One-pot native chemical ligation of peptide hydrazides enables total synthesis of modified histones†
Abstract
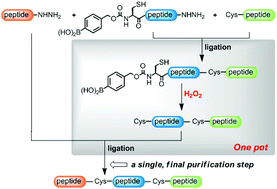
One of the rising demands in the field of protein chemical synthesis is the development of facile strategies that yield the protein in workable quantities and homogeneity, with fewer handling steps. Although the native chemical ligation of peptide hydrazides has recently been shown to be useful for the chemical synthesis of proteins carrying acid-sensitive modification groups, previous hydrazide-based protein synthesis studies have used sequential ligation strategies. Here, we report a practical method for a “one-pot” native chemical ligation of peptide hydrazides that would circumvent the need for the isolation of the intermediate products. This method employed a fast and selective arylboronate oxidation reaction mediated by H2O2, which draws attention to the potential applications of the thus far under-exploited boron-based functionalities in protein chemical synthesis. To demonstrate the practicality and efficiency of the new one-pot method, we report its application to a scalable total synthesis of modified histones (with five analogues of H3 and H4 as examples) on a multi-milligram scale, with good homogeneity.

 Please wait while we load your content...
Please wait while we load your content...