Photo-reactive nanogels as a means to tune properties during polymer network formation†
JianCheng
Liu
a,
Ima Y.
Rad
a,
Fang
Sun
bc and
Jeffrey W.
Stansbury
*ad
aDepartment of Chemical and Biological Engineering, University of Colorado, Boulder, Colorado 80309, USA. E-mail: jeffrey.stansbury@ucdenver.edu
bState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, PR China
cCollege of Science, Beijing University of Chemical Technology, Beijing 100029, PR China
dDepartment of Craniofacial Biology, University of Colorado, Aurora, Colorado 80045, USA
First published on 9th August 2013
Abstract
Photo-reactive nanogels with an integrated photoinitiator-based functionality were synthesized via a Reversible Addition–Fragmentation chain Transfer (RAFT) process. Without additional free initiators, these nanogels are capable of radical generation and initiating polymerization of a secondary monomer (i.e. dimethacrylate) that infiltrates and disperses the nanogel particles. Due to the presence of a RAFT functionality and the fact that all initiating sites are initially located within the nanogel structure, gelation can be delayed by sequencing the polymerization from the nanogel to the bulk matrix. During polymerization of a nanogel-filled resin system, a progressive delay of gelation conversion from about 2% for conventional chain growth polymerization to 18% for the same monomer containing 20 wt% nanogel additive was achieved. A significant delay of stress development was also observed with much lower final stress achieved with the nanogel-modified systems due to the change in network formation mechanics. Compared with the nanogel-free dimethacrylate control, which contained a uniformly distributed free initiator, the flexural modulus and mechanical strength results were maintained for the photopolymers with nanogel contents greater than 10 wt%. There appears to be a critical interparticle spacing for the photo-reactive nanogel that provides effective photopolymerization while providing delayed gelation and substantial stress reduction.
Introduction
Free radical polymerization has been used extensively to form thermosets from vinyl monomers including styrenics and (meth)acrylates. With a wide range of materials and versatile reaction conditions, it gives the opportunity to form polymers with quite diverse properties. Photopolymerization is an important mode for the generation of initiating radicals with many advantages such as energy efficiency, accommodation of heat sensitive additives/substrates, as well as temporal and spatial control over the polymerization process. These features make photopolymerization an ideal choice for broad applications including films and coatings,1 biomaterials,2 and photolithography.3,4 These materials are typically composed of crosslinked, often densely crosslinked, polymeric structures. The utilization of free radical polymerization that involves a chain-growth mechanism in di- or multi-vinyl materials is usually accompanied by gelation at a very low extent of reaction. Features such as cyclization, primary chain length and differential reactivity of free monomers compared with pendant vinyl groups affect the gel point but bulk polymerization of multi-functional (meth)acrylates undergoes macrogelation at conversion levels typically below 5%.5 A practical consequence of the early gel point is that polymerization-induced stress starts to accumulate in the system during the transition from the liquid to the solid state. Stress increases exponentially along with storage modulus as conversion increases. For a highly crosslinked material with a high storage modulus, stress generated during polymerization results in the formation of internal and interfacial defects, warped structures as well as materials that are less tough and fatigue resistant. Many approaches have been developed to achieve reduced polymerization stress. As an example, multi-phase generation6–8 can create internal phase boundaries that when combined with control of local modulus or a sequence of property development within each phase can result in partial volume recovery and stress relaxation during polymer formation. Polymer additives have also shown the ability to reduce stress with the replacement of some monomers with a variety of pre-polymers including oligomers,9 nanogels9,10 or dendrimers.11 Other techniques include design of new monomers,12 functionalization of inorganic fillers,13 or post-gel relaxation based on covalently adaptable networks.14Delaying gelation is an efficient method for reducing stress because stress is only generated after the transition from a liquid to a viscoelastic solid during polymerization. Beyond gelation, the extent of relaxation decreases while the relaxation time scale increases dramatically as the reaction proceeds. Chain transfer agents (CTAs)15 have shown the capability to suppress gelation by favoring limited chain length and intramolecular cyclization. RAFT agents not only behave like CTAs in controlling chain length,16,17 but also suppress early gelation by uniform growth of primary chains.18 Scranton and coworkers19 developed a multi-stage illumination process for shrinkage stress reduction of acrylate coatings based on illumination with micro/macro-patterned light during the initial stage. This allowed monomer migration from the dark regions to partially compensate for the local shrinkage effect. Since bulk stress buildup requires a continuous network structure, only when the entire sample was flood cured to form the final crosslinked network did stress begin to evolve and significant shrinkage stress reduction was achieved through this approach.
Initiator-functionalized nanogels (macroinitiators) are able to initiate polymerization under designated conditions. They have certain advantages compared to free initiators, such as predetermined nanoscale spatial control and the ability to generate complex polymer architectures. They have been used for surface modification,20,21 block copolymer synthesis,22 and as star-shaped nanogels.23 Here we report a novel method to limit stress formation during polymerization by incorporation of a photo-reactive nanogel with RAFT functionality with the aim to create nano-scale (5–10 nm) spatially isolated reaction domains in the initial phase of polymerization. This approach unites both the advantages of localized polymerization effects through initiation in the nanogel phase and RAFT characteristics to control primary chain length and homogeneity towards delaying gelation. A versatile procedure was used to synthesize the nanogel architecture through a solution RAFT polymerization of a mono-vinyl monomer and a photo-initiator functionalized crosslinker. This nanogel is readily dispersible in resin systems, and upon UV irradiation, is capable of initiating polymerization to form crosslinked materials in the presence of multi-vinyl monomers. This study characterized the reaction kinetics, gelation behavior, polymerization stress, modulus development and dynamic mechanical properties of these nanogel-modified materials.
Experimental methods
Materials
Isobornyl methacrylate (IBMA, technical grade), 2-mercaptoethanol (99%, ME), 2-cyano-2-propyl dithiobenzoate (97%, CPBD), tin(II) 2-ethylhexanoate (95%), azobisisobutyronitrile (98%, AIBN) and triethylene glycol dimethacrylate (95%, TEGDMA) were purchased from Sigma-Aldrich. Ethoxylated bisphenol-A dimethacrylate (>87%, BisEMA) was from Esstech. 2-Isocyanatoethyl methacrylate (98%, IEM) was obtained from TCI America. 2-Hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone (97%, Irgacure 2959 or I2959) was from BASF. AIBN was purified by recrystallization twice in methanol. Inhibitors in IBMA were removed with basic alumina. Dichloromethane (DCM) was dried using molecular sieves (4 Å, Fisher Scientific). All other materials were used without further purification.Synthesis of I2959-IEM dimethacrylate crosslinker (I2959-IEM-DMA)
I2959 (2.89 g, 12.9 mmol) was mixed with IEM (4.00 g, 25.8 mmol) and DCM (16.0 ml) in a sealed 50 ml vial. Two drops of tin(II) 2-ethylhexanoate were added as the catalyst. The reaction (Scheme S1†) was allowed to continue for 1 day at room temperature. An aliquot of the sample was analyzed by Fourier transform infrared (FT-IR) spectroscopy to confirm the disappearance of the isocyanate peak (2270 cm−1, stretch) after the reaction. DCM was removed under reduced pressure afterwards and the product (I2959-IEM-DMA) was used without need for further purification as verified spectroscopically: 1H NMR (400 MHz, CDCl3) (Fig. S1a†) (ppm) 8.04 (d, J = 8.8 Hz, 2H), 6.87 (d, J = 8.9 Hz, 2H), 6.09 (d, J = 6.2 Hz, 2H), 5.58 (d, J = 3.1 Hz, 2H), 5.10 (dt, J = 27.5, 5.9 Hz, 2H), 4.53–4.34 (m, 2H), 4.21 (dt, J = 15.5, 4.9 Hz, 4H), 4.00 (t, J = 5.0 Hz, 2H), 3.51 (q, J = 5.0, 4.5 Hz, 2H), 3.31 (q, J = 5.4 Hz, 2H), 1.93 (dd, J = 1.6, 0.9 Hz, 6H), 1.67 (s, 6H); 13C NMR (400 MHz, CDCl3) (Fig. S1b†) (ppm) 18.4, 25.7, 40.3, 61.3, 63.7, 66.5, 84.1, 114.1, 126.17, 127.93, 131.1, 136.0, 154.9, 156.2, 161.7, 167.3, 197.7; mass spectrometry (Fig. S1c†) m/z: 557.2 [M + Na]+, 535.2 [M + H]+, 517.2, 362.2, 307.1.Synthesis of nanogel materials
The RAFT nanogel was prepared from a mixture of IBMA (13.96 g, 62.8 mmol) and I2959-IEM-DMA (3.73 g, 6.98 mmol) with AIBN initiator (0.11 g, 0.68 mmol) in a magnetically stirred 250 ml round-bottom flask. CPBD (0.77 g, 3.49 mmol) was added as a RAFT agent with ethyl acetate (100 ml) used as the solvent (Scheme 1). The reaction was carried out at 80 °C for 20 h in a N2 environment. The resulting nanogel was precipitated in methanol (600 ml) three times and the solid materials were retrieved following centrifugation. The isolated nanogel materials were obtained after residual solvent removal under reduced pressure (nanogel conversion: 85%; yield: 80%).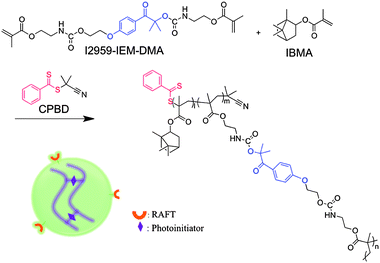 | ||
| Scheme 1 Reaction scheme for nanogel synthesis. The nanogel contains RAFT agents at chain ends and photoinitiators in crosslinking sections. | ||
BisEMA and TEGDMA were combined at a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 mass ratio as the resin system to disperse the photo-reactive nanogels at various concentrations. Free I2959 was added to the control resin (no nanogel).
30 mass ratio as the resin system to disperse the photo-reactive nanogels at various concentrations. Free I2959 was added to the control resin (no nanogel).
The nanogel was characterized by GPC, UV-Vis spectroscopy and rheology. The polymerization kinetics was monitored with either near-IR or mid-IR spectroscopy. Near-IR coupling with a rheometer was applied for determination of material modulus development and gelation behavior. Near-IR coupling with a tensometer was used for measurement of stress generation profiles along with conversion. Dynamic mechanical analysis (DMA) was employed to obtain thermomechanical properties. The detailed characterization methodology can be found in the accompanying ESI.†
Results and discussion
Photo-reactive nanogel synthesis and characterization
From NMR spectra, the CPBD concentration is 40% that of the I2959 functionality with the ratio of the incorporated monomers (IBMA and I2959-IEM-DMA) equivalent to the feed ratio ([IBMA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I2959-IEM-DMA] = 9
[I2959-IEM-DMA] = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the nanogel synthesis. With the assumption that each primary chain in this nanogel contains one RAFT functionality, the primary chains are estimated to consist of approximately 25 monomer units. From the triple-detection GPC analysis, a weight average molecular weight (Mw) of 35 kDa (polydispersity index of 2.5) and an average hydrodynamic radius of 3.3 nm were obtained for this nanogel, which corresponds to a calculated average of 14 photo-reactive crosslinker groups present per nanogel particle.
1) in the nanogel synthesis. With the assumption that each primary chain in this nanogel contains one RAFT functionality, the primary chains are estimated to consist of approximately 25 monomer units. From the triple-detection GPC analysis, a weight average molecular weight (Mw) of 35 kDa (polydispersity index of 2.5) and an average hydrodynamic radius of 3.3 nm were obtained for this nanogel, which corresponds to a calculated average of 14 photo-reactive crosslinker groups present per nanogel particle.
Viscosity
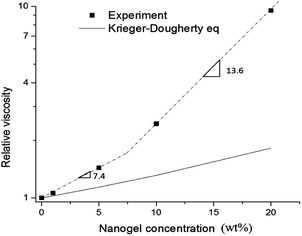
Steady shear flow tests (Fig. S2†) of the monomer-dispersed nanogel from 0 to 20 wt% show Newtonian liquid behavior due to lack of extended interparticle entanglement. This indicates that a 20% nanogel dispersion is below the dense packing critical point. With an increase in nanogel concentration, the zero shear viscosity (η) increased accordingly from 0.082 Pa s for the monomeric resin to 0.78 Pa s for the 20% nanogel composition. By fitting of the data, the relative viscosity (ηr) is found to be an exponential function of nanogel concentration with an exponent of 7.4 for loading levels below 10% and an exponent of 13.6 from 10% to 20% (Fig. 1). This concentration dependent slope change indicates a change in polymer interaction.24 Below 10 wt%, the weaker nanogel–solvent interactions dominate the much larger particle–particle interactions. When the nanogel concentration is greater than 10%, the average interparticle spacing is reduced with interparticle effects no longer negligible. The extended interactions between nanogel domains start to contribute to rapid viscosity change with continued loading. For this particular nanogel/monomer combination, we expect a 10–20% nanogel loading is close to the percolation threshold. The Krieger–Dougherty model25 was applied to fit the rheological behavior of hard sphere particles and microgel particles26,27 in solutions:where Φ is the particle concentration, Φm is the volume fraction of randomly close packed systems (64%), and [η] is the intrinsic viscosity of hard spheres ([η] = 2.5 based on Einstein equation). A much higher viscosity profile was found for equivalent loading of the nanogel compared with the theoretical values for hard spheres. The nanogel, as a highly internally branched and cyclized polymer formed by solution polymerization, may have a lower elastic modulus versus a comparable microgel, and is capable of significant swelling, which enlarges the nanogel effective volume fraction with decreased interparticle spacing that potentially increases viscosity behavior.
Kinetics study
From UV-Vis experiments, the modified crosslinker, I2959-IEM-DMA, has much lower absorbance than the unmodified I2959 initiator (Fig. S3a†). Other studies have proved that modification of the tertiary –OH group in this type of photoinitiator through esterification causes low quantum yields28,29 and long triplet state lifetimes.28 However, previous reports30,31 have shown that functionalization of the primary –OH group in I2959 while leaving the tertiary hydroxyl group infact does not significantly affect the initiator efficiency. Here, the divinyl version of the initiator was applied to internally crosslink the nanogel in order to enhance the initial localized polymerization only within the nanogel domain by avoiding primary radical diffusion into the matrix. On the other hand, the photo-reactive nanogel showed a greater UV absorbance than equivalent concentrations of the free photo-reactive crosslinker monomer apparently due to the light absorption associated with the included RAFT functionality. Upon exposure to 365 nm light, the monomeric crosslinker has only one-tenth the absorbance of I2959 at the same effective molar concentration (Fig. S3b†) at this specific wavelength (365 nm). To normalize the absorptivities, a ten-fold molar excess was applied considering the free, monomeric crosslinker as the initiator (4.2 wt%, [I2959] = 88.3 mM) compared with the conventional unmodified initiator control (0.18 wt% I2959, 8.8 mM). From the real-time IR kinetic results of resin photopolymerization (Fig. 2), the photo-reactive monomeric crosslinker, even at the higher concentration, has a much longer induction time and a slower rate of polymerization compared with the control, which indicates that the modified initiator has a much lower efficiency than free, non-derivatized I2959 under 365 (±10) nm irradiation. This is probably due to the alkyl radical generated from I2959 being more stable than the analogous radical from the crosslinker because of the stronger electron-donating capability of –OH groups from I2959 as opposed to the urethane group. In contrast, the nanogel (overall modified I2959 concentration of 44.2 mM) showed almost no induction time (Fig. 2). This is due to the potential of RAFT agents to cleave into radicals and initiate polymerization under UV light.32,33 It was confirmed that a variety of RAFT agents, including dithioester (Fig. S4†) and trithiocarbonate (data not shown), were able to initiate monomer polymerization under the same irradiation conditions even in the absence of a photoinitiator. RAFT agents typically restrict polymerization rates due to the reduction of active radical concentration.34,35 In addition, prior investigations have shown that UV irradiation with wavelengths greater than 313 nm may not be suitable for activation of direct initiation via RAFT agents.36 However, this experiment demonstrates that some RAFT agents are able to initiate polymerization under relatively long wavelength UV irradiation (i.e. 365 nm or 320–500 nm) without significant O2 inhibition and achieve reasonably high conversion in a limited time during network formation.Gelation measurements and stress evaluation
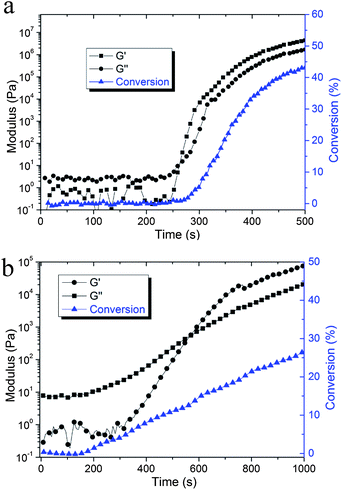
Rheometry is a versatile technique for quantitatively measuring gelation of a crosslinked system where the power law preserves (G′ ∼ G′′ ∼ ω1/2).37 The crossover point of the dynamic storage modulus (G′) and loss modulus (G′′) evolution during polymerization is one of the generally accepted criteria used to determine the gel point.38,39 An early gelation in terms of conversion (∼2% for dimethacrylates) is normally observed for bulk chain growth network formation. For the nanogel with the photo-initiator integrated as internal crosslinks, multiple initiators and terminal RAFT agents are spatially confined within the discrete nanogel structures with the average distance between the initiating sites controlled by the nanogel loading level. Based on the solution polymerization formation of nanogels, they are readily swollen by monomers of appropriate solubility parameters. Initially, localized polymerization would be expected to take place exclusively inside and around the dispersed nanogels. The RAFT functionality can prevent the inter-nanogel coupling at early conversion by control of radical concentration that leads to more uniform chain growth and limiting chain lengths. After the nanogel-based polymerizing domains become interconnected through continued network formation, macrogelation occurs and polymerization stress begins to accumulate. Therefore, potentially lower final stress can be achieved with a delayed stress development profile. From the rheology experiments, the crossover point between G′ and G′′ of the control system formed at very low conversion (∼2%, Fig. 3a). For the 10 wt% CPBD nanogel composition, the rheological analysis indicated a rise in viscosity, which coincided with the G′/G′′ crossover that occurred at ∼12% conversion due to the localized polymerization effect (Fig. 3b). There is a progressive increase in the conversion at gelation with increasing nanogel concentration; the gel point conversion was delayed from 4.7% for 1 wt% added nanogel to 18.2% for the 20 wt% nanogel loading level (Table 1). Even if the initiation process is initially confined to in and around the nanogel structure, the reaction still follows a chain growth mechanism that radicals generated from the nanogel migrate mainly via propagation into the matrix to eventually interconnect and form a continuous gel. More highly loaded nanogel systems have a higher overall concentration of RAFT groups and greater proportions of the internalized monomer that helps to confine the polymerization regime through the formation of shorter chains. Moreover, higher viscosity and faster kinetic rates can also suppress diffusion that may cause inter-nanogel crosslinking and lead to a relatively earlier gelation. This may be why the 20% nanogel dispersion has a greater conversion at gel point than the other systems. To validate that the gel points of these systems are similar to the crossover points between G′ and G′′, polymerization of the 20% CPBD nanogel system was carried out between salt plates while monitoring conversion using IR. From multiple runs, this material reached physically evident gelation at 14–15% conversion, which is in reasonably good agreement with the value of 18% conversion determined by the coupled real-time rheometer/NIR experimental technique.| Nanogel content (wt%) | G′/G′′ crossover conversion (%) |
|---|---|
| 0 (Control) | 2.0 (0.6) |
| 1 | 4.7 (0.9) |
| 5 | 10.1 (1.3) |
| 10 | 12.0 (0.3) |
| 20 | 18.2 (0.5) |
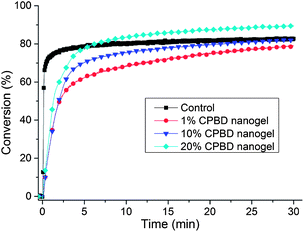
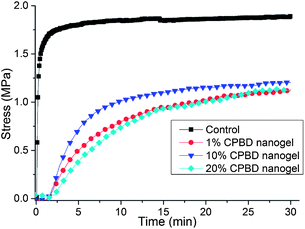
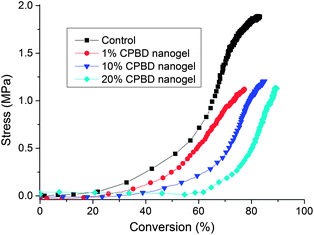
Stress reduction for nanogel-filled resin systems has been reported to be more or less proportional to the nanogel concentration.9,10 This is due to the replacement of the monomer by the pre-formed nanogel, which contributes to limited reactive group concentrations and negligible volume change during network formation. For nanogels containing photo-initiator groups, localized polymerization is another factor that influences stress generation during reaction. From other studies,40 light intensities and, therefore, reaction rates appear to have little effect on the stress-conversion correlation profile. Due to the lower efficiency, a higher curing light intensity was used here for the CPBD-based nanogel systems in order to achieve similar final conversion values between the control and experimental materials to facilitate direct comparisons of polymerization stress. In accord with the delayed gelation results, a delay in stress generation was observed compared with the control (final stress value: 1.86 ± 0.05 MPa). The delay in the onset of stress development as well as the point at which rapid stress rise is observed indicates that both macrogelation and bulk vitrification are suppressed as a consequence of the nanogel-based network formation strategy. The photo-active nanogel as the initiator also produced much lower final stress for the 1 wt% nanogel system (1.08 ± 0.06 MPa). From Fig. 4, it can be observed that the nanogel-based materials reached a similar conversion after 30 min reaction compared with the control by adjusting the respective light intensities. The nanogel systems generated much lower stress (Fig. 5). Both the 10% and 20% nanogel materials reached similar final stress values (1.25 ± 0.15 MPa, 1.12 ± 0.07 MPa, respectively) while attaining higher conversion than either the 1% nanogel composition or the control. By correlating the stress with conversion (Fig. 6), it is evident that the nanogel-based photopolymerization produces a significant delay in the onset of stress compared with the control. The 20% nanogel had the highest conversion and it only accumulated about a quarter of the stress relative to the control at 80% conversion. The nanogel concentration played an important role in delaying stress generation and in the final stress value. From Fig. 7, it can be seen that the storage modulus for the 1% nanogel system develops more slowly than the control system in terms of conversion at the early stage. This indicates a more heterogeneous network development for the nanogel system with crosslinking between nanogels taking place at higher conversion leading to a delay of gelation. The 10% nanogel-modified resin has lower modulus at low conversion than the control or the 1% nanogel material due to further delay of gelation.
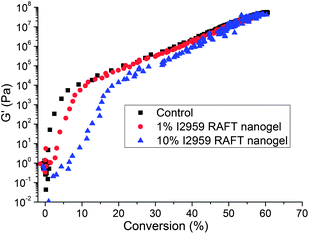 | ||
| Fig. 7 Storage modulus (G′) vs. conversion for the free initiator control (BisEMA/TEGDMA with 0.5 wt% I2959) as well as the 1% and 10% nanogel dispersions (in BisEMA/TEGDMA). | ||
Mechanical properties
Nanogel-initiated polymerization is prone to a more heterogeneous material since the nanogel-surrounded regions are polymerized at the early stage and, therefore, achieves a higher conversion and crosslinking density, while regions between nanogels may have a lower crosslinking density due to more limited conversion. From the flexural tests (Fig. 8), it is evident that the 1% nanogel system has much lower flexural strength and modulus compared with the control system, most likely due to the lower level of conversion achieved within the interparticle polymer matrix region. With the increase in nanogel content, both the flexural strength and modulus increased to values similar to the control, while conversion increased. At a nanogel loading greater than 10%, nanogel interaction starts to increase, which helps to enhance both the physical and chemical crosslinking. The higher initiator concentration also leads to a higher final conversion for 10% and 20% systems. Moreover, the RAFT functionality in the nanogel structure helps to reduce termination reaction and promote relatively homogeneous network formation.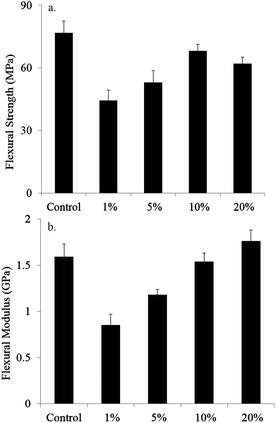 | ||
| Fig. 8 (a) Flexural strength and (b) flexural modulus results for the control and systems at 1, 5, 10 and 20 wt% nanogel concentrations. | ||
From DMA tests, nanogel systems are found to be of similar glassy modulus (G′) at room temperature compared with the control resin at around 3 GPa (Fig. S5†). However, the nanogel systems have a lower rubbery modulus (∼58 MPa) at 200 °C as compared with the control (113 MPa). From the correlation between rubbery modulus and crosslinking density
| E = 3υeRT |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ profiles (Fig. S5†), it is evident that the 20 wt% nanogel-based polymer has a glass transition temperature (Tg) approximately 10 °C below that of the control with the other nanogel contents yielding polymers with slightly lower Tg.
δ profiles (Fig. S5†), it is evident that the 20 wt% nanogel-based polymer has a glass transition temperature (Tg) approximately 10 °C below that of the control with the other nanogel contents yielding polymers with slightly lower Tg.
Conclusion
A photo-reactive nanogel with RAFT functionality was synthesized with UV photo-initiator groups integrated within the structure. This nanogel was added to a dimethacrylate resin at various concentrations. It was shown that this nanogel has the ability to initiate polymerization with reasonable (although reduced relative to the control) reaction rate and final conversion without addition of any free initiators. It was demonstrated that the RAFT functionality is capable of assisting in the initiation of polymerization under longer wavelength UV irradiation. By this approach, the desired localized polymerization effect was achieved with a delay in gelation up to 18% conversion with 20 wt% nanogel loading. These experimental resins have delayed stress development profiles with much lower final stress values. At appropriate nanogel loading levels, mechanical properties are similar to the control photopolymer obtained with a free initiator. The RAFT functionality in the nanogel structure is important due to its ability to initiate polymerization, extend the gel point conversion and moderate stress generation while contributing to the formation of a more homogeneous network.Acknowledgements
This study was supported by NIH/NIDCR R01 DE022348.Notes and references
- C. Decker, Macromol. Rapid Commun., 2002, 23, 1067–1093 CrossRef CAS
.
- K. T. Nguyen and J. L. West, Biomaterials, 2002, 23, 4307–4314 CrossRef CAS
.
- D. Bratton, D. Yang, J. Y. Dai and C. K. Ober, Polym. Adv. Technol., 2006, 17, 94–103 CrossRef CAS
.
- K. S. Schanze, T. S. Bergstedt, B. T. Hauser and C. S. P. Cavalaheiro, Langmuir, 2000, 16, 795–810 CrossRef CAS
.
- L. V. Natarajan, C. K. Shepherd, D. M. Brandelik, R. L. Sutherland, S. Chandra, V. P. Tondiglia, D. Tomlin and T. J. Bunning, Chem. Mater., 2003, 15, 2477–2484 CrossRef CAS
.
- L. F. Francis, A. V. McCormick, D. M. Vaessen and J. A. Payne, J. Mater. Sci., 2002, 37, 4717–4731 CrossRef CAS
.
- Y. J. Cheng, J. M. Antonucci, S. D. Hudson, N. J. Lin, X. R. Zhang and S. Lin-Gibson, Adv. Mater., 2011, 23, 409–413 CrossRef CAS PubMed
.
- C. R. Szczepanski, C. S. Pfeifer and J. W. Stansbury, Polymer, 2012, 53, 4694–4701 CrossRef CAS PubMed
.
- J. C. Liu, G. D. Howard, S. H. Lewis, M. D. Barros and J. W. Stansbury, Eur. Polym. J., 2012, 48, 1819–1828 CrossRef CAS PubMed
.
- R. R. Moraes, J. W. Garcia, M. D. Barros, S. H. Lewis, C. S. Pfeifer, J. C. Liu and J. W. Stansbury, Dent. Mater., 2011, 27, 509–519 CrossRef CAS PubMed
.
- R. Mezzenga, L. Boogh and J. A. E. Manson, Compos. Sci. Technol., 2001, 61, 787–795 CrossRef CAS
.
- J. H. Ge, M. Trujillo-Lemon and J. W. Stansbury, Macromolecules, 2006, 39, 8968–8976 CrossRef CAS PubMed
.
- S. Ye, N. B. Cramer, I. R. Smith, K. R. Voigt and C. N. Bowman, Macromolecules, 2011, 44, 9084–9090 CrossRef CAS PubMed
.
- C. J. Kloxin, T. F. Scott and C. N. Bowman, Macromolecules, 2009, 42, 2551–2556 CrossRef CAS PubMed
.
- C. S. Pfeifer, N. D. Wilson, Z. R. Shelton and J. W. Stansbury, Polymer, 2011, 52, 3295–3303 CrossRef CAS PubMed
.
- Y. Zheng, B. Newland, H. Y. Tai, A. Pandit and W. X. Wang, Chem. Commun., 2012, 48, 3085–3087 RSC
.
- B. L. Liu, A. Kazlauciunas, J. T. Guthrie and S. Perrier, Macromolecules, 2005, 38, 2131–2136 CrossRef CAS
.
- Q. Yu, S. H. Xu, H. W. Zhang, Y. H. Ding and S. P. Zhu, Polymer, 2009, 50, 3488–3494 CrossRef CAS PubMed
.
- A. B. Scranton, P. D. Ganahl, C. M. Smith and C. N. Coretsopoulos, Polym. Mater. Sci. Eng. Prepr., 2009, 101, 1019–1020 CAS
.
- Y. Liu, V. Klep, B. Zdyrko and I. Luzinov, Langmuir, 2004, 20, 6710–6718 CrossRef CAS PubMed
.
- Y. Nakayama and T. Matsuda, Macromolecules, 1996, 29, 8622–8630 CrossRef CAS
.
- Y. J. Fan, G. P. Chen, J. Tanaka and T. Tateishi, Biomacromolecules, 2005, 6, 3051–3056 CrossRef CAS PubMed
.
- Y. Amamoto, M. Kikuchi, H. Masunaga, S. Sasaki, H. Otsuka and A. Takahara, Macromolecules, 2010, 43, 1785–1791 CrossRef CAS
.
- P. Gupta, C. Elkins, T. E. Long and G. L. Wilkes, Polymer, 2005, 46, 4799–4810 CrossRef CAS PubMed
.
- I. M. Krieger and T. J. Dougherty, Trans. Soc. Rheol., 1959, 3, 137–152 CrossRef CAS
.
- S. P. Meeker, W. C. K. Poon and P. N. Pusey, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1997, 55, 5718–5722 CrossRef CAS
.
- R. Borrega, M. Cloitre, I. Betremieux, B. Ernst and L. Leibler, Europhys. Lett., 1999, 47, 729–735 CrossRef CAS
.
- S. Jockusch, M. S. Landis, B. Freiermuth and N. J. Turro, Macromolecules, 2001, 34, 1619–1626 CrossRef CAS
.
- J. Lalevee, X. Allonas, S. Jradi and J. P. Fouassier, Macromolecules, 2006, 39, 1872–1879 CrossRef CAS
.
- D. Ruhlmann, K. Zahouily and J. P. Fouassier, Eur. Polym. J., 1992, 28, 1063–1067 CrossRef CAS
.
- N. S. Allen, M. C. Marin, M. Edge, D. W. Davies, J. Garrett, F. Jones, S. Navaratnam and B. J. Parsons, J. Photochem. Photobiol., A, 1999, 126, 135–149 CrossRef CAS
.
- J. F. Quinn, L. Barner, C. Barner-Kowollik, E. Rizzardo and T. P. Davis, Macromolecules, 2002, 35, 7620–7627 CrossRef CAS
.
- H. Wang, Q. B. Li, J. W. Dai, F. F. Du, H. T. Zheng and R. K. Bai, Macromolecules, 2013, 46, 2576–2582 CrossRef CAS
.
- Q. Yu, Y. S. Zhu, Y. H. Ding and S. P. Zhu, Macromol. Chem. Phys., 2008, 209, 551–556 CrossRef CAS
.
- C. Barner-Kowollik, M. Buback, B. Charleux, M. L. Coote, M. Drache, T. Fukuda, A. Goto, B. Klumperman, A. B. Lowe, J. B. Mcleary, G. Moad, M. J. Monteiro, R. D. Sanderson, M. P. Tonge and P. Vana, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5809–5831 CrossRef CAS
.
- L. C. Lu, N. F. Yang and Y. L. Cai, Chem. Commun., 2005, 5287–5288 RSC
.
- H. H. Winter and F. Chambon, J. Rheol., 1986, 30, 367–382 CrossRef CAS
.
- H. H. Winter, Polym. Eng. Sci., 1987, 27, 1698–1702 CAS
.
- B. S. Chiou and S. A. Khan, Macromolecules, 1997, 30, 7322–7328 CrossRef CAS
.
- H. Lu, J. W. Stansbury and C. N. Bowman, J. Dent. Res., 2005, 84, 822–826 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed characterization, reaction scheme, 1H NMR, 13C NMR and mass spectrometry information of crosslinker (I2959-IEM-DMA); UV/Vis spectra and DMA scans. See DOI: 10.1039/c3py00870c |
| This journal is © The Royal Society of Chemistry 2014 |

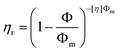
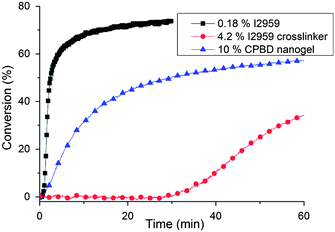
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch (1640 cm−1) was monitored by IR with 2 wavenumber resolution. Continuous irradiation with 365 nm light at 35 mW cm−2 was applied.
C stretch (1640 cm−1) was monitored by IR with 2 wavenumber resolution. Continuous irradiation with 365 nm light at 35 mW cm−2 was applied.