Friedel–Crafts alkylations of electron-rich aromatics with 3-hydroxy-2-oxindoles: scope and limitations†‡
Abstract
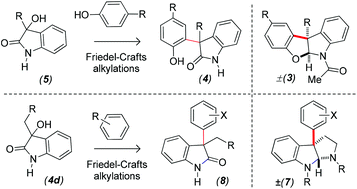
A Lewis acid-catalyzed nucleophilic addition of electron rich aromatics with 3-hydroxy-2-oxindoles 5 was developed. The reaction is believed to proceed through the 2H-indol-2-one ring system 9, which eventually reacts with various electron-rich aromatics to afford a variety of 2-oxindoles with an all-carbon quaternary center at the pseudobenzylic position (4, 8, 13, and 16) in high yields. The methodology provides an expeditious route to the tetracyclic core (3) of diazonamide (1), and azonazine (2) as well as the tricyclic core of asperazine (6a), idiospermuline (6b), and calycosidine (6c) viz. C(3a)-arylpyrroloindolines 7 having an all-carbon quaternary center on further synthetic elaboration.

 Please wait while we load your content...
Please wait while we load your content...