19957522, 2001 Search PubMed; Chem. Abstr. 2001 135 5378 Search PubMed.
- Besides Otto Roloen's first generation catalyst, for the other two generation catalysts used in industry, see: (a) J. Falbe, New Syntheses with Carbon Monoxides, Springer-Verlag, Berlin, Germany, 1980, p. 158 Search PubMed; (b) H.-J. Blankertz, A. V. Grenacher, F. Sauer, H. Schwahn and W. Schönmann, (BASF Aktiengesellschaft), WO Patent, 98/12235, 1998 Search PubMed; Chem. Abstr. 1998 128 230883 Search PubMed; (c) H.-W. Bohnen and B. Cornils, Adv. Synth. Catal., 2002, 47, 1 CAS.
- D. Evans, J. A. Osborn, F. H. Jardine and G. Wilkinson, Nature, 1965, 208, 1203 CrossRef CAS.
- For Bisbi type ligands, see: (a) T. J. Devon, G. W. Phillips, T. A. Puckette, J. L. Stavinoha and J. J. Vanderbilt, US Patent, 4694109, 1987 Search PubMed; (b) W. A. Herrmann, C. W. Kohlpaintner, E. Herdtweck and P. Kiprof, Inorg. Chem., 1991, 30, 4271 CrossRef CAS; (c) C. P. Casey, G. T. Whiteker;, M. G. Melville, L. M. Lori, J. A. Gavney and D. R. Powell, J. Am. Chem. Soc., 1992, 114, 5535 CrossRef CAS; (d) W. A. Herrmann, R. Schmid, C. W. Kohlpaintner and T. Priermeier, Organometallics, 1995, 14, 1961 CrossRef CAS; (e) C. P. Casey, E. L. Paulsen, E. W. Beuttenmueller, B. R. Proft, L. M. Petrovich, B. A. Matter and D. R. Powell, J. Am. Chem. Soc., 1997, 119, 11817 CrossRef CAS.
- For Xantphos type ligands, see: (a) M. Kranenburg, Y. E. M. van der Burgt, P. C. J. Kamer and P. W. N. M. van Leeuwen, Organometallics, 1995, 14, 3081 CrossRef CAS; (b) L. A. Vander Veen, M. D. Boele, F. R. Bregman, P. C. Paul, P. W. N. M. van Leeuwen, K. Goubitz, J. Fraanje, H. Schenk and C. Bo, J. Am. Chem. Soc., 1998, 120, 11616 CrossRef CAS; (c) J. J. Carb, F. Maseras, C. Bo and P. W. N. M. van Leeuwen, J. Am. Chem. Soc., 2001, 123, 7630 CrossRef PubMed.
- For Biphephos, see: (a) E. Billig, A. G. Abatjoglou and D. R. Bryant, (UCC), US Patent, 4769498, 1988 Search PubMed; (b) G. D. Cuny and S. Buchwald, J. Am. Chem. Soc., 1993, 115, 2066 CrossRef CAS.
- For calix[4]arene bisphosphite, see: R. Paciello, L. Siggel and M. Rçper, Angew. Chem., Int. Ed., 1999, 38, 1920 CrossRef CAS.
- For Naphos, see: H. Klein, R. Jackstell, K. D. Wiese, C. Borgmann and M. Beller, Angew. Chem., Int. Ed., 2001, 40, 3408 CrossRef CAS.
- For a bisphosphoramidite ligand, see: S. C. van der Slot, J. Duran, J. Luten, P. C. J. Kamer and P. W. N. M. van Leeuwen, Organometallics, 2002, 21, 3873 CrossRef CAS.
- For self-assembled bisphosphorus ligands, see: (a) B. Breit and W. Seiche, J. Am. Chem. Soc., 2003, 125, 6608 CrossRef CAS PubMed; (b) V. F. Slagt, J. N. H. Reek, P. C. J. Kamer and P. W. N. M. van Leeuwen, Angew. Chem., Int. Ed., 2001, 40, 4271 CrossRef CAS; (c) V. F. Slagt, P. W. N. M. van Leeuwen and J. N. H. Reek, Angew. Chem., Int. Ed., 2003, 42, 5619 CrossRef CAS PubMed; (d) V. F. Slagt, P. C. J. Kamer, P. W. N. M. van Leeuwen and J. N. H. Reek, J. Am. Chem. Soc., 2004, 126, 1526 CrossRef CAS PubMed; (e) B. Breit and W. Seiche, Angew. Chem., Int. Ed., 2005, 44, 1640 CrossRef CAS PubMed; (f) A. T. Straub, M. Otto, I. Usui and B. Breit, Adv. Synth. Catal., 2013, 355, 2071 CrossRef CAS.
- For a spiroketal-based ligand, see: (a) X. Jia, Z. Wang, C. Xia and K. Ding, Chem. – Eur. J., 2012, 18, 15288 CrossRef CAS PubMed; (b) X. Jia, Z. Wang, C. Xia and K. Ding, Catal. Sci. Technol., 2013, 3, 1901 RSC.
- (a) Y. Yan, X. Zhang and X. Zhang, J. Am. Chem. Soc., 2006, 128, 16058 CrossRef CAS PubMed; (b) Y. Yan, X. Zhang and X. Zhang, Adv. Synth. Catal., 2007, 349, 1582 CrossRef CAS; (c) S. Yu, Y. Chie and X. Zhang, Adv. Synth. Catal., 2009, 351, 537 CrossRef CAS; (d) S. Yu, X. Zhang, Y. Yan, C. Cai, L. Dai and X. Zhang, Chem. – Eur. J., 2010, 16, 4938 CrossRef CAS PubMed.
- C. Chen, Y. Qiao, H. Geng and X. Zhang, Org. Lett., 2013, 15, 1048 CrossRef CAS PubMed.
- S. Lou and G. C. Fu, Adv. Synth. Catal., 2010, 352, 2081 CrossRef CAS PubMed.
- K. Weissermel and H.-J. Arpe, Industrial Organic Chemistry, VCH, Weinheim, Germany, 1988, p. 69 Search PubMed.
- K.-D. Wiese, G. Protzmann, J. Koch and W. Büschken, (Oxeno Olefinchemie GmbH), German Patent, DE 19957522, 2001 Search PubMed; Chem. Abstr. 2001 135 5378 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4qo00132j |
| This journal is © the Partner Organisations 2014 |