Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Absolut “copper catalyzation perfected”; robust living polymerization of NIPAM: Guinness is good for SET-LRP†
Christopher
Waldron
,
Qiang
Zhang
,
Zaidong
Li
,
Vasiliki
Nikolaou
,
Gabit
Nurumbetov
,
Jamie
Godfrey
,
Ronan
McHale
,
Gokhan
Yilmaz
,
Rajan K.
Randev
,
Mony
Girault
,
Kayleigh
McEwan
,
David M.
Haddleton
*,
Martijn
Droesbeke
,
Alice J.
Haddleton
,
Paul
Wilson
,
Alexandre
Simula
,
Jennifer
Collins
,
Danielle J.
Lloyd
,
James A.
Burns
,
Christopher
Summers
,
Claudia
Houben
,
Athina
Anastasaki
,
Muxiu
Li
,
C. Remzi
Becer
,
Jenny K.
Kiviaho
and
Nuttapol
Risangud
Dept. of Chemistry, University of Warwick, Library Road, Coventry, UK CV4 7AL. E-mail: D.M.Haddleton@warwick.ac.uk; Tel: +44 (0) 2476 523256
First published on 11th September 2013
Abstract
The controlled polymerization of N-isopropyl acrylamide (NIPAM) is reported in a range of international beers, wine, ciders and spirits utilizing Cu(0)-mediated living radical polymerization (SET-LRP). Highly active Cu(0) is first formed in situ by the rapid disproportionation of [Cu(I)(Me6-Tren)Br] in the commercial water–alcohol mixtures. Rapid, yet highly controlled, radical polymerization follows (Đ values as low as 1.05) despite the numerous chemicals of diverse functionality present in these solvents e.g. alpha acids, sugars, phenols, terpenoids, flavonoids, tannins, metallo-complexes, anethole etc. The results herein demonstrate the robust nature of the aqueous SET-LRP protocol, underlining its ability to operate efficiently in a wide range of complex chemical environments.
Controlled/living polymerisation has made significant impact in the field of polymer science owed to its ability to regulate molecular weight, dispersity, polymer architecture, end group functionality etc.1–4 Amongst numerous techniques, metal-catalysed radical approaches, such as single-electron transfer living radical polymerisation (SET-LRP),1,5 have made significant contributions to the field, allowing rapid access to quantitative monomer conversions, extremely low dispersity (Đ < 1.10) and high end group fidelity.6,7 The range of monomer and solvent choice is ever expanding as catalysts and other mediators become increasingly tolerant to the full library of chemical functionality, allowing access to novel polymer synthesis in increasingly relevant media for a wider variety of applications.8
Precision macromolecular synthesis in wholly aqueous systems has recently been reported in our laboratory at Warwick.9 This Cu-mediated SET-LRP approach represents a step-change in our ability to control the synthesis of functional water-soluble polymers with a facile process. Excellent control was maintained over chain length and molecular weight distribution (Đ ≤ 1.10) in the polymerization of a variety of acrylic and acrylamide monomers including poly(N-isopropylacrylamide), N,N-dimethylacrylamide, poly(ethylene glycol) acrylate, 2-hydroxyethyl acrylate (HEA) and an acrylamido glyco-monomer. Remarkably, the polymerizations were performed at or below ambient temperature with very high conversions attained in minutes. Polymers exhibited near quantitative chain end fidelity (∼99%) and are capable of further chain extension and/or multi-block copolymerization via iterative monomer addition after full conversion. The scope of this robust technique has also been extended to polymerization in more biologically relevant conditions (PBS buffer and sheep blood serum).9,10
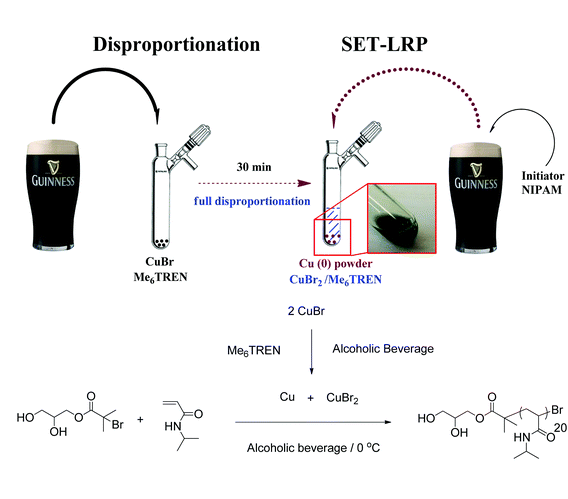
The possibility of this polymerization protocol operating in a wide variety of chemical environments was illustrated in the initial study via controlled polymerization of NIPAM in a complex water–alcohol mixture (tequila (38% ethanol); Mn 9900 g mol−1; Đ 1.08; >99% monomer conversion within 30 min).9 Not only does this illustrate the robustness of this technique to the considerable unknown chemical functionality in a commercial alcoholic beverage, the use of water–alcohol mixtures also promises to broaden the ultimate application of this protocol, allowing the use of monomers, initiators and/or ligands which may not be soluble in wholly aqueous media.
Considering the promising results when tequila was employed as solvent, we decided to investigate further. Taking advantage of the multi-national spread of researchers in the laboratory, and our considerable interest (and expertise) in alcoholic beverages, it was decided to initiate a search to study alcoholic solvents from each person's country of origin for this precision polymer synthesis. We were particularly interested in the effects of residual sugars, left over from the fermentation process (which will vary considerably depending on the source of sugar used), the effect of the different alcohol contents and whether the residual chemicals present from complex brewing and distillation processes would be detrimental to the polymerization. The solvents are compared in terms of both kinetics (time to reach high (>95%) monomer conversion) and control over the molecular weight distribution (Đ). The polymerisation of NIPAM, a commonly studied biocompatible polymer for applications in tissue engineering and controlled drug delivery,11–13 has been conducted in 25 alcoholic beverages from 17 countries using our recently reported method.
The ability of the various solvents to support rapid disproportionation of Cu(I) to Cu(0) and Cu(II) was initially investigated. After deoxygenating via N2 bubbling (freeze–pump–thaw cycles were also used for more carbonated solvents), solutions of Cu(I)Br and Me6-Tren were prepared and continuously bubbled with N2 for a further 30 minutes (Scheme 1). In all cases either a turquoise or green colour was observed instantaneously, indicating the rapid formation of Cu(II) under these conditions (a blue colour from Cu(II)(aq) was observed previously in water9). A fine Cu(0) precipitate was also evident, although this was difficult to see in some of the darker solutions e.g. Guinness14 and red wine.
The polymerization of N-isopropyl acrylamide (NIPAM) was subsequently investigated in these various solvents. Deoxygenated solutions of NIPAM and the water-soluble initiator (Scheme 1) were added to the aforementioned disproportionation mixture via cannula transfer at 0 °C. Monomer conversion was monitored via1H NMR of regular samples in D2O, with many polymerizations reaching near quantitative monomer conversion (≥99%) within 30 minutes (Table 1; 19 out of 25 polymerizations reached ≥87% monomer conversion). Remarkably, 20 out of the 25 polymerizations produced poly(NIPAM) with a Đ value ≤1.10. Whilst very fast, highly controlled polymerization was recorded with both low and high alcoholic contents, a trend towards slower propagation and lower overall monomer conversion was observed at higher alcohol contents in most instances. Nonetheless, the solvent with the highest alcohol content (A'Bunadh Single Malt whiskey from Scotland; 59% alc.) yielded 99% monomer conversion within 60 minutes and an associated Đ value of 1.08. The fastest measured attainment of 99% conversion was 10 minutes for Singha beer (5.0% alc.) from Thailand, while 96% conversion was attained in 9 minutes in Alpha beer (5.0% alc.) from Greece.
| Country | “Solvent” | % Alc. | Chemist(s) | % Conv. (min) | M n (g mol−1) | Đ |
|---|---|---|---|---|---|---|
| a M n measured versus PMMA standards; Mn,theo. = 2504 g mol−1. b Repeated on reviewer request. c Reaction performed in the absence of CuBr. | ||||||
| England | Banks's Bitter | 3.8 | PW, CS, DMH | 99 (30) | 3900 | 1.06 |
| England | Banks's Mild | 3.5 | PW | 99 (30) | 4000 | 1.08 |
| England | Burrow Hill Cider | 6.0 | JC | 63 (30) | 2300 | 1.08 |
| England | Kingston Black Apple Apperitif | 18.0 | JC | 87 (30) | 1900 | 1.09 |
| England | Pimm's | 25.0 | JC | 99 (15) | 3900 | 1.06 |
| Ireland | Guinness | 4.2 | RM | 99 (22) | 3600 | 1.08 |
| China | Tsingtao | 4.7 | RR, QZ, ZL | 99 (104) | 3600 | 1.20 |
| China | Tsingtao | 4.7 | DJL, JAB | 99 (30) | 3500 | 1.09b |
| France | Verveine Verte | 55 | AS, MG | 62 (30) phase separation | 1600 | 1.05 |
| France | Chateau Laubes Bordeaux 2009 | 12.5 | AS | 87 (90) | 2600 | 1.16 |
| France | Napoleon Brandy Bordeaux 1973 | 40.0 | CW | 83 (60) phase separation | 3900 | 1.05 |
| Germany | Krombacher | 4.7 | CH | 70 (90) | 3000 | 1.28 |
| Thailand | Singha | 5.0 | NR | 99 (10) | 3300 | 1.12 |
| Belgium | Leffe | 6.6 | MD | 93 (40) | 3000 | 1.10 |
| Wales | Brains Rev. James | 4.5 | JG | 99 (45) | 3200 | 1.10 |
| Greece | Ouzo | 38.0 | AA | 67 (60) | 3800 | 1.07 |
| Greece | Alpha Beer | 5.0 | VN | 96 (9) | 3200 | 1.06 |
| Greece | Tsipouro | 40.0 | VN | 94 (30) phase separation | 3200 | 1.06 |
| Romania | Homemade Brandy | ?? | AH | 97 (60) | 3000 | 1.07 |
| Scotland | McEwan's Champion | 7.3 | KM | 99 (30) | 3400 | 1.09 |
| Scotland | A'Bunadh Single Malt | 59.0 | JG | 99 (60) phase separation | 2800 | 1.08 |
| Finland | Karhu | 4.6 | JK | 99 (45) | 3600 | 1.07 |
| Switzerland | Morand Abricotine | 43.0 | AS | 96 (30) | 2800 | 1.14 |
| Turkey | Yeni Raki | 45.0 | GY, RB | 99 (60) phase separation | 5000 | 1.06 |
| Russia/Poland | Imperial Vodka | 37.5 | ML | 99 (30) phase separation | 4000 | 1.05 |
| Sweden | Absolut Elyx Vodka | 40.0 | CW | 83 (60) | 3000 | 1.08 |
| Sweden | Absolut Elyx Vodkab | 40.0 | CW | — | — | —c |
Such rapid propagation rates were accompanied by excellent control over dispersity with Đ values of 1.12 and 1.07 attained in Singha and Alpha respectively (see Fig. 1 for typical molecular weight distribution). Other notable solvents giving full monomer conversion included Banks's beer (Mild and Bitter; England), Pimm's (England), Guinness (Ireland) and McEwan's Champion (Scotland) which all yielded 99% monomer conversion within 30 minutes and associated Đ values between 1.06 and 1.09. Welsh, Belgian, Romanian, Turkish and Finnish solvents gave monomer conversions ≥97% in all cases and Đ values in the range of 1.06–1.10.
 | ||
| Fig. 1 Typical SEC trace of poly(NIPAM) formed in an alcoholic beverage using DMF as SEC eluent. Solvent = Guinness; 99% conversion in 22 minutes; Mn = 3600 g mol−1; Đ = 1.08. | ||
The lowest recorded Đ value (1.05), hence greatest control, was observed in Imperial Vodka (40% alc.) and two high alcohol content French solvents, Verveine Verte liqueur (55% alc.) and 40 year old Napoleon Brandy (40% alc.). Notably, however, full conversion was attained only in the case of the vodka (∼99% within 30 min) while the French liqueur gave 62% conversion and the brandy 83%. Phase separation of poly(NIPAM) from the reaction medium was observed in all three of these solvents,15,16 which is a probable cause of the lower final conversions in the latter (see Fig. S1† for photograph of phase separation in Verveine Verte). Similar phase separation was also observed in Tsipouro (Greece; 40% alc.), Yeni Raki (Turkey; 45% alc.) and A'Bunadh (Scotland; 59% alc.), which, like Imperial vodka, achieved near quantitative monomer conversion (94–99% conversion within 60 minutes) whilst also maintaining excellent control (Đ = 1.06–1.08). This observation is in keeping with previous findings in our laboratory wherein poly(nBA) was observed to continue controlled propagation after phase separation from DMSO.17 Lower monomer conversions in Ouzo (Greece; 38% alc.; 67% monomer conversion) and Absolut Elyx vodka (Sweden; 40% alc.; 83% monomer conversion) may also be attributable to some form of micro-precipitation or phase separation although such behaviour was not apparent with the naked eye. It is noted that Absolut Elyx vodka is marketed as “Copper Catalyzation Perfected” in reference to the copper stills used during the distillation process, a phrase we thought particularly apt and in the spirit of the current work. At the request of a referee and considering this distillation process, a polymerization was performed in the absence of CuBr to determine whether any residual copper leeched from the stills could mediate the polymerization. However, no polymer was observed on the usual time scale of the reaction (30 min) and even after a prolonged period (3 h) only monomer was seen by 1H NMR.
Slightly higher Đ values were observed in the French red wine (1.16) and the Swiss liqueur Abricotine (1.14). Whilst reasons for this small loss in control are not immediately apparent it is probable that the high phenolic content of red wines18 may have a role to play in the former. Overall, the fruit containing beverages in the samples, including the Abricotine (apricots), Burrow Hill Cider and Kingston Black Apple Apperitif (both apples) exhibited either slower propagation or slightly less control over dispersity, which may be attributable to an unfavourable interaction between their relatively high L-ascorbic acid (vitamin C) contents and the catalytic polymerization process. The Romanian brandy and Pimm's, however, which contain plums and possible fruit juices respectively, exhibited no detrimental effects in this regard. 1H NMR analysis of all solvents was conducted to illustrate the range of possible chemical functions present during polymerization (see Fig. 2 for 1H NMR of Banks's Bitter and SI for a further representative sample of NMRs). The primary chemical functionalities observed in all cases were likely carbohydrate/sugar peaks. Perhaps most telling from this NMR analysis was the fact that all NMR spectra showed significant differences between beverages, thus highlighting the diverse range of chemical environments used in the current study.
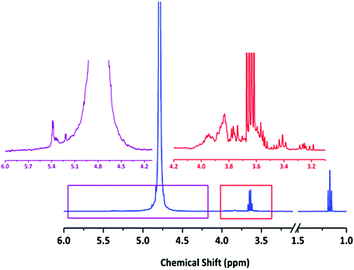 | ||
| Fig. 2 Typical 1H NMR of alcoholic beverage in D2O – Banks's Bitter. Note ethanol peaks at 1.18 (t) and 3.66 (q) ppm. Magnifications illustrate likely carbohydrate/sugar peaks between 3.2 and 4.0 ppm and other unknown peaks between 5.0 and 5.5 ppm. See ESI† for further 1H NMR of solvents. | ||
Other outliers in terms of dispersity include the Chinese and German beers, Tsingtao (1.20) and Krombacher (1.28). With no obvious reasoning for this loss of control relative to other solvents, readers should note that it was purposely decided to conduct each polymerization only once and to use a wide variety of experience of chemists ranging from undergraduate to professorial level to examine the ease of operation of aqueous SET-LRP. While such an approach might normally be expected to yield significant discrepancies in the data set, the fact that >90% of polymerizations gave polymers with Đ values ≤1.16 highlights not only the ability for this protocol to operate in a wide variety of chemical environments/impurities (thus removing the necessity for prolonged purification), but also its robustness to handling by a wide range of people of varying experience, many of whom had no prior experimental exposure to this chemistry. The polymerization in Tsingtao was repeated to challenge the initial result. Pleasingly, 99% conversion was obtained within 30 min and the Đ measured (1.09) was in line with other low alcohol containing beverages suggesting that the scientist was perhaps the most significant variable in this set of experiments which does give an indication of expectations.
In conclusion, precision polymer chemistry is possible in an extensive range of international alcoholic beverages containing a diverse range of chemical functionality. This observed tolerance is similar to that described during for the application of ‘click’ chemistry19 in polymer and materials science and particular to the preparation of hydrogels using this chemistry.20 The aqueous SET-LRP technique recently developed in our lab is highly robust, operating efficiently in >90% of solvents surveyed, from homemade Romanian brandy to English bitter, from Irish stout to Swiss liqueur and many drinks in between. It is possible to attain very low Đ poly(acrylamides) at very high monomer conversions in the presence of many unknown impurities, thus circumventing lengthy and expensive purification in a very robust and simple process. Most importantly, whilst chemistry is often perceived as challenging21 (and sometimes undoubtedly is), it is crucial, if we are to attract and retain the brightest and best, that it also remains all-inclusive and fun whilst demonstrating chemical advances.
We thank Prof. Virgil Percec, University of Pennsylvania, for supplying the home-made Romanian brandy, Prof. Sébastien Perrier, University of Sydney, for the Verveine Verte and Mr Albert Emerson for helping source the Absolut Elyx. Whilst alcohol appears good for SET-LRP, excessive amounts of alcohol are harmful and binge drinking is not advocated by (most of) the authors or their institution. No drinks company was involved in the conception or funding of this study; the collection, analysis, or interpretation of data; or in the preparation of this article.
Notes and references
- B. M. Rosen and V. Percec, Chem. Rev., 2009, 109, 5069–5119 CrossRef CAS PubMed.
- J. Nicolas, Y. Guillaneuf, C. Lefay, D. Bertin, D. Gigmes and B. Charleux, Prog. Polym. Sci., 2013, 38, 63–235 CrossRef CAS PubMed.
- M. Ouchi, T. Terashima and M. Sawamoto, Chem. Rev., 2009, 109, 4963–5050 CrossRef CAS PubMed.
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015–4039 CrossRef CAS.
- V. Percec, T. Guliashvili, J. S. Ladislaw, A. Wistrand, A. Stjerndahl, M. J. Sienkowska, M. J. Monteiro and S. Sahoo, J. Am. Chem. Soc., 2006, 128, 14156–14165 CrossRef CAS PubMed.
- A. Anastasaki, C. Waldron, V. Nikolaou, P. Wilson, R. McHale, T. Smith and D. M. Haddleton, Polym. Chem., 2013, 4, 4113–4119 RSC.
- A. Anastasaki, C. Waldron, P. Wilson, R. McHale and D. M. Haddleton, Polym. Chem., 2013, 4, 2672–2675 RSC.
- V. Percec, S. Samanta, A. Anastasaki, C. Waldron and D. Haddleton, Polym. Chem., 2013 10.1039/C3PY00901G.
- Q. Zhang, P. Wilson, Z. Li, R. McHale, J. Godfrey, A. Anastasaki, C. Waldron and D. M. Haddleton, J. Am. Chem. Soc., 2013, 135, 7355–7363 CrossRef CAS PubMed.
- Q. Zhang, Z. Li, P. Wilson and D. M. Haddleton, Chem. Commun., 2013, 49, 6608–6610 RSC.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163–249 CrossRef CAS.
- Y. Xia, X. Yin, N. A. D. Burke and H. D. H. Stöver, Macromolecules, 2005, 38, 5937–5943 CrossRef CAS.
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173–1222 CrossRef CAS PubMed.
- D. Kotz, L. G. Glynn, C. D. Mallen and J. W. L. Cals, J. Food Sci., 2011, 76, S121–S125 CrossRef CAS PubMed.
- R. O. R. Costa and R. F. S. Freitas, Polymer, 2002, 43, 5879–5885 CrossRef CAS.
- F. M. Winnik, M. F. Ottaviani, S. H. Bossmann, M. Garcia-Garibay and N. J. Turro, Macromolecules, 1992, 25, 6007–6017 CrossRef CAS.
- C. Boyer, A. Atme, C. Waldron, A. Anastasaki, P. Wilson, P. B. Zetterlund, D. Haddleton and M. R. Whittaker, Polym. Chem., 2013, 4, 106–112 RSC.
- O. Dóka and D. Bicanic, Anal. Chem., 2002, 74, 2157–2161 CrossRef.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- M. Malkoch, R. Vestberg, N. Gupta, L. Mespouille, P. Dubois, A. F. Mason, J. L. Hedrick, Q. Liao, C. W. Frank, K. Kingsbury and C. J. Hawker, Chem. Commun., 2006, 2774–2776 RSC.
- C. S. Carter and N. W. Brickhouse, J. Chem. Educ., 1989, 66, 223 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H NMR of solvents etc. See DOI: 10.1039/c3py01075a |
| This journal is © The Royal Society of Chemistry 2014 |