Synthesis and properties of fluorous benzoquinones and their application in deprotection of silyl ethers†
Abstract
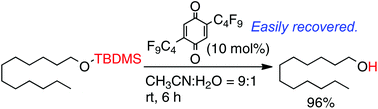
1,4-Benzoquinone derivatives bearing trifluoromethyl, perfluorobutyl and perfluorohexyl groups were prepared and employed in the deprotection of silyl ethers. The fluorous character of these compounds was examined by measuring the partition coefficient between the fluorous and organic solvents. The benzoquinone derivatives showed significant fluorous character, indicating that they can be recovered from the reaction mixtures using a fluorous/organic biphasic system. The oxidising ability of the fluorous benzoquinones was estimated by cyclic voltammetry, and these compounds were found to be strong oxidisers. The fluorous benzoquinones were utilised in the oxidative desilylation of silyl ethers to afford the deprotected alcohols in high yield. In addition, the reduced fluorous benzoquinones were recovered from the reaction mixtures in good yields using a fluorous/organic biphasic system.

 Please wait while we load your content...
Please wait while we load your content...