Linear synthesis of the branched pentasaccharide repeats of O-antigens from Shigella flexneri 1a and 1b demonstrating the major steric hindrance associated with type-specific glucosylation†
Abstract
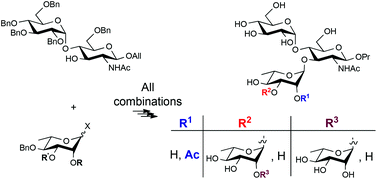
Shigella flexneri serotypes 1b and 1a are Gram-negative enteroinvasive bacteria causing shigellosis in humans. The O-antigen from S. flexneri 1b is a {→2)-[3Ac/4Ac]-α-L-Rhap-(1→2)-α-L-Rhap-(1→3)-[2Ac]-α-L-Rhap-(1→3)-[α-D-Glcp-(1→4)]-β-D-GlcpNAc-(1→}n branched polysaccharide ({AcABAcC(E)D}n). It is identical to that from S. flexneri 1a, except for the 2C-acetate. A concise synthesis of the disaccharide ED, trisaccharides AcC(E)D and C(E)D, tetrasaccharides BAcC(E)D and BC(E)D, and pentasaccharides ABAcC(E)D and ABC(E)D is described starting from a 2-N-acetyl-D-glucosaminide acceptor and using the imidate glycosylation chemistry. The E residue was efficiently introduced via a potent stereoselective [E + D] coupling. In contrast, harsh conditions and appropriate tuning of the donor were required for a high yielding [C + ED] glycosylation. Irrespective of the level of steric bulk at residue C, glycosylation at O-3D of the ED acceptor generated a major change of conformation of the D residue within the obtained C(E)D trisaccharide, as attested by NMR data. Proper manipulation of the constrained C(E)D trisaccharide was necessary to proceed with the stepwise chain elongation at O-3C of an acceptor having the 2C-O-acetyl already in place. The protected intermediates went through a one- to three-step deprotection sequence to give the propyl glycoside targets, as portions of the O-antigens from both S. flexneri 1a and 1b. Protecting group removal was clearly associated with conformational relief, yielding oligosaccharides, for which NMR data were consistent with a 4C1 conformation for the 3,4-di-O-glycosylated residue D, as in the native bacterial polymers.

 Please wait while we load your content...
Please wait while we load your content...