Convergent chemoenzymatic synthesis of a library of glycosylated analogues of pramlintide: structure–activity relationships for amylin receptor agonism†
Abstract
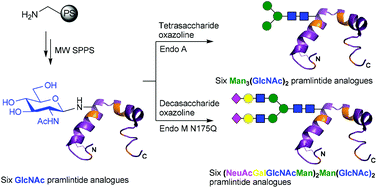
Pramlintide (Symlin®), a synthetic analogue of the naturally occurring pancreatic hormone amylin, is currently used with insulin in adjunctive therapy for type 1 and type 2 diabetes mellitus. Herein we report a systematic study into the effect that N-glycosylation of pramlintide has on activation of amylin receptors. A highly efficient convergent synthetic route, involving a combination of solid phase peptide synthesis and enzymatic glycosylation, delivered a library of N-glycosylated variants of pramlintide bearing either GlcNAc, the core N-glycan pentasaccharide [Man3(GlcNAc)2] or a complex biantennary glycan [(NeuAcGalGlcNAcMan)2Man(GlcNAc)2] at each of its six asparagine residues. The majority of glycosylated versions of pramlintide were potent receptor agonists, suggesting that N-glycosylation may be used as a tool to optimise the pharmacokinetic properties of pramlintide and so deliver improved therapeutic agents for the treatment of diabetes and obesity.

 Please wait while we load your content...
Please wait while we load your content...