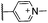Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Huisgen-based conjugation of water-soluble porphyrins to deprotected sugars: towards mild strategies for the labelling of glycans†
Francesca
Giuntini‡
a,
Francesca
Bryden
a,
Robin
Daly
b,
Eoin M.
Scanlan
b and
Ross W.
Boyle
*a
aDepartment of Chemistry, University of Hull, Kingston-upon-Hull, East Yorkshire HU6 7RX, UK. E-mail: R.W.Boyle@hull.ac.uk
bSchool of Chemistry, Trinity Biomedical Sciences Institute, Trinity College, 152-160 Pearse Street, Dublin 2, Ireland
First published on 2nd January 2014
Abstract
Fully deprotected alkynyl-functionalised mono- and oligosaccharides undergo CuAAC-based conjugation with water-soluble porphyrin azides in aqueous environments. The mild reaction conditions are fully compatible with the presence of labile glycosidic bonds. This approach provides an ideal strategy to conjugate tetrapyrroles to complex carbohydrates.
Introduction
Advances in the field of functional glycomics shed light on the structural and functional diversity of carbohydrates exposed on the cell membrane, and their fundamental role in a variety of biological processes, including protein folding, self-recognition, cell migration, modulation of signalling pathways, and trafficking.1,2 The weak but highly specific binding of surface-exposed glycans with carbohydrate-binding proteins (e.g. lectins) underpins diverse phenomena such as immune system modulation, pathogen invasion, cell migration and proliferation. Variations in the structure, expression, or binding affinity of glycans and glycoproteins have been associated with the state and invasiveness of neoplastic lesions3,4 and non-malignant diseases,5 and with bacterial virulence and invasion of host tissues,6,7 as a consequence, the biochemistry of glycan–lectin interactions has been object of investigation as a potential route for the identification of new therapeutic targets, and the development of expeditious diagnostic methods.8–11Recently, the possibility of exploiting glycan–lectin interactions for analytical and sensing purposes has also received a great deal of attention, with a consistent body of data demonstrating how viruses, bacterial strains, and bacterial spores can be detected by exploiting the specificity of glycan–lectin interactions. Many high-throughput strategies to probe glycan–lectin interactions have been devised, the majority of which are based on fluorescence-assisted arrays, renewing the interest in the synthesis of fluorescently labelled glycans and lectins.12–17 While labelling of proteins is readily achievable, the derivatisation of glycans remains less explored. The complex structure of glycans and the recurring presence of labile moieties (e.g. sialic acid)18 pose a challenge to their chemical modification, and although various labelling strategies have been reported, a versatile and reliable approach remains to be devised.13,19,20
Following our current interest in mild bio-orthogonal approaches for the conjugation of tetrapyrrolic photosensitisers to macromolecules,21–24 we undertook a study aimed at identifying a suitable method for the conjugation of porphyrins to glycans. A straightforward ligation strategy to label glycans with porphyrins would not merely afford new luminescent lectin markers for in vitro assays, but could lead to phototherapeutic agents targeted to cells over-expressing lectins, to viruses, and to bacteria organised in biofilms. Mannose containing glycopeptides labelled with fluorescein have previously been used in cellular immunology screens for the development of carbohydrate vaccines.25 Endowed with high absorption coefficients and high luminescence and singlet oxygen quantum yields, porphyrins have found a variety of applications in the biomedical field.26 Their versatility towards chemical modification, which allows the tuning of the molecule's physico-chemical properties, contributed to make these species excellent candidates as photodynamic therapy drugs, reporters for fluorescent diagnosis, and investigative tools.
The association of porphyrins with carbohydrates has been widely explored with the purpose of obtaining water-soluble or targeted species.27 The synthesis of porphyrin–carbohydrate conjugates is however hampered by their conflicting solubility, which often imposes the use of solvent mixtures, high temperatures, or alternatively requires the use of protected carbohydrates.28–34
While these approaches prove efficient for the synthesis of porphyrin–monosaccharides conjugates, mild methods that preserve the integrity of labile polysaccharides remain substantially unavailable.
In a previous study23 we showed that water-soluble porphyrin-azides can be conjugated to polyacrylamide nanoparticles in aqueous environment and in mild conditions, by copper-catalysed azide–alkyne cycloaddition (CuAAC).35,36 We reasoned that, in a similar way, CuAAC could provide the optimal ligation chemistry for conjugation of porphyrins to glycans. CuAAC has proved to be an efficient tool for the conjugation of porphyrins to peptides,37 polymers, and carbohydrates;30,32,34,38 furthermore, as a result of intense investigations recently carried out in the field, the feasibility of this approach is facilitated by the improved synthetic accessibility of azide-containing porphyrins22,23,39 and alkynyl-carbohydrates.40
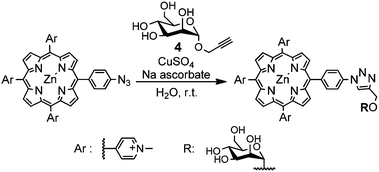
We first assessed the efficiency of the cycloaddition using water-soluble porphyrin 1 and a simple deprotected monosaccharide bearing a terminal alkyne. Porphyrin 1 was synthesised from 5-(4-aminophenyl)-10,15,20-triphenylporphyrin, which was converted into the corresponding azide by treatment with sodium nitrite and sodium azide in water, according to a modified literature procedure.39 The resulting porphyrin-azide was treated with iodomethane, to afford the water-soluble tricationic species. In order to avoid sequestration of copper ions by the tetrapyrrole during the cycloaddition, the porphyrin free base were converted into the corresponding zinc complex.23
Treatment of an aqueous solution of porphyrin 1 with 1-α-propargyloxy mannose 439 in the presence of CuSO4·5H2O and sodium ascorbate at room temperature, led to the complete conversion of 1 into cycloadduct 11 within 15 minutes. (Scheme 1, Table 1).
Isolation of the desired product from the reaction mixture was achieved by treating the reaction mixture with NH4PF6, which allowed the recovery of the water-insoluble hexafluorophosphate salt of the porphyrin by filtration, and subsequent conversion of the hexachlorophosphate salt into the corresponding trichloride by treatment of with tetra-n-butylammonium chloride in acetone.23
Encouraged by this positive result, we investigated the applicability of the conjugation to different deprotected carbohydrate and porphyrin substrates (Fig. 1). Gratifyingly, 1-β-propargyloxy-monosaccharides 5 and 6 and 1-β-propargyloxy-N-acetyl-lactosamine 7 underwent CuAAC with 1 to afford, respectively, adducts 10, 12 and 17 in excellent yields, and negatively charged porphyrin 4 reacted with 1-α-propargyloxy mannose 4 under the conditions described above, to afford the desired conjugates in equally good yield (Table 1). Similarly, the presence of a PEG chain as a spacer between the porphyrin and the azide group did not affect the conjugation efficiency, as shown by the formation of cycloadducts 14–16 in high yields from the reaction of porphyrin 3 with 1-α-propargyloxy-hexoses 4–6.39 The excellent yields indicate that our approach has the potential of becoming a reliable bioconjugation tool of general applicability.
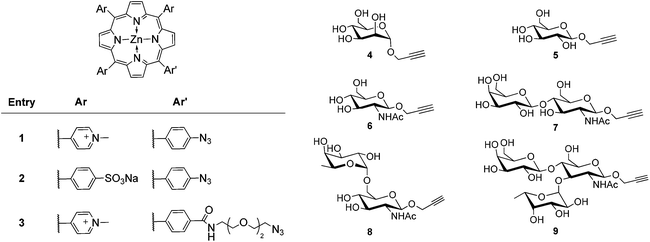 | ||
| Fig. 1 Porphyrin azides and propargyloxy sugars employed in the cycloadditions. Cationic porphyrins 1 and 3 are used as the trichlorides. | ||
We next proceeded to verify the compatibility of the ligation conditions with the presence of labile glycosidic bonds: we chose propargyloxy-bearing α-Fuc-(1–6)-GlcNAc 8 and Lewisx trisaccharide 941 as models for a glycan substrate, as the link α-(1–6) is known to be unstable to acidic and basic conditions, and high temperatures. When porphyrin 1 was treated with 8 or 9 under the conditions reported above, the desired cycloadducts were isolated (Table 2), and the spectroscopic data confirmed that the oligosaccharide moieties were intact in both 18 and 19. To the best of our knowledge, this is the first report of the conjugation of a porphyrin to a totally deprotected α-Fuc-(1–6)-GlcNAc and Lewisx trisaccharide; the integrity of the labile link α-(1–6) in the conjugates further confirms the suitability this of ligation method for glycan labelling.
Conclusions
In conclusions, we devised a CuAAC-based method for the ligation of water-soluble azide-bearing porphyrins to alkyne-bearing fully deprotected carbohydrates. The reaction takes place in aqueous solution and affords the desired conjugates in good-to-excellent yields, and involves minimal manipulation for the isolation of the cycloaddition product. Variations of the structure of the carbohydrate and of the porphyrin are well tolerated and do not impair the efficiency of the reaction. Ligation of porphyrins to totally deprotected carbohydrates containing the labile α-(1–6) glycosidic bond, a recurrent feature in the structure of glycans, are achievable with this method. It is envisaged that this approach will complement the recent chemo-enzymatic strategies for glycan modification.42 Although it did not represent an issue in the present work, the presence of the copper ion is certainly a caveat for any CuAAC-based labelling strategy to be carried out in biological environment: we anticipate that the new porphyrins reported here will be equally reactive under copper-free conditions,43 which has already been successfully applied to cyclooctyne-functionalised carbohydrate substrates.44 The application of this approach to more complex carbohydrates is the object of current studies in our laboratories.Acknowledgements
The authors thank EPSRC for funding the project (EP/H000151/1). Mass spectrometry data was acquired at the EPSRC UK National Mass Spectrometry Facility at Swansea University.Notes and references
- J. Li and J. Richards, in Functional Glycomics, ed. J. Li, Humana Press, 2010, vol. 600, pp. 1–8 Search PubMed.
- G. W. Hart and R. J. Copeland, Cell, 2010, 143, 672–676 CrossRef CAS PubMed.
- C. Slawson and G. W. Hart, Nat. Rev. Cancer, 2011, 11, 678–684 CrossRef CAS PubMed.
- N. D. S. Rambaruth and M. V. Dwek, Acta Histochem., 2011, 113, 591–600 CrossRef CAS PubMed.
- A. Kuno, Y. Ikehara, Y. Tanaka, T. Angata, S. Unno, M. Sogabe, H. Ozaki, K. Ito, J. Hirabayashi, M. Mizokami and H. Narimatsu, Clin. Chem., 2011, 57, 48–56 CAS.
- G. A. Rabinovich and M. A. Toscano, Nat. Rev. Immunol., 2009, 9, 338–352 CrossRef CAS PubMed.
- R. C. Davicino, R. J. Eliçabe, M. S. Di Genaro and G. A. Rabinovich, Int. Immunopharmacol., 2011, 11, 1457–1463 CrossRef CAS PubMed.
- J. Katrlík, J. Švitel, P. Gemeiner, T. Kožár and J. Tkac, Med. Res. Rev., 2010, 30, 394–418 Search PubMed.
- A. Bernardi, J. Jimenez-Barbero, A. Casnati, C. De Castro, T. Darbre, F. Fieschi, J. Finne, H. Funken, K.-E. Jaeger, M. Lahmann, T. K. Lindhorst, M. Marradi, P. Messner, A. Molinaro, P. V. Murphy, C. Nativi, S. Oscarson, S. Penades, F. Peri, R. J. Pieters, O. Renaudet, J.-L. Reymond, B. Richichi, J. Rojo, F. Sansone, C. Schaffer, W. B. Turnbull, T. Velasco-Torrijos, S. Vidal, S. Vincent, T. Wennekes, H. Zuilhof and A. Imberty, Chem. Soc. Rev., 2013, 42, 4709–4727 RSC.
- H. An and C. Lebrilla, in Functional Glycomics, ed. J. Li, Humana Press, 2010, vol. 600, pp. 199–213 Search PubMed.
- D. H. Dube and C. R. Bertozzi, Nat. Rev. Drug Discovery, 2005, 4, 477–488 CrossRef CAS PubMed.
- D. Grünstein, M. Maglinao, R. Kikkeri, M. Collot, K. Barylyuk, B. Lepenies, F. Kamena, R. Zenobi and P. H. Seeberger, J. Am. Chem. Soc., 2011, 133, 13957–13966 CrossRef PubMed.
- X. Song, Y. Lasanajak, B. Xia, D. F. Smith and R. D. Cummings, ACS Chem. Biol., 2009, 4, 741–750 CrossRef CAS PubMed.
- S. Park, J.-W. Sung and I. Shin, ACS Chem. Biol., 2009, 4, 699–701 CrossRef CAS PubMed.
- X. Song, J. Heimburg-Molinaro, N. Dahms, D. Smith and R. Cummings, in Carbohydrate Microarrays, ed. Y. Chevolot, Humana Press, 2012, vol. 808, pp. 137–148 Search PubMed.
- X. Wang, O. Ramstrom and M. Yan, Chem. Commun., 2011, 47, 4261–4263 RSC.
- X. Song, B. Xia, S. R. Stowell, Y. Lasanajak, D. F. Smith and R. D. Cummings, Chem. Biol., 2009, 16, 36–47 CrossRef CAS PubMed.
- C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357–2364 CrossRef CAS.
- K. W. Dehnert, B. J. Beahm, T. T. Huynh, J. M. Baskin, S. T. Laughlin, W. Wang, P. Wu, S. L. Amacher and C. R. Bertozzi, ACS Chem. Biol., 2011, 6, 547–552 CrossRef CAS PubMed.
- L. R. Ruhaak, G. Zauner, C. Huhn, C. Bruggink, A. M. Deelder and M. Wuhrer, Anal. Bioanal. Chem., 2010, 397, 3457–3481 CrossRef CAS PubMed.
- C. M. A. Alonso, A. Palumbo, A. J. Bullous, F. Pretto, D. Neri and R. W. Boyle, Bioconjugate Chem., 2010, 21, 302–313 CrossRef CAS PubMed.
- F. Bryden and R. W. Boyle, Synlett, 2013, 1978–1982 CAS.
- F. Giuntini, F. Dumoulin, R. Daly, V. Ahsen, E. M. Scanlan, A. S. P. Lavado, J. W. Aylott, G. A. Rosser, A. Beeby and R. W. Boyle, Nanoscale, 2012, 4, 2034–2045 RSC.
- R. Hudson, M. Carcenac, K. Smith, L. Madden, O. J. Clarke, A. Pelegrin, J. Greenman and R. W. Boyle, Br. J. Cancer, 2005, 92, 1442–1449 CrossRef CAS PubMed.
- M. A. Brimble, R. Kowalczyk, P. W. R. Harris, P. R. Dunbar and V. J. Muir, Org. Biomol. Chem., 2008, 6, 112–121 CAS.
- R. K. Pandey and G. Zheng, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, London, 2000, vol. 6, pp. 157–230 Search PubMed.
- X. Zheng and R. K. Pandey, Anti-Cancer Agents Med. Chem., 2008, 8, 241–268 CrossRef CAS.
- J. P. C. Tomé, E. M. P. Silva, A. M. V. M. Pereira, C. M. A. Alonso, M. A. F. Faustino, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, S. A. P. Tavares, R. R. Duarte, M. F. Caeiro and M. L. Valdeira, Bioorg. Med. Chem., 2007, 15, 4705–4713 CrossRef PubMed.
- R. Daly, G. Vaz, A. M. Davies, M. O. Senge and E. M. Scanlan, Chem.–Eur. J., 2012, 18, 14671–14679 CrossRef CAS PubMed.
- G. Garcia, D. Naud-Martin, D. Carrez, A. Croisy and P. Maillard, Tetrahedron, 2011, 67, 4924–4932 CrossRef CAS PubMed.
- D. Kushwaha and V. K. Tiwari, J. Org. Chem., 2013, 78, 8184–8190 CrossRef CAS PubMed.
- S. v. Ballut, D. Naud-Martin, B. Loock and P. Maillard, J. Org. Chem., 2011, 76, 2010–2028 CrossRef CAS PubMed.
- S. Achelle, P. Couleaud, P. Baldeck, M.-P. Teulade-Fichou and P. Maillard, Eur. J. Org. Chem., 2011, 1271–1279 CrossRef CAS.
- M. G. H. Vicente, E. Hao and T. J. Jensen, J. Porphyrins Phthalocyanines, 2009, 13, 51–59 CrossRef.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed.
- F. Giuntini, C. M. A. Alonso and R. W. Boyle, Photochem. Photobiol. Sci., 2011, 10, 759–791 CAS.
- B. L. Carpenter, E. Feese, H. Sadeghifar, D. S. Argyropoulos and R. A. Ghiladi, Photochem. Photobiol., 2012, 88, 527–536 CrossRef CAS PubMed.
- O. B. Locos, C. C. Heindl, A. Corral, M. O. Senge and E. M. Scanlan, Eur. J. Org. Chem., 2010, 1026–1028 CrossRef CAS.
- P. H. Seeberger, N. Finney, D. Rabuka and C. R. Bertozzi, in Essential of Glycobiology, ed. A. Varki, R. D. Cummings, J. D. Esko, H. H. Freeze, P. Stanley, C. R. Bertozzi, G. W. Hart and M. E. Etzeler, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2nd edn, 2009, pp. 691–703 Search PubMed.
- R. Daly, T. McCabe and E. M. Scanlan, J. Org. Chem., 2012, 78, 1080–1090 CrossRef PubMed.
- E. Boeggeman, B. Ramakrishnan, M. Pasek, M. Manzoni, A. Puri, K. H. Loomis, T. J. Waybright and P. K. Qasba, Bioconjugate Chem., 2009, 20, 1228–1236 CrossRef CAS PubMed.
- P. V. Chang, J. A. Prescher, E. M. Sletten, J. M. Baskin, I. A. Miller, N. J. Agard, A. Lo and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1821–1826 CrossRef CAS PubMed.
- I. Singh, C. Freeman, A. Madder, J. S. Vyle and F. Heaney, Org. Biomol. Chem., 2012, 10, 6633–6639 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ob42306a |
| ‡ Present address: School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Liverpool L3 3AF, UK. |
| This journal is © The Royal Society of Chemistry 2014 |