Synthesis of a chiral building block for highly functionalized polycyclic ethers†
Abstract
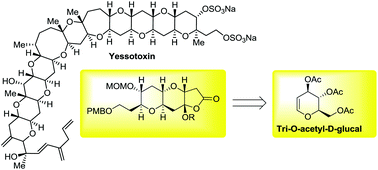
An efficient procedure for preparing enantiopure polycyclic ethers is reported. The protocol is based on the photo-oxidation/conjugate addition sequence over a chiral functionalized furan, which was prepared from commercially available tri-O-acetyl-D-glucal. The Michael addition step afforded two products with the same absolute configuration from a mixture of diastereomers.

 Please wait while we load your content...
Please wait while we load your content...