A triplet–triplet annihilation based up-conversion process investigated in homogeneous solutions and oil-in-water microemulsions of a surfactant
Marta
Penconi
,
Pier Luigi
Gentili
,
Giuseppina
Massaro
,
Fausto
Elisei
and
Fausto
Ortica
*
Dipartimento di Chimica, Università di Perugia, 06123 Perugia, Italy. E-mail: fausto.ortica@unipg.it; Fax: +39 075 585 5598; Tel: +39 075 585 5576
First published on 15th October 2013
Abstract
The triplet–triplet annihilation based up-conversion process, involving a platinum octaethyl-porphyrin (PtOEP) as a sensitizer and tetraphenyl-pyrene (TPPy) as an emitter, has been investigated in homogeneous solutions of toluene, bromobenzene and anisole, and oil-in-water microemulsions of the TX-100 surfactant, where toluene constitutes the non-polar phase. In homogeneous solutions, the highest up-conversion quantum yield (of the order of 20%) has been achieved in toluene, being the solvent that has the lowest viscosity among those explored. The up-conversion emission from the PtOEP–TPPy pair has been then investigated in a toluene based oil-in-water microemulsion at three different concentrations of the solutes, showing quantum yields up to the order of 1%, under the same irradiation conditions, but different deoxygenating procedures. The results herein reported might represent a good starting point for a future investigation in microheterogeneous systems. An optimization of the microemulsion composition, in terms of surfactant, co-surfactant and toluene concentrations, could allow us to increase the sensitizer and emitter concentrations and set up the best operative conditions to obtain even higher up-conversion efficiencies.
Introduction
Up-conversion of solar radiation represents an interesting phenomenon and one of the most promising solutions to the issue of low energy photon loss in the process of solar light harvesting by the common large band-gap photocatalysts.1–6 Even though the transformation of low frequency coherent light into higher energy radiation is a process which dates back to the 1960s7 and that has been achieved in several ways afterwards,8 its fulfilment with non-coherent radiation, such as the light coming from the Sun, is more difficult to pursue. Nevertheless, after the early pioneering experiments carried out by Parker and Hatchard,9–10 several important attempts have been made and reported in the literature and, in this scenario, triplet–triplet annihilation (TTA) as a mechanism to accomplish the up-conversion of non-coherent radiation has drawn notable attention over the last decade.11–44 In these studies, quite often the essential ingredients for TTA have been organic molecules such as metallated porphyrins and aromatic polycyclic hydrocarbons. The former plays the role of sensitizers, owing to their red-shifted absorption spectra and significant quantum yields of triplet formation, while the latter, characterized by quantum yields of fluorescence often close to unity, act as higher-energy emitters. In the light of possible applications in the field of solar energy exploitation and practical realization of devices with technological interest, such as dye-sensitized solar cells, TTA has been investigated not only in fluid solution but also in rigid systems.5,21,25,30,31,42,45–50 The sensitizers and the emitters are usually dispersed in rubber-like polymeric materials of various compositions and rigidity. Unfortunately, one of the major drawbacks of this technique is a decreased efficiency of the up-conversion activity with respect to the fluid solution, due to a reduced diffusion rate of the molecules in the rigid environment.46 In these media the intensities of the up-conversion signals are strongly affected by the properties of the rigid host, such as the Tg of the polymeric material, and they are even weaker than those in inorganic materials based on up-converter rare earth ions.30 One of the possible ways to overcome these issues might be either by the use of soft polymeric hosts21 or by the synthesis of a multichromophoric system, containing both the donor and the acceptor species, thus avoiding the requirement of a diffusion controlled bimolecular process.51 Another interesting alternative, which we present in this work, might be by dissolving the organic molecules for TTA into a microheterogeneous fluid system, such as an oil-in-water microemulsion, where cage and segregation effects can make the sensitizer–emitter encounters easier, without a significant decrease of the diffusion rate of the solutes. Recently, Baluschev52,53 and co-workers demonstrated the possibility to perform a TTA-assisted photon up-conversion in a predominantly aqueous environment with appreciable efficiencies. In ref. 52 they employed a polyoxyethanyl–tocopheryl sebacate micellar solution to obtain a highly efficient up-conversion emission from a meso-tetraphenyl-tetrabenzoporphine palladium–dibenz[de,kl]anthracene (perylene) couple, whose photon flux in the water environment was estimated to be only four-fold less than that of the same system in toluene, while in ref. 53 the up-converting dye system was enclosed in a synthesized nanocapsule, in an aqueous dispersion. In another paper,54 Chujo and co-workers studied a sensitizer–emitter upconverting pair accumulated into water-soluble dendrimers; moreover, the possibility to use an aqueous medium would no doubt be of great importance in the search for biocompatible sensitizer–emitter couples55 as well. The role of microheterogeneous systems in photophysical and photochemical processes has been thoroughly investigated and it is well documented in the literature.56–62 Microemulsions are rather complex systems and their peculiar structure and composition has been the subject of several studies56,63–71 with a two-fold interest: on the one hand, the characterization of their morphology and structure is carried out using molecular probes; on the other hand, the microemulsions represent a particular microheterogeneous medium where interesting relaxation processes after photoexcitation can be investigated.Recently, we were synthesizing and characterizing solid solutions of metal oxides to be employed as heterogeneous photo-catalysts for hydrogen production from water.72–74 Since our photocatalysts do not absorb wavelengths longer than 500 nm, to reduce the waste of solar visible light, we turned to the TTA-UC process.75 In this work, we present the first study of the up-conversion emission from a 2,3,7,8,12,13,17,18-octaethyl-21H,23H-porphine platinum(II) (PtOEP)–1,3,6,8-tetraphenylpyrene (TPPy) sensitizer–emitter pair in an oil-in-water microemulsion of 4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol (TX-100) (Scheme 1), which has been reported to be the best TX-series surfactant for enhancing toluene solubilization.76 To understand the effects of the microheterogeneous environment on the up-conversion emission of the PtOEP–TPPy system, our investigation was first carried out in homogeneous solvents having different viscosities, such as toluene, which also constitutes the non-polar component of the oil-in-water microemulsions prepared, bromobenzene, that also bears Br as a heavy atom, and anisole.
Experimental
Materials
The compounds 2,3,7,8,12,13,17,18-octaethyl-21H,23H-porphine platinum(II) (PtOEP) (98%, purchased from Sigma), 1,3,6,8-tetraphenylpyrene (TPPy) (Sigma), 4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol (TX-100) (Fluka) and sodium sulphite (anhydrous, ≥99%, Carlo Erba Reagents) were used as received after checking their degree of purity by recording their absorption, emission and excitation spectra and/or by HPLC. The solvents toluene, bromobenzene, anisole (all of high purity, from Fluka) and 1-pentanol (≥99%, Sigma) were used as received. Water was deionized and distilled before use.Instruments
The absorption spectra were recorded using a Perkin-Elmer Lambda 800 double-beam spectrophotometer.The emission spectra were collected using a Spex Fluorolog-2 1680/1 equipped with a system for spectral correction; the quantum yields were determined using 9,10-diphenylanthracene (DPA) in cyclohexane (quantum yield: ΦF = 0.9077) as a standard. When recording the up-conversion emission signals, a cut-off filter was always inserted between the excitation monochromator and the sample holder, to avoid direct excitation of the emitter molecule due to the second order component passing through the monochromator. Measurements were performed in right angle (RA) geometry, by collecting the up-conversion emission at 90° with respect to the direction of the incident excitation beam, which was always focused on the centre of the cuvette (1 cm optical path).
Emission lifetimes were measured by means of different experimental set-ups, depending on the lifetime range of the species involved.
The PtOEP phosphorescence decay, in the range of tens of microseconds, was measured using a Varian Cary Eclipse spectrofluorimeter provided with an 80 Hz pulsed xenon lamp, whereas the fluorescence lifetime of the TPPy emitter was recorded using an Edinburgh Instruments 199S fluorescence spectrometer, based on the time-correlated single-photon counting technique.
Methods
The oil-in-water microemulsions were prepared by using water, toluene (as the internal organic phase for solubilizing the sensitizer–emitter couple), a surfactant, to reduce the interface tension between the two media, and a co-surfactant, to ensure flexibility to the interfacial layer of the supramolecular structure. TX-100 was chosen as the surfactant agent, because of its aforementioned ability to form microemulsions with a large toluene content,76 characterized by relatively wide non-polar cavities with respect to those obtained from other surfactants of the same family. 1-Pentanol was used as the co-surfactant agent. These optically transparent oil-in-water microemulsions, which turned out to be thermodynamically and chemically stable, contained an excess of water (71.1 wt%), toluene (1.4 wt%), TX-100 (24.2 wt%) and 1-pentanol (3.3 wt%).The emission quantum yields (Φ(X)) were obtained by comparing corrected areas of the sample (AreaX) and the standard (AreaSt) emissions, using eqn (1):
 | (1) |
In the up-conversion experiments in the right angle geometry, where highly absorbing solutions were used, the up-conversion quantum yield (ΦUC) was calculated using the modified eqn (2):
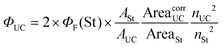 | (2) |
Potassium ferrioxalate actinometry was used to determine the radiation intensity of the excitation source; the light was focused on an area of 0.3 cm2.
The deoxygenating procedure is a crucial point in these measurements, given the well-known quenching effects of molecular oxygen on the excited triplet states of the molecules. In the case of homogeneous solutions the deoxygenating procedure consisted of argon bubbling, whilst in the case of microheterogeneous media, sodium sulphite79 was employed to avoid foam production in the cell, affecting the optical transparency of the sample. Na2SO3 allows sample deoxygenation without altering the spectral properties of the solutions in the visible region. It was added to the microemulsions in concentrations equal to 0.1 mol dm−3 and left to react for a reasonable time (about 15 min), until the emission signal reached a stable intensity maximum. The concentration of sodium sulphite was chosen taking into account the concentration of oxygen in a toluene air-equilibrated solution at room temperature and atmospheric pressure (about 10−3 mol dm−380).
The luminescence decay curves, I(t), were analysed by two methods: the non-linear least-squares method and the maximum entropy method (MEM) using the MemExp Software available online.81,82 In the maximum entropy method the experimental decay I(t) is fitted by the following function:
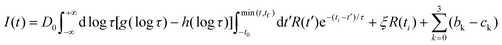 | (3) |
| Q = S − λC − αI | (4) |
 where f is the image that includes both the g and the h lifetime distributions, whereas F is the MEM prior distribution used to incorporate prior knowledge into the solution. C is a measure of the quality of the fit F to the data. When we analyzed the data of phosphorescence decays with normally distributed noise, C is the χ2. For Poisson-distributed data like those collected by the single photon counting technique, C is the Poisson deviance. I is a normalization factor; λ and α are Lagrange multipliers. In our group, we have proved that the maximum entropy method is a valuable tool in describing the poly-exponential character of the luminescence decays for samples consisting of different conformers dissolved in solution,38 fluorophores embedded in polymers84,85 and for microcrystalline compounds86,87 at room temperature.
where f is the image that includes both the g and the h lifetime distributions, whereas F is the MEM prior distribution used to incorporate prior knowledge into the solution. C is a measure of the quality of the fit F to the data. When we analyzed the data of phosphorescence decays with normally distributed noise, C is the χ2. For Poisson-distributed data like those collected by the single photon counting technique, C is the Poisson deviance. I is a normalization factor; λ and α are Lagrange multipliers. In our group, we have proved that the maximum entropy method is a valuable tool in describing the poly-exponential character of the luminescence decays for samples consisting of different conformers dissolved in solution,38 fluorophores embedded in polymers84,85 and for microcrystalline compounds86,87 at room temperature.
Results and discussion
The pair of compounds used as a sensitizer and an emitter for the up-conversion study was chosen after a series of preliminary measurements carried out in our laboratory, where several potential candidates for these roles had been tested. In particular, the choice of PtOEP allowed us to optimize the conditions for the up-conversion process, the triplet energies of the sensitizer (PtOEP) and the emitter (TPPy) being very close to each other (221 cm−1 = 1kT). Furthermore, the presence of a heavy atom such as Pt favours the spin–orbit coupling, thereby increasing the kinetic constant of the collisional Dexter energy transfer involving the triplet states of PtOEP and TPPy. Both these issues result in an enhancement of the up-conversion quantum yield.75PtOEP–TPPy in homogeneous solution
The compounds PtOEP and TPPy were first investigated in the three aromatic solvents: toluene, bromobenzene and anisole. These solvents have closely related structures but different viscosities: toluene is the least viscous, whereas anisole is the most viscous, respectively (see Table 3). Bromobenzene has also a heavy atom (Br) in its structure. Our intention was to test the possible action of these parameters on the up-conversion quantum yield.The molar extinction coefficients of the porphyrin have been measured in the three solvents and they are very similar (Fig. 1), indicating that the excitation of the sensitizer to its excited singlet state occurs with the same probability in the three cases. The lifetimes and quantum yields of phosphorescence have also been determined in argon de-aerated solutions (Table 1).
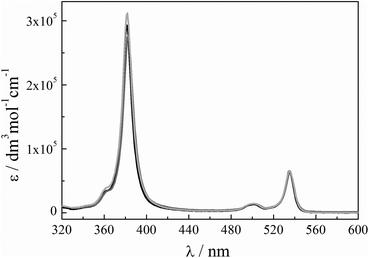 | ||
| Fig. 1 Quantitative absorption spectra of PtOEP in toluene (black), bromobenzene (grey) and anisole (light grey). | ||
| Solvent | ε/dm3 mol−1 s−1 | τ P/μs | Φ P | k P/×104 s−1 | |
|---|---|---|---|---|---|
| λ = 382 nm | λ = 536 nm | ||||
| Toluene | 2.93 × 105 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
90 | 0.41 | 0.45 |
| Bromobenzene | 2.74 × 105 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
84 | 0.98 | 1.16 |
| Anisole | 3.12 × 105 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
75 | 0.87 | 1.16 |
The quantum yield of phosphorescence determined in toluene is in agreement with the value reported in the literature.88 From Table 1, it can be inferred that both the ΦP and the kP values in toluene are smaller than those found in the other two solvents. The quantum yields of fluorescence of the TPPy acceptor alone have also been measured in the three solvents explored, where unitary values were found in all cases.
The study of the up-conversion features of the PtOEP–TPPy pair has then been carried out in toluene, bromobenzene and anisole.
A plausible mechanism for the up-conversion process is the following (eqn (5)–(9)):
| S0(PtOEP) + hν → S1(PtOEP) | (5) |
| S1(PtOEP) → T1(PtOEP) | (6) |
| T1(PtOEP) + S0(TPPy) → S0(PtOEP) + T1(TPPy) | (7) |
| T1(TPPy) + T1(TPPy) → S0(TPPy) + S1(TPPy) | (8) |
| S1(TPPy) → S0(TPPy) + hν′ | (9) |
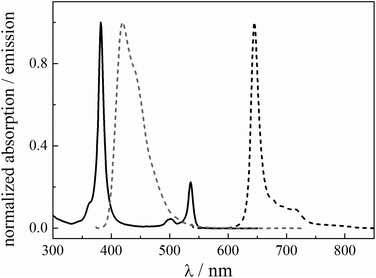 | ||
| Fig. 2 Normalized absorption (full line) and emission (dashed line) spectra of PtOEP (black) and TPPy (grey) in toluene. | ||
The excitation of the PtOEP–TPPy system was carried out in de-aerated (after bubbling with argon) toluene solution at 536 nm, and the emission of the up-converted photons was collected in the violet-blue region. The concentration of the sensitizer was kept constant ([PtOEP] = 10−5 mol dm−3), whilst that of the acceptor (TPPy) was varied within the range 2 × 10−5–2 × 10−3 mol dm−3. The emission spectra, corrected for the inner filter effects, based on the procedure reported in the Experimental section, are shown in Fig. 3.
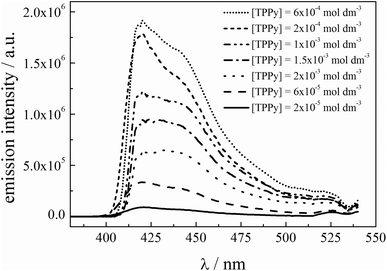 | ||
| Fig. 3 Up-conversion emission spectra as a function of the TPPy acceptor concentration, in toluene; [PtOEP] = 1.1 × 10−5 mol dm−3. | ||
The spectra reported in Fig. 3 were measured regulating the incident power by means of a grey filter on excitation, thus obtaining an excitation intensity similar to the reference value: Iexc = 194 W m−2. This value is close to the solar intensity integrated over the absorption range of the porphyrin “Q band” at A.M. = 1.5.
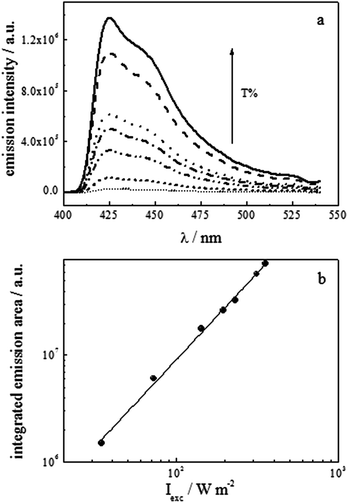
For the concentrations of PtOEP (10−5 mol dm−3) and TPPy (6 × 10−4 mol dm−3) giving the highest value of ΦUC, we studied the effect of excitation intensity to verify when ΦUC becomes independent of the Iexc (Fig. 4a). The plot of the integrated up-conversion emission intensity as a function of the absorbed power is shown on the logarithmic scale in Fig. 4b.
From a minimum least squares method treatment of the data in Fig. 4b, a slope of (1.63 ± 0.04; R2 = 1) was obtained. Such a value is intermediate between the two regimes of quadratic (slope = 2) and linear (slope = 1) dependence of the integrated emission area on Iexc, respectively. The first case is normally encountered in a low intensity range, due to the two-photon mechanism involved in the up-conversion process. In contrast, upon increasing the excitation intensity, a progressive shift towards a linear dependence of the integrated emission area on the Iexc value is observed. In the latter situation, the quantum yield of up-conversion emission ΦUC is independent of the excitation intensity and its plot vs. Iexc would exhibit a plateau.36 The results shown in Fig. 4 were obtained under excitation intensity values which did not allow us to reach this limit.
Particular attention was devoted to step (7) of the overall mechanism of up-conversion, that is, the energy transfer involving the low-lying triplet states of the sensitizer and the emitter molecules. This spin-forbidden process, adequately described by a Dexter collisional mechanism,39 can be treated in terms of a Stern–Volmer equation (eqn (10)):
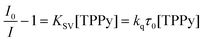 | (10) |
 | ||
| Fig. 5 (a) Quenching of PtOEP phosphorescence upon increasing the TPPy concentration, in the range 2 × 10−5 ÷ 2 × 10−3 mol dm−3 and (b) the corresponding Stern–Volmer diagram. | ||
From the slope of the Stern–Volmer plot (eqn (10) and Fig. 4b), a KSV = 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ± 100 mol−1 dm3 was found. The corresponding kq, which in the present case can be identified with the rate constants of energy transfer, due to the low excitation power and high emitter–sensitizer concentration ratio used, could then be obtained once the phosphorescence lifetimes (τ0, that is, τP of Table 1) was measured. The phosphorescence decay, well fitted by a monoexponential function, gave a τ0 of 90 ± 3 μs, which is in fair agreement with the value 80 ± 5 μs reported in the literature.89 The rate constant for the energy transfer process was then found to be kq = 3.24 × 108 mol−1 dm3 s−1 (Table 2).
300 ± 100 mol−1 dm3 was found. The corresponding kq, which in the present case can be identified with the rate constants of energy transfer, due to the low excitation power and high emitter–sensitizer concentration ratio used, could then be obtained once the phosphorescence lifetimes (τ0, that is, τP of Table 1) was measured. The phosphorescence decay, well fitted by a monoexponential function, gave a τ0 of 90 ± 3 μs, which is in fair agreement with the value 80 ± 5 μs reported in the literature.89 The rate constant for the energy transfer process was then found to be kq = 3.24 × 108 mol−1 dm3 s−1 (Table 2).
| [TPPy]/mol dm−3 | Φ UC | ||
|---|---|---|---|
| Toluene | Bomobenzene | Anisole | |
| 2 × 10−3 | 7.34 × 10−2 | 1.87 × 10−2 | 1.80 × 10−3 |
| 1.5 × 10−3 | 1.03 × 10−1 | 2.78 × 10−2 | 2.57 × 10−3 |
| 1 × 10−3 | 1.33 × 10−1 | 3.32 × 10−2 | 3.42 × 10−3 |
| 6 × 10−4 | 1.91 × 10−1 | 4.08 × 10−2 | 5.76 × 10−3 |
| 2 × 10−4 | 1.72 × 10−1 | 2.28 × 10−2 | 1.82 × 10−3 |
| 6 × 10−5 | 3.57 × 10−2 | 7.56 × 10−3 | 1.03 × 10−3 |
| 2 × 10−5 | 8.68 × 10−3 | 1.64 × 10−3 | 3.31 × 10−4 |
To achieve a better comparison among the three solvents used, the measurements were carried out under the same experimental conditions, in terms of the concentrations of both the sensitizer ([PtOEP] = 10−5 mol dm−3) and the emitter ([TPPy] = 2 × 10−5 ÷ 2 × 10−3 mol dm−3) and in terms of excitation intensity (194 W m−2) (see below, Fig. 6 and Table 2).
The values of KSV were determined by collecting the phosphorescence intensity of PtOEP as a function of the TPPy concentration. From the slope of the Stern–Volmer equation (eqn (10)) a KSV = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 100 mol−1 dm3 was found. The phosphorescence decay, well fitted by a monoexponential function, gave a τ0 of 84 ± 3 μs and the resulting rate constant for the energy transfer process was then found to be kq = 2.01 × 108 mol−1 dm3 s−1.
000 ± 100 mol−1 dm3 was found. The phosphorescence decay, well fitted by a monoexponential function, gave a τ0 of 84 ± 3 μs and the resulting rate constant for the energy transfer process was then found to be kq = 2.01 × 108 mol−1 dm3 s−1.
The values of KSV were determined by collecting the phosphorescence intensity of PtOEP as a function of the TPPy concentration. From the slope of the Stern–Volmer treatment (eqn (10)), a KSV = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 100 mol−1 dm3 was found. The phosphorescence decay, well fitted by a monoexponential function, gave a τ0 of 75 ± 3 μs, while the resulting rate constant for the energy transfer process was kq = 2.96 × 108 mol−1 dm3 s−1.
200 ± 100 mol−1 dm3 was found. The phosphorescence decay, well fitted by a monoexponential function, gave a τ0 of 75 ± 3 μs, while the resulting rate constant for the energy transfer process was kq = 2.96 × 108 mol−1 dm3 s−1.
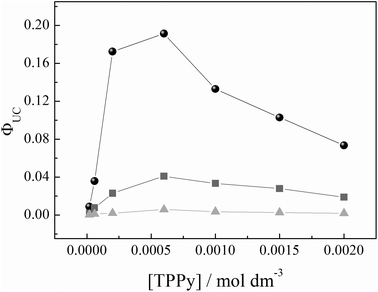
The higher ΦUC obtained in toluene with respect to bromobenzene and anisole can be explained based on various parameters. A factor which is no doubt favourable is the low viscosity of toluene, which renders the molecular diffusion easier, thereby enhancing the efficiency of the energy transfer between the triplet state of the PtOEP and that of the TPPy. Moreover, both the ΦP and the kP values in toluene are smaller than those found in the other two solvents (see Table 1). This is an indication that the radiative deactivation of the triplet state is unfavoured with respect to other non-radiative paths, such as the energy transfer process to an acceptor molecule. This fact, already reported by other authors22 who documented the detrimental effect of the sensitizer phosphorescence on the triplet–triplet energy transfer and the up-conversion emission process, represents a further explanation for the higher ΦUC measured in toluene. In all of the solvents explored, the highest quantum yield value was achieved for a concentration of the acceptor [TPPy] = 6 × 10−4 mol dm−3. The observed trends of ΦUCvs. [TPPy] are not so common in the literature, where a monotonic increase of the quantum yield with increasing the acceptor concentration is generally reported.36 However, the experimental data we present are reproducible and have already been obtained in a previous investigation in our laboratory75 and by S. Baluschev and co-workers,24 who reported a decrease of the up-conversion quantum yield after the acceptor concentration had reached the “threshold” value of 10−3 mol dm−3. A possible explanation for this issue might be the formation of TPPy complexes, since this concentration has been reported as the onset value at which aggregation of TPPy takes place.90
The efficiency of the energy transfer process is quantified in terms of the rate constant of bimolecular quenching, kq, obtained from the Stern–Volmer treatment (Fig. 7 and Table 3).
 | ||
| Fig. 7 Stern–Volmer plots for the three solvents explored: black dots, toluene; grey squares, bromobenzene; light grey triangles, anisole ([PtOEP ∼ 10−5 mol dm−3]). | ||
| Solvent | η (Pa s) | [PtOEP] (mol dm−3) | K SV (dm3 mol−1) | k q (dm3 mol−1 s−1 × 108) |
|---|---|---|---|---|
| Toluene | 0.5859 | 1.1 × 10−5 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
3.24 |
| Bromobenzene | 1.196 | 1.1 × 10−5 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.01 |
| Anisole | 1.32 | 1.0 × 10−5 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.96 |
The kq value obtained in toluene, higher than those estimated in bromobenzene and anisole, as well as the longer lifetime of the PtOEP, plays a positive role in determining an increase of the up-conversion quantum yield with respect to the other two solvents. The differences found in bromobenzene and anisole, solvents having similar viscosity values, can be accounted for based on a comparison of the other parameters. From the KSV, similar kq values are obtained for these two solvents; therefore the larger ΦUC exhibited in bromobenzene may be attributed to a heavy atom effect, which increases the probability of the spin-forbidden transition from the excited singlet state of the sensitizer, populated upon light absorption, to its triplet state, through inter-system crossing. In addition to that, the lifetime of PtOEP in bromobenzene is longer than that in anisole.
The PtOEP–TPPy couple in oil-in-water microemulsion
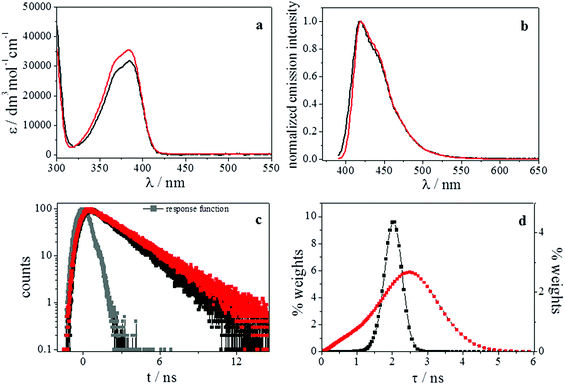
The possibility to confine both the sensitizer and the emitter species into a restricted environment, to favour the encounter process and therefore the energy transfer, with a possible increase of the up-conversion emission quantum yield, induced us to test the effect of the inclusion of PtOEP and TPPy in an oil-in-water microemulsion. Based on the results obtained in a homogeneous solution, we chose toluene as the organic phase, in light of the higher value of up-conversion quantum yield observed in this solvent. Owing to the necessity to work with optically transparent microemulsions and with the sensitizer and the acceptor species completely dissolved, microemulsions containing 6.5 × 10−6 mol dm−3 PtOEP and 6.5 × 10−5 mol dm−3 TPPy as the highest concentrations, respectively, were prepared. We chose to work with an excess of [TPPy] because in this condition we observed the largest ΦUC in solution; moreover, a high ratio of [TPPy] over [PtOEP] guarantees that the TTA involves mainly pairs of TPPy triplets.The spectra displayed in Fig. 8 do not show any significant modification in the PtOEP spectral features passing from a toluene homogeneous solution to a toluene based microemulsion. An increase in the phosphorescence quantum yield (Table 4) was observed in microemulsion with respect to the value found in toluene. This phenomenon might be ascribed to different environments where the solute molecules can be localized within the microemulsion structure. In most of these microenvironments, the triplet lifetime and the quantum yield undergo an increase with respect to the homogeneous solution. A further support of this hypothesis comes from the maximum entropy method (MEM) analysis performed on the PtOEP based microemulsions (Fig. 8); the results are summarized in Table 4. The analysis of the PtOEP phosphorescence decays by MEM reveals that the kinetics in toluene has a marked mono-exponential character (see Fig. 8d). Its lifetimes distribution appears like a Delta Dirac function peaked at 88 μs, in very good agreement with the value obtained by the least squares analysis (see Table 4). On the other hand, the kinetics of microemulsion is described by a much broader distribution of lifetimes (see Fig. 8d) centered at 104 μs (note that the least squares method gives a good result by a mono-exponential fitting function having a lifetime of 103 μs). Clearly, when PtOEP is in microemulsion, it experiences a large spectrum of micro-environments. In fact, the distribution includes lifetimes which are equal to but also longer and shorter than those found in pure toluene.
| Sensitizer/emitter | Medium | Φ P/F | τ P/F | |
|---|---|---|---|---|
| LSM | MEM | |||
| PtOEP | Toluene | 0.41 | 90 μs | 88 μs |
| Microemulsion | 0.71 | 103 μs | 104 μs | |
| TPPy | Toluene | 1 | 2.0 ns | 2.0 ns |
| Microemulsion | 0.62 | 0.17 ns; 2.5 ns | 2.5 ns | |
The TPPy emitter was also studied in microemulsion without the presence of the PtOEP sensitizer. The quantitative absorption and fluorescence emission spectra are displayed in Fig. 9(a) and (b), respectively, along with the corresponding results obtained in toluene homogeneous solution.
From a comparison of the results obtained in microemulsion and in toluene, no significant changes could be detected concerning the spectral shapes of both the absorption and emission spectra; nevertheless, a slight increase of the molar extinction coefficient was found in microemulsion. In contrast, the fluorescence quantum yields (Table 4) exhibited a decrease when compared with the value measured in a toluene homogeneous solution (ΦF = 1). This fact may be probably be explained considering the possibility of TPPy aggregation.90 By means of the single photon counting technique, the fluorescence lifetimes in microemulsion were also determined and well fitted by a double-exponential function (weights of 10% and 90% for the fast and the slow component decays, respectively). As already described for PtOEP, the presence of different environments within the microemulsion structure as possible sites of solubilization for TPPy can be the reason for these results. The maximum entropy method (MEM) analysis performed on the TPPy based microemulsions is displayed in Fig. 9 and the results are summarized in Table 4. The TPPy fluorescence decays in toluene and microemulsion are shown in Fig. 9c. When TPPy is dissolved in pure toluene, it gives rise to a pretty restricted and symmetrical distribution of lifetimes peaked at 2 ns (see Fig. 9d). This result is in agreement with the lifetime of the mono-exponential fitting function achieved by the least squares analysis (see Table 4). The presence of a TPPy lifetimes distribution even in solution of pure toluene may be due to different conformers of the rather flexible molecular skeleton (a similar effect due to conformers was found in ref. 83). In microemulsion, TPPy exhibits a much broader collection of lifetimes (see Fig. 9d). These lifetimes are equal but also longer and shorter than those estimated in pure solvent, as it occurs in the case of PtOEP. Even the least squares method gives the best fitting of the experimental data by a bi-exponential function with 0.17 ns and 2.5 ns as lifetimes. Therefore, when TPPy is dissolved in the microheterogeneous medium, it experiences a large distribution of micro-environments, as PtOEP does. These spreading effects exerted by micro-heterogeneous environments on the electronic excited states lifetimes have been already observed also in other cases (see ref. 84–86).
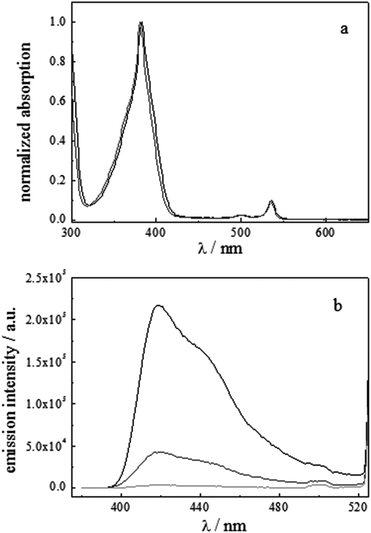
The absorption spectrum of a TX-100 based microemulsion containing the PtOEP and TPPy couple does not show substantial variations with respect to the spectrum recorded in toluene homogeneous solution, as can be inferred from Fig. 10a.
The up-conversion fluorescence spectra originating from 536 nm excitation of three microemulsions containing different combinations of the sensitizer and the emitter and deoxygenated by Na2SO3 are shown in Fig. 10b. The up-conversion intensity exhibits an expected increase upon raising the solute concentrations.
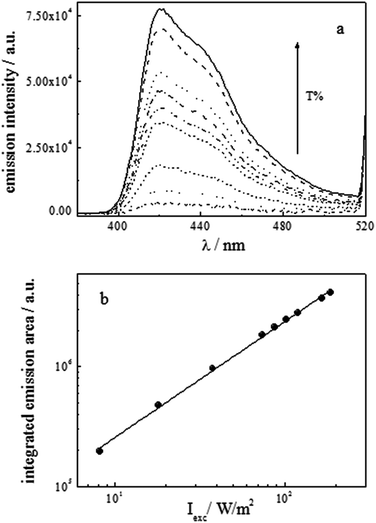
The effect of the excitation intensity on the up-conversion emission was also analyzed by using various grey filters with different values of transmittance (Fig. 11).
From the plot of the integrated up-conversion emission intensity as a function of the absorbed power, on the logarithmic scale (Fig. 11b), a slope of 0.97 ± 0.06 (R2 = 1) was found, thereby indicating that the regime of linearity with Iexc was reached and, therefore, the region of independence of ΦUC from the excitation intensity was also attained. This finding was further confirmed when values of ΦUC very similar to those found at Iexc = 194 W m−2 were obtained employing two different excitation intensities for irradiation (42 and 87 W m−2, see Table 5).
| Microemulsion | [PtOEP] (mol dm−3) | [TPPy] (mol dm−3) | Φ UC | ||
|---|---|---|---|---|---|
| I exc = 42 W m−2 | I exc = 87 W m−2 | I exc = 194 W m−2 | |||
| A | 6.5 × 10−6 | 6.5 × 10−5 | 9.54 × 10−3 | 1.02 × 10−2 | 9.48 × 10−3 |
As already pointed out above, the substantial agreement among the ΦUC measured with different excitation intensities nicely confirms the attainment of the region where the up-conversion quantum yields are independent of Iexc. The lower up-converting efficiency with respect to the corresponding toluene homogeneous solution might have been foreseen, considering the difficulty of dissolving organic molecules in a prevalently aqueous environment and the results of Baluschev and co-workers, who observed a four-fold decrease of the up-conversion photon flux in a surfactant aqueous solution with respect to toluene.52 It has to be noted that another previous literature example concerning a TTA-based up-conversion process in a water environment54 reported a marked decrease of four orders of magnitude in the up-conversion efficiency when comparing the results in the aqueous solution with those obtained in an organic solvent. Moreover, it should also be emphasized that different deoxygenation procedures were employed in homogeneous (argon bubbling) and microheterogeneous (Na2SO3) media and this could contribute to the discrepancy observed. However, the use of oil-in-water microemulsions demonstrated a significant possibility to pave the way for a TTA-based up-conversion process even under these unfavourable conditions. The increase of the sensitizer and emitter concentrations in a TX-100 microemulsion could provide an interesting way to achieve high solute concentrations in a restricted space, thus favouring the TTA process, even in comparison with homogenous environments. Such an increase of the solute content would require either a change in the nature of the sensitizer–emitter couple or a modification of the oil-in-water microemulsion structure, where the size of the cavities could be regulated, for instance, upon changing the co-surfactant/surfactant ratio. This could allow us to reduce the well known drawbacks observed in completely rigid media, such as solids, polymers, films, rubbers, etc., where the drastic decrease of the diffusion rate strongly affects the up-conversion process.
Conclusions
A TTA based up-conversion study of the couple PtOEP–TPPy was carried out in homogeneous and microheterogeneous media. The highest quantum yield for the up-conversion emission in homogeneous solution was found for a concentration of the TPPy acceptor of 6 × 10−4 mol dm−3 in toluene (ΦUC = 0.19), the solvent having the lowest viscosity among those explored. The PtOEP in toluene exhibits the longest lifetime (90 μs), the highest kq (3.24 × 108 dm3 mol−1 s−1) and the lowest phosphorescence quantum yield (ΦP = 0.40). All these factors have a positive influence on the resulting ΦUC value observed.PtOEP and TPPy were first characterized separately in a toluene based oil-in-water microemulsion. The sensitizer showed an increase of both the ΦP and the τP values with respect to the homogeneous case, probably due to the presence of different environments where the solute molecules can be localized within the microemulsion structure. In most of these microenvironments, the triplet lifetime and the quantum yield show an increase with respect to the homogeneous solution. A support for this hypothesis comes from the maximum entropy method (MEM) analysis. In contrast, TPPy exhibited a decrease of the ΦF, which can probably be ascribed to aggregation processes favoured by the confinement effect exerted by the microheterogeneous medium.
The up-conversion emission from the PtOEP–TPPy couple has then been investigated in microemulsion at three different concentrations. The molecular pair constituted by PtOEP as a sensitizer and TPPy as an emitter demonstrated significant up-conversion efficiency in microheterogeneous media. Microemulsions of the surfactant turned out to be an interesting environment where the TTA-based up-conversion can be accomplished, with quantum yields up to the order of 10−2, upon irradiation at 536 nm with an excitation intensity of 194 W m−2. The results herein reported might represent a good starting point for further investigation of the TTA based up-conversion process in these microheterogeneous systems. An optimization of the relative concentrations of the surfactant, the co-surfacting agent, and the water and the toluene content in such an environment, could allow us to increase the sensitizer and emitter concentrations and set up the best operative conditions to obtain high up-conversion efficiencies. This study might well be extended to other possible sensitizer–acceptor couples.
Acknowledgements
The authors gratefully acknowledge the financial support of the Ministero per l'Università e la Ricerca Scientifica e Tecnologica (Rome, Italy), the University of Perugia [PRIN 2010–2011, 2010FM738P] and the Progetto Nazionale FISR “Vettore Idrogeno: Sistemi Innovativi di Produzione di Idrogeno da Energie Rinnovabili”.References
- T. Trupke, M. A. Green and P. Würfel, Improving solar cell efficiencies by up-conversion of sub-band-gap light, J. Appl. Phys., 2002, 92, 4117–4122 CrossRef CAS.
- T. Trupke, A. Shalav, B. S. Richards, P. Würfel and M. A. Green, Efficiency enhancement of solar cells by luminescent up-conversion of sunlight, Sol. Energy Mater. Sol. Cells, 2006, 90, 3327–3338 CrossRef CAS PubMed.
- A. Shalav, B. S. Richards and M. A. Green, Luminescent layers for enhanced silicon solar cell performance: Up-conversion, Sol. Energy Mater. Sol. Cells, 2007, 91, 829–842 CrossRef CAS PubMed.
- H. Shpaisman, O. Niitsoo, I. Lubomirsky and D. Cahen, Can up-and down-conversion and multi-exciton generation improve photovoltaics?, Sol. Energy Mater. Sol. Cells, 2008, 92, 1541–1546 CrossRef CAS PubMed.
- J. de Wild, A. Meijerink, J. K. Rath, W. G. J. H. M. Van Sark and R. E. I. Schropp, Upconverter solar cells: materials and applications, Energy Environ. Sci., 2011, 4, 4835–4848 CAS.
- Y. Yap Cheng, T. Khoury, R. G. C. R. Clady, M. J. Y. Tayebjee, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, On the efficiency limit of triplet–triplet annihilation for photochemical upconversion, Phys. Chem. Chem. Phys., 2010, 12, 66–71 RSC.
- P. A. Franken, A. E. Hill, C. W. Peters and G. Weinreich, Generation of optical harmonics, Phys. Rev. Lett., 1961, 7, 118–120 CrossRef.
- M.-F. Joubert, Photon avalanche upconversion in rare earth laser materials, Opt. Mater., 1999, 11, 181–203 CrossRef CAS.
- C. A. Parker and C. G. Hatchard, Delayed fluorescence from solutions of anthracene and phenanthrene, Proc. Chem. Soc., London, 1962, 147 CAS.
- C. A. Parker and C. G. Hatchard, Sensitized Anti-Stokes delayed fluorescence, Proc. Chem. Soc., London, 1962, 386–387 CAS.
- A. Nattestad, Y. Y. Cheng, R. W. Mac Queen, T. F. Schulze, F. W. Thompson, A. J. Mozer, B. Fückel, T. Khoury, M. J. Crossley, K. Lips, G. G. Wallace and T. W. Schmidt, Dye-Sensitized Solar Cell with Integrated Triplet–Triplet Annihilation Upconversion System, J. Phys. Chem. Lett., 2013, 4, 2073–2078 CrossRef CAS.
- F. Deng, J. R. Sommer, M. Myahkostupov, K. S. Schanze and F. N. Castellano, Near-IR phosphorescent metalloporphyrin as a photochemical upconversion sensitizer, Chem. Commun., 2013, 49, 7406–7408 RSC.
- K. Börjesson, D. Dzebo, B. Albinsson and K. Moth-Poulsen, Photon upconversion facilitated molecular solar energy storage, J. Mater. Chem. A, 2013, 1, 8521–8524 Search PubMed.
- X. Cao, B. Hu and P. Zhang, High Upconversion Efficiency from Hetero Triplet–Triplet Annihilation in Multiacceptor Systems, J. Phys. Chem. Lett., 2013, 4, 2334–2338 CrossRef CAS.
- S. H. Lee, J. R. Lott, Y. C. Simon and C. Weder, Melt-processed polymer glasses for low-power upconversion via sensitized triplet–triplet annihilation, J. Mater. Chem. C, 2013, 1, 5142–5148 RSC.
- C. Zhang, J. Zhao, S. Wu, Z. Wang, W. Wu, J. Ma, S. Guo and L. Huang, Intramolecular RET Enhanced Visible Light-Absorbing Bodipy Organic Triplet Photosensitizers and Application in Photooxidation and Triplet–Triplet Annihilation Upconversion, J. Am. Chem. Soc., 2013, 135, 10566–10578 CrossRef CAS PubMed.
- S. K. Sugunan, C. Greenwald, M. F. Paige and R. P. Steer, Efficiency of Noncoherent Photon Upconversion by Triplet–Triplet Annihilation: The C60 Plus Anthanthrene System and the Importance of Tuning the Triplet Energies, J. Phys. Chem. A, 2013, 117, 5419–5427 CrossRef CAS PubMed.
- W. Wu, J. Sun, X. Cui and J. Zhao, Observation of the room temperature phosphorescence of Bodipy in visible light-harvesting Ru(II) polyimine complexes and application as triplet photosensitizers for triplet–triplet-annihilation upconversion and photocatalytic oxidation, J. Mater. Chem. C, 2013, 1, 4577–4589 RSC.
- C. Wohnhaas, K. Friedemann, D. Busko, K. Landfester, S. Baluschev, D. Crespy and A. Turshatov, All Organic Nanofibers As Ultralight Versatile Support for Triplet–Triplet Annihilation Upconversion, ACS Macro Lett., 2013, 2, 446–450 CrossRef CAS.
- F. Deng, J. Blumhoff and F. N. Castellano, Annihilation Limit of a Visible-to-UV Photon Upconversion Composition Ascertained from Transient Absorption Kinetics, J. Phys. Chem. A, 2013, 117, 4412–4419 CrossRef CAS PubMed.
- A. Monguzzi, F. Bianchi, A. Bianchi, M. Mauri, R. Simonutti, R. Ruffo, R. Tubino and F. Meinardi, High Efficiency Up-Converting Single Phase Elastomers for Photon Managing Applications, Adv. Energy Mater., 2013, 3, 680–686 CrossRef CAS.
- J. Zhao, S. Ji and H. Guo, Triplet–triplet annihilation based upconversion: from triplet sensitizers and triplet acceptors to upconversion quantum yields, RSC Adv., 2011, 1, 937–950 RSC.
- Y. Y. Cheng, B. Fückel, T. Khoury, R. G. C. R. Clady, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, Entropically Driven Photochemical Upconversion, J. Phys. Chem. A, 2011, 115, 1047–1053 CrossRef CAS PubMed.
- S. Baluschev, V. Yakutkin, T. Miteva, G. Wegner, T. Roberts, G. Nelles, A. Yasuda, S. Chernov, S. Aleshchenkov and A. Cheprakov, A general approach for non-coherently excited annihilation up-conversion: transforming the solar-spectrum, New J. Phys., 2008, 10, 013007_1–013007_12 CrossRef.
- T. Miteva, V. Yakutkin, G. Nelles and S. Baluschev, Annihilation assisted upconversion: all-organic, flexible and transparent multicolour display, New J. Phys., 2008, 10, 103002_1–103002_10 CrossRef.
- S. Baluschev, T. Miteva, V. Yakutkin, G. Nelles, A. Yasuda and G. Wegner, Up-Conversion Fluorescence: Noncoherent Excitation by Sunlight, Phys. Rev. Lett., 2006, 97, 143903_1–143903_3 CrossRef.
- S. Baluschev, V. Yakutkin, T. Miteva, Y. Avlasevich, S. Chernov, S. Aleshchenkov, G. Nelles, A. Cheprakov, A. Yasuda, K. Müllen and G. Wegner, Blue-Green Up-Conversion: Noncoherent Excitation by NIR Light, Angew. Chem., Int. Ed., 2007, 46, 7693–7696 CrossRef CAS PubMed.
- S. Baluschev, V. Yakutkin, G. Wegner, B. Minch, T. Miteva, G. Nelles and A. Yasuda, Two pathways for photon upconversion in model organic compound systems, J. Appl. Phys., 2007, 101, 023101_1–023101_5 CrossRef.
- S. Baluschev, V. Yakutkin, G. Wegner, T. Miteva, G. Nelles, A. Yasuda, S. Chernov, S. Aleshchenkov and A. Cheprakov, Upconversion with ultrabroad excitation band: Simultaneous use of two sensitizers, J. Appl. Phys., 2007, 90, 181103_1–181103_4 Search PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Photon upconversion based on sensitized triplet–triplet annihilation, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS PubMed.
- R. R. Islangulov, J. Lott, C. Weder and F. N. Castellano, Noncoherent Low-Power Upconversion in Solid Polymer Films, J. Am. Chem. Soc., 2007, 129, 12652–12653 CrossRef CAS PubMed.
- R. R. Islangulov, D. V. Kozlov and F. N. Castellano, Low power upconversion using MLCT sensitizers, Chem. Commun., 2005, 3776–3778 RSC.
- T. N. Singh-Rachford and F. N. Castellano, Low Power Visible-to-UV Upconversion, J. Phys. Chem. A, 2009, 113, 5912–5917 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Supra-Nanosecond Dynamics of a Red-to-Blue Photon Upconversion System, Inorg. Chem., 2009, 48, 2541–2548 CrossRef CAS PubMed.
- T. N. Singh-Rachford, A. Nayak, M. L. Muro-Small, S. Goeb, M. J. Therien and F. N. Castellano, Supermolecular-Chromophore-Sensitized Near-Infrared-to-Visible Photon Upconversion, J. Am. Chem. Soc., 2010, 132, 14203–14211 CrossRef CAS PubMed.
- A. Monguzzi, R. Tubino, S. Hoseinkhani, M. Campione and F. Meinardi, Low power, non-coherent sensitized photon up-conversion: modelling and perspectives, Phys. Chem. Chem. Phys., 2012, 14, 4322–4332 RSC.
- A. Monguzzi, M. Frigoli, C. Larpent, R. Tubino and F. Meinardi, Low-Power-Photon Up-Conversion in Dual-Dye-Loaded Polymer Nanoparticles, Adv. Funct. Mater., 2012, 14, 139–143 CrossRef.
- A. Monguzzi, R. Tubino and F. Meinardi, Non-coherent low-power up-conversion in multicomponent organic systems, Nuovo Cimento B, 2010, 125, 569–578 Search PubMed.
- A. Monguzzi, R. Tubino and F. Meinardi, Upconversion-induced delayed fluorescence in multicomponent organic systems: Role of Dexter energy transfer, Phys. Rev. B: Condens. Matter, 2008, 77, 155122_1–155122_4 CrossRef.
- A. Monguzzi, R. Tubino and F. Meinardi, Diffusion Enhanced Upconversion in Organic Systems, Int. J. Photoenergy, 2008, 684196 Search PubMed.
- A. Monguzzi, J. Mezyk, F. Scotognella, R. Tubino and F. Meinardi, Upconversion-induced fluorescence in multicomponent systems: Steady-state excitation power threshold, Phys. Rev. B: Condens. Matter, 2008, 78, 195112_1–195112_5 CrossRef.
- A. Monguzzi, R. Tubino and F. Meinardi, Multicomponent Polymeric Film for Red to Green Low Power Sensitized Up-Conversion, J. Phys. Chem. A, 2009, 113, 1171–1174 CrossRef CAS PubMed.
- S. K. Sugunan, U. Tripathy, S. M. K. Brunet, M. F. Paige and R. P. Steer, Mechanisms of Low-Power Noncoherent Photon Upconversion in Metalloporphyrin-Organic Blue Emitter Systems in Solution, J. Phys. Chem. A, 2009, 113, 8548–8556 CrossRef CAS PubMed.
- P. Ceroni, Energy Up-Conversion by Low-Power Excitation: New Applications of an Old Concept, Chem.–Eur. J., 2011, 17, 9560–9564 CrossRef CAS PubMed.
- T. N. Singh-Rachford, J. Lott, C. Weder and F. N. Castellano, Influence of Temperature on Low-Power Upconversion in Rubbery Polymer Blends, J. Am. Chem. Soc., 2009, 131, 12007–12014 CrossRef CAS PubMed.
- J. Mezyk, R. Tubino, A. Monguzzi, A. Mech and F. Meinardi, Effect of an External Magnetic Field on the Up-Conversion Photoluminescence of Organic Films: The Role of Disorder in Triplet-Triplet Annihilation, Phys. Rev. Lett., 2009, 102, 087404_1–087404_4 CrossRef.
- P. E. Keivanidis, S. Baluschev, G. Lieser and G. Wegner, Inherent photon energy recycling effects in the up-converted delayed luminescence dynamics of poly(fluorine)-Pt(II)octaethyl porphyrin blends, ChemPhysChem, 2009, 10, 2316–2326 CrossRef CAS PubMed.
- P. B. Merkel and J. P. Dinnocenzo, Low-power green-to-blue and blue-to-UV upconversion in rigid polymer films, J. Lumin., 2009, 129, 303–306 CrossRef CAS PubMed.
- S. Baluschev, F. Yu, T. Miteva, S. Ahl, A. Yasuda, G. Nelles, W. Knoll and G. Wegner, Metal-Enhanced Up-Conversion Fluorescence: Effective Triplet-Triplet Annihilation near Silver Surface, Nano Lett., 2005, 5, 2482–2484 CrossRef CAS PubMed.
- J. A. O'Brien, S. Rallabandi, U. Tripathy, M. F. Paige and R. P. Steer, Efficient S2 state production in ZnTPP–PMMA thin films by triplet–triplet annihilation: Evidence of solute aggregation in photon upconversion systems, Chem. Phys. Lett., 2009, 475, 220–222 CrossRef CAS PubMed.
- G. Bergamini, P. Ceroni, P. Fabbrizi and S. Cicchi, A multichromophoric dendrimer: from synthesis to energy up-conversion in a rigid matrix, Chem. Commun., 2011, 47, 12780–12782 RSC.
- A. Turshatov, D. Busko, S. Baluschev, T. Miteva and K. Landfester, Micellar carrier for triplet–triplet annihilation-assisted photon energy upconversion in a water environment, New J. Phys., 2011, 13, 083035_1–083035_11 CrossRef.
- C. Wohnhaas, A. Turshatov, V. Mailänder, S. Lorenz, S. Baluschev, T. Miteva and K. Landfester, Annihilation Upconversion in Cells by Embedding the Dye System in Polymeric Nanocapsules, Macromol. Biosci., 2011, 11, 772–778 CrossRef CAS PubMed.
- K. Tanaka, K. Inafuku and Y. Chujo, Environment-responsive upconversion based on dendrimer-supported efficient triplet–triplet annihilation in aqueous media, Chem. Commun., 2010, 46, 4378–4380 RSC.
- C. Wohnhaas, V. Mailänder, M. Dröge, M. A. Filatov, D. Busko, Y. Avlasevich, S. Baluschev, T. Miteva, K. Landfester and A. Turshatov, Triplet–Triplet Annihilation Upconversion Based Nanocapsules for Bioimaging Under Excitation by Red and Deep-Red Light, Macromol. Biosci., 2013, 13, 1422–1430 CrossRef CAS PubMed.
- K. Kalyanasundaram, Photochemistry in microheterogeneous sytems, Academic Press Inc., London, 1987 Search PubMed.
- V. Ramamurthy, Photochemistry in Organized and Constrained Media, Wiley VCH, 1991 Search PubMed.
- L. I. Osipow, Surface Chemistry, Reinhold Publishing Corporation, New York, 1962 Search PubMed.
- P. C. Hiemenz, Principle of Colloid and surface chemistry, Marcel Dekker Inc., New York, 1977 Search PubMed.
- N. J. Turro, M. Grätzel and A. M. Braun, Photophysical and Photochemical Processes in Micellar Systems, Angew., Chem., Int. Ed. Engl, 1980, 19, 675–696 CrossRef.
- N. J. Turro, G. S. Cox and M. A. Paczkowski, Photochemistry in micelles, Top. Curr. Chem., 1985, 129, 57–97 CrossRef CAS.
- J. K. Thomas and M. Almgren, in Solution chemistry of surfactants, ed. K. L. Mittal, Plenum Press, New York, 1979, vol. 2 Search PubMed.
- T. Hellweg, Phase structures of microemulsions, Curr. Opin. Colloid Interface Sci., 2002, 7, 50–56 CrossRef CAS.
- B. K. Paul and S. P. Moulik, Uses and applications of microemulsions, Curr. Sci. India, 2001, 80, 990–1001 CAS.
- M. Almgren, F. Grieser and J. K. Thomas, Photochemical and Photophysical Studies of Organized Assemblies. Interaction of Oils, Long-chain Alcohols, and Surfactants Forming Microemulsions, J. Am. Chem. Soc., 1980, 102, 3188–3193 CrossRef CAS.
- S. S. Atik and J. K. Thomas, Photoprocesses in Cationic Microemulsion Systems, J. Am. Chem. Soc., 1981, 103, 4367–4371 CrossRef CAS.
- Y. Tricot, J. Kiwi, W. Niederberger and M. Grätzel, Application of 13C NMR, Fluorescence, and Light-Scattering Techniques for Structural Studies of Oil-in-Water Microemulsions, J. Phys. Chem., 1961, 85, 862–870 CrossRef.
- A. K. Panda, S. P. Moulik, B. B. Bhowmik and A. R. Dasy, Dispersed Molecular Aggregates II. Synthesis and Characterization of Nanoparticles of Tungstic Acid in H2O/(TX-100+Alkanol)/n-Heptane W/O Microemulsion Media, J. Colloid Interface Sci., 2001, 235, 218–226 CrossRef CAS PubMed.
- P. Lianos, J. Lang, C. Strazielle and R. Zana, Fluorescence Probe Study of Oil-in-Water Microemulsions. 1. Effect of Pentanol and Dodecane or Toluene on Some Properties of Sodium Dodecyl Sulfate Micelles, J. Phys. Chem., 1982, 86, 1019–1025 CrossRef CAS.
- P. Lianos, J. Lang and R. Zana, Fluorescence Probe Study of Oil-in-Water Microemulsions. 2. Effect of the Nature of Alcohol, Oil, and Surfactant on the Surfactant Aggregation Number in the Aggregates, J. Phys. Chem., 1982, 86, 4809–4814 CrossRef CAS.
- J. Sjöblom, R. Lindbergh and S. E. Friberg, Microemulsions – phase equilibria characterization, structures, applications and chemical reactions, Adv. Colloid Interface, 1996, 95, 125–287 CrossRef.
- P. L. Gentili, M. Penconi, F. Costantino, P. Sassi, F. Ortica, F. Rossi and F. Elisei, Structural and photophysical characterization of some La2xGa2yIn2zO3 solid solutions, to be used as photocatalysts for H2 production from water/ethanol solutions, Sol. Energy Mater. Sol. Cells, 2010, 94, 2265–2274 CrossRef CAS PubMed.
- P. L. Gentili, M. Penconi, F. Ortica, F. Cotana, F. Rossi and F. Elisei, Synergistic effects in hydrogen production through water sonophotolysis catalyzed by new La2xGa2yIn2(1−x−y)O3 solid solutions, Int. J. Hydrogen Energy, 2009, 34, 9042–9049 CrossRef CAS PubMed.
- P. L. Gentili, F. Rossi, M. Penconi, F. Ortica and F. Elisei, Hydrogen production through sono-photolysis of water in the presence of solid solutions of metal oxides as photocatalysts, Expert Commentary, in Hydrogen Production, ed. D. Honnery and P. Moriarty, Nova Science Publishers, Inc., 2012, ch. 14, pp. 411–420 Search PubMed.
- M. Penconi, F. Ortica, F. Elisei and P. L. Gentili, New molecular pairs for low power non-coherent triplet-triplet annihilation based upconversion: dependence on the triplet energies of sensitizer and emitter, J. Lumin., 2013, 135, 265–270 CrossRef CAS PubMed.
- L. Liu, S. Tian and P. Ning, Phase behavior of TXs/toluene/water microemulsion systems for solubilization absorption of toluene, J. Environ. Sci., 2010, 22, 271–276 CrossRef CAS.
- G. Bartocci, F. Masetti, U. Mazzucato, A. Spalletti, I. Baraldi and F. Momicchioli, Photophysical and theoretical studies of photoisomerism and rotamerism of trans-styrylphenanthrenes, J. Phys. Chem., 1987, 91, 4733–4743 CrossRef CAS.
- M. Kubista, R. Sjoback, S. Eriksson and B. Albinsson, Experimental Correction for the Inner-filter Effect in Fluorescence Spectra, Analyst, 1994, 119, 417–419 RSC.
- M. E. Diaz Garcia and A. Sanz-Medel, Facile Chemical Deoxygenation of Micellar Solutions for Room Temperature Phosphorescence, Anal. Chem., 1986, 58, 1436–1440 CrossRef CAS.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of Photochem, CRC Press, Boca Raton, 2006 Search PubMed.
- P. J. Steinbach, R. Ionescu and C. R. Matthews, Analysis of Kinetics Using a Hybrid Maximum-Entropy/Nonlinear-Least-Squares Method: Application to Protein Folding, Biophys. J., 2002, 82, 2244–2255 CrossRef CAS.
- P. J. Steinbach, Inferring Lifetime Distributions from Kinetics by Maximizing Entropy Using a Bootstrapped Model, J. Chem. Inf. Comput. Sci., 2002, 42, 1476–1478 CrossRef CAS PubMed.
- P. L. Gentili, F. Ortica and G. Favaro, Static and Dynamic Interaction of a Naturally Occurring Photochromic Molecule with Bovine Serum Albumin Studied by UV-Visible Absorption and Fluorescence Spectroscopy, J. Phys. Chem. B, 2008, 112, 16793–16801 CrossRef CAS PubMed.
- M. R. di Nunzio, P. L. Gentili, A. Romani and G. Favaro, Role of the microenvironment on the fluorescent properties of a spirooxazine, Chem. Phys. Lett., 2010, 491, 80–85 CrossRef CAS PubMed.
- M. R. di Nunzio, P. L. Gentili, A. Romani and G. Favaro, Photochromism and Thermochromism of some Spirooxazines and Naphthopyrans in the Solid State and in Polymeric Film, J. Phys. Chem. C, 2010, 114, 6123–6131 CAS.
- P. L. Gentili, C. Clementi and A. Romani, Ultraviolet–Visible Absorption and Luminescence Properties of Quinacridone–Barium Sulfate Solid Mixtures, Appl. Spectrosc., 2010, 64, 923–929 CrossRef CAS PubMed.
- F. Costantino, P. L. Gentili and N. Audebrand, A new dual luminescent pillared cerium(IV)sulfate–diphosphonate, Inorg. Chem. Commun., 2009, 12, 406–408 CrossRef CAS PubMed.
- F. Nifiatis, W. Su, J. E. Haley, J. E. Slagle and T. M. Cooper, Comparison of the Photophysical Properties of a Planar, PtOEP, and a Nonplanar, PtOETPP, Porphyrin in Solution and Doped Films, J. Phys. Chem. A, 2011, 115, 13764–13772 CrossRef CAS PubMed.
- J. Yang, P. W. Cyr, Y. Wang, R. Soong, P. M. Macdonald, L. Chen, I. Manners and M. A. Winnik, Quenching Platinum Octaethylporphine Phosphorescence in Solution by Poly(ferrocenylsilane), Photochem. Photobiol., 2006, 82, 262–267 CrossRef CAS PubMed.
- T. Oyamada, S. Akiyama, M. Yahiro, M. Saigou, M. Shiro, H. Sasabe and C. Adachi, Unusual photoluminescence characteristics of tetraphenylpyrene (TPPy) in various aggregated morphologies, Chem. Phys. Lett., 2006, 421, 295–299 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2014 |