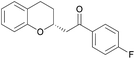
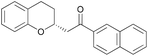
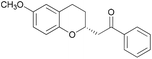
Asymmetric chroman synthesis via an intramolecular oxy-Michael addition by bifunctional organocatalysts†
Ryota
Miyaji
,
Keisuke
Asano
* and
Seijiro
Matsubara
*
Department of Material Chemistry, Graduate School of Engineering, Kyoto University, Kyotodaigaku-Katsura, Nishikyo, Kyoto 615-8510, Japan. E-mail: asano.keisuke.5w@kyoto-u.ac.jp; matsubara.seijiro.2e@kyoto-u.ac.jp; Fax: +81 75 383 2438; Tel: +81 75 383 7571 Tel: +81 75 383 7130
First published on 29th October 2013
Abstract
Cinchona-alkaloid-urea-based bifunctional organocatalysts facilitate the catalytic asymmetric synthesis of chroman derivatives via an intramolecular oxy-Michael addition reaction. Phenol derivatives bearing an easily available (E)-α,β-unsaturated ketone or a thioester moiety are useful substrates for the title transformation. This method represents a facile synthesis of various optically active 2-substituted chromans in high yield.
Introduction
Chiral 2-substituted chromans are found in an extremely wide range of bioactive compounds, as typified by vitamin E, and their biological activities have attracted much attention (Fig. 1).1 Thus, the enantioselective synthetic methods toward chroman derivatives are in high demand; indeed, a number of strategies have been reported, addressing this need.2–5 Among them, the intramolecular oxy-Michael addition is a promising method for constructing the desired framework from easily available phenol derivatives bearing an α,β-unsaturated carbonyl; the remaining carbonyl group in the product allows for further structural modification, which may lead to the synthesis of various pharmacological compounds. However, only a few examples of such approaches have been reported to date.4,5 In addition, most of these strategies display a significant limitation in that the substrate, typically an α,β-unsaturated ester moiety, must be in its (Z)-isomer form, and that the (Z)-forms of more electron-deficient olefins (α,β-unsaturated ketones and thioesters) are extremely difficult to prepare by means of simple methods such as Wittig reactions using stabilized ylides (Scheme 1). These problems must be solved to expand the scope of synthetically accessible chromans.We have recently established a useful strategy for the asymmetric synthesis of five- or six-membered oxygen heterocycles via an intramolecular oxy-Michael addition starting from (E)-α,β-unsaturated carbonyl substrates (Scheme 2).6,7 This method utilizes multipoint substrate recognition by bifunctional organocatalysts through hydrogen bonding;8,9 the mild characteristics of activation through hydrogen bonding facilitate concerted catalysis efficient for obtaining high enantioselectivity even in rapid intramolecular processes for cycloetherifications.6d The efficiency of this protocol prompted us to explore the use of bifunctional organocatalysts for the intramolecular oxy-Michael addition from phenol derivatives bearing an (E)-α,β-unsaturated carbonyl moiety.5,10 In this study, we present a novel enantioselective synthesis of 2-substituted chromans via an intramolecular oxy-Michael addition using cinchona-alkaloid-urea-based bifunctional organocatalysts.
 | ||
| Scheme 2 Asymmetric synthesis of 2-substituted THF and THP via an intramolecular oxy-Michael addition by a bifunctional organocatalyst. | ||
Results and discussion
We initiated our investigation using substrate 1a with 10 mol% quinidine-derived bifunctional aminourea catalyst 3a in CH2Cl2 at 25 °C over 24 h; chroman product 2a was obtained with moderate enantioselectivity (Table 1, entry 1). Solvent screening revealed that THF was effective for both enantioselectivity and yield (Table 1, entry 9). A decrease in reaction temperature improved the enantioselectivity (Table 1, entry 10). However, the use of a smaller amount of catalyst (5 mol%) led to lower enantioselectivity, which was likely due to the competing non-catalytic reaction (Table 1, entry 11). A time of 12 h was found to be sufficient for reaction completion, and higher enantioselectivity could be obtained (Table 1, entry 12). In this reaction, the urea catalyst 3a was revealed to be more efficient than the corresponding thiourea catalyst 3b (Table 1, entries 12 and 13).7a Further screening of urea catalysts showed that quinine-derived 3d was an efficient catalyst for obtaining the opposite enantiomer of 2a with good enantioselectivity (Table 1, entry 15).| Entry | Catalyst | Solvent | Yieldb (%) | ee (%) |
|---|---|---|---|---|
a Reactions were run using 1a (0.1 mmol) and the catalyst (0.01 mmol) in the solvent (0.2 mL).
b Isolated yields.
c CPME = cyclopentyl methyl ether.
d Reactions were run at 0 °C.
e Reaction was run using 5 mol% of 3a (0.005 mmol).
f Reactions were run for 12 h. 
|
||||
| 1 | 3a | CH2Cl2 | 99 | 63 |
| 2 | 3a | Benzene | 87 | 60 |
| 3 | 3a | Pyridine | 99 | 58 |
| 4 | 3a | DMSO | 88 | 25 |
| 5 | 3a | Et2O | 91 | 69 |
| 6 | 3a | t-BuOMe | 99 | 55 |
| 7 | 3a | CPMEc | 93 | 63 |
| 8 | 3a | 1,4-Dioxane | 96 | 72 |
| 9 | 3a | THF | 99 | 72 |
| 10d | 3a | THF | 99 | 81 |
| 11d,e | 3a | THF | 99 | 76 |
| 12d,f | 3a | THF | 95 | 84 |
| 13d,f | 3b | THF | 95 | 75 |
| 14d,f | 3c | THF | 86 | 82 |
| 15d,f | 3d | THF | 98 | −80 |
| 16d,f | 3e | THF | 86 | −78 |
With catalyst 3a and optimized reaction conditions identified, we next investigated the reactions of substrates bearing other (E)-Michael-acceptor moieties.11 Electron-rich substrates were also effective, providing the chroman products in high yield and comparable enantioselectivity (Table 2, entries 2 and 3). A starting material bearing an electron-withdrawing group afforded the corresponding product in high yield, although the enantiomeric excess was slightly lower (Table 2, entry 4). A substrate with a p-bromophenyl substituent yielded the corresponding product quantitatively in high enantioselectivity (Table 2, entry 5); however, a 2-naphthyl-substituted enone gave the resultant product in lower yield and stereoselectivity (Table 2, entry 6). Unfortunately, a methylketone proved to be an unsuccessful substrate (Table 2, entry 7). Substituents on the phenol moiety were also investigated, and a substrate with a methoxy group gave the corresponding product in good yield with moderate enantioselectivity (Table 2, entry 8), although a phenol derivative with a bromo group resulted in lower yield and stereoselectivity (Table 2, entry 9). To our delight, an α,β-unsaturated thioester participated in the cyclization reaction, yielding a chroman derivative suitable for various subsequent transformations, demonstrating the synthetic utility of our method (Scheme 3). The absolute configuration of 2e was determined as (R) using X-ray analysis (see ESI† for details), and the configurations of all other examples were assigned accordingly.
Conclusions
In summary, we have presented a novel asymmetric chroman synthesis via an intramolecular oxy-Michael addition employing bifunctional aminourea catalysts. In this method, substrates bearing an easily available (E)-Michael acceptor including α,β-unsaturated ketones and thioesters could be used, thereby leading to a facile and versatile approach to optically active chromans. Further studies on the expansion of the substrate scope and the application of this methodology toward other heterocyclic scaffolds are currently underway in our laboratory and will be reported in due course.Acknowledgements
We thank Professor Takuya Kurahashi (Kyoto University) for X-ray crystallographic analysis. This work was supported financially by the Japanese Ministry of Education, Culture, Sports, Science and Technology.Notes and references
- For reviews, see: (a) Chromenes, Chromanones, and Chromones, ed. G. P. Ellis, Wiley-Interscience, New York, 1977 Search PubMed; (b) Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon, Oxford, 1996 Search PubMed.
- For reviews, see: (a) H. C. Shen, Tetrahedron, 2009, 65, 3931 CrossRef CAS PubMed; (b) M. Núñez, P. García, R. F. Moro and D. Díez, Tetrahedron, 2010, 66, 2089 CrossRef PubMed.
- For selected examples, see: (a) Y. Uozumi, K. Kato and T. Hayashi, J. Am. Chem. Soc., 1997, 119, 5063 CrossRef CAS; (b) E. Mizuguchi and K. Achiwa, Chem. Pharm. Bull., 1997, 45, 1209 CrossRef CAS; (c) B. M. Trost and F. D. Toste, J. Am. Chem. Soc., 1998, 120, 9074 CrossRef CAS; (d) B. M. Trost and N. Asakawa, Synthesis, 1999, 1491 CrossRef CAS PubMed; (e) B. M. Trost, H. C. Shen and J.-P. Surivet, Angew. Chem., Int. Ed., 2003, 42, 3943 CrossRef CAS PubMed; (f) B. M. Trost, H. C. Shen, L. Dong and J.-P. Surivet, J. Am. Chem. Soc., 2003, 125, 9276 CrossRef CAS PubMed; (g) B. M. Trost, H. C. Shen, L. Dong, J.-P. Surivet and C. Sylvain, J. Am. Chem. Soc., 2004, 126, 11966 CrossRef CAS PubMed; (h) J.-R. Labrosse, C. Poncet, P. Lhoste and D. Sinou, Tetrahedron: Asymmetry, 1999, 10, 1069 CrossRef CAS; (i) K. Ishihara, S. Nakamura and H. Yamamoto, J. Am. Chem. Soc., 1999, 121, 4906 CrossRef CAS; (j) S. Nakamura, K. Ishihara and H. Yamamoto, J. Am. Chem. Soc., 2000, 122, 8131 CrossRef CAS; (k) H. Ishibashi, K. Ishihara and H. Yamamoto, J. Am. Chem. Soc., 2004, 126, 11122 CrossRef CAS PubMed; (l) L. F. Tietze, K. M. Sommer, J. Zinngrebe and F. Stecker, Angew. Chem., Int. Ed., 2005, 44, 257 CrossRef CAS PubMed; (m) L. F. Tietze, F. Stecker, J. Zinngrebe and K. M. Sommer, Chem.–Eur. J., 2006, 12, 8770 CrossRef CAS PubMed; (n) K. Liu, A. Chougnet and W.-D. Woggon, Angew. Chem., Int. Ed., 2008, 47, 5827 CrossRef CAS PubMed; (o) Y. K. Chung and G. C. Fu, Angew. Chem., Int. Ed., 2009, 48, 2225 CrossRef CAS PubMed; (p) L.-Q. Lu, F. Li, J. An, J.-J. Zhang, X.-L. An, Q.-L. Hua and W.-J. Xiao, Angew. Chem., Int. Ed., 2009, 48, 9542 CrossRef CAS PubMed; (q) X.-F. Wang, Q.-L. Hua, Y. Cheng, X.-L. An, Q.-Q. Yang, J.-R. Chen and W.-J. Xiao, Angew. Chem., Int. Ed., 2010, 49, 8379 CrossRef CAS PubMed; (r) X.-F. Wang, J. An, X.-X. Zhang, F. Tan, J.-R. Chen and W.-J. Xiao, Org. Lett., 2011, 13, 808 CrossRef CAS PubMed; (s) L.-Q. Lu, Z.-H. Ming, J. An, C. Li, J.-R. Chen and W.-J. Xiao, J. Org. Chem., 2012, 77, 1072 CrossRef CAS PubMed; (t) X.-G. Song, S.-F. Zhu, X.-L. Xie and Q.-L. Zhou, Angew. Chem., Int. Ed., 2013, 52, 2555 CrossRef CAS PubMed; (u) H. Wu, Y.-P. He and L.-Z. Gong, Org. Lett., 2013, 15, 460 CrossRef CAS PubMed; (v) W. Yang, Y. Yang and D.-M. Du, Org. Lett., 2013, 15, 1190 CAS; (w) Z.-X. Jia, Y.-C. Luo, X.-N. Cheng, P.-F. Xu and Y.-C. Gu, J. Org. Chem., 2013, 78, 6488 CrossRef CAS PubMed.
- (a) A. Merschaert, P. Delbeke, D. Daloze and G. Dive, Tetrahedron Lett., 2004, 45, 4697 CrossRef CAS PubMed; (b) N. Saito, A. Ryoda, W. Nakanishi, T. Kumamoto and T. Ishikawa, Eur. J. Org. Chem., 2008, 2759 CrossRef CAS.
- Analogous intramolecular oxy-Michael additions to form chromans from (E)-substrates by a bifunctional organocatalyst have been recently reported, and the utility is specific to slow reactions from α,β-unsaturated amides, see: Y. Kobayashi, Y. Taniguchi, N. Hayama, T. Inokuma and Y. Takemoto, Angew. Chem., Int. Ed., 2013, 52, 11114 CrossRef CAS PubMed.
- (a) K. Asano and S. Matsubara, J. Am. Chem. Soc., 2011, 133, 16711 CrossRef CAS PubMed; (b) K. Asano and S. Matsubara, Org. Lett., 2012, 14, 1620 CrossRef CAS PubMed; (c) T. Okamura, K. Asano and S. Matsubara, Chem. Commun., 2012, 48, 5076 RSC; (d) Y. Fukata, R. Miyaji, T. Okamura, K. Asano and S. Matsubara, Synthesis, 2013, 1627 CAS.
- For our related works on intramolecular aza-Michael addition by bifunctional organocatalysts, see: (a) R. Miyaji, K. Asano and S. Matsubara, Org. Lett., 2013, 15, 3658 CrossRef CAS PubMed; (b) Y. Fukata, K. Asano and S. Matsubara, Chem. Lett., 2013, 355 CrossRef CAS; (c) Y. Fukata, K. Asano and S. Matsubara, J. Am. Chem. Soc., 2013, 135, 12160 CrossRef CAS PubMed.
- (a) T. Okino, Y. Hoashi and Y. Takemoto, J. Am. Chem. Soc., 2003, 125, 12672 CrossRef CAS PubMed; (b) T. Okino, Y. Hoashi, T. Furukawa, X. Xu and Y. Takemoto, J. Am. Chem. Soc., 2005, 127, 119 CrossRef CAS PubMed; (c) B. Vakulya, S. Varga, A. Csámpai and T. Soós, Org. Lett., 2005, 7, 1967 CrossRef CAS PubMed; (d) A. Hamza, G. Schubert, T. Soós and I. Pápai, J. Am. Chem. Soc., 2006, 128, 13151 CrossRef CAS PubMed; (e) S. J. Connon, Chem.–Eur. J., 2006, 12, 5418 CrossRef PubMed; (f) J.-L. Zhu, Y. Zhang, C. Liu, A.-M. Zheng and W. Wang, J. Org. Chem., 2012, 77, 9813 CrossRef CAS PubMed.
- For reviews on asymmetric catalysis based on hydrogen bonding, see: (a) Hydrogen Bonding in Organic Synthesis, ed. P. M. Pihko, Wiley-VCH, Weinheim, 2009 Search PubMed; (b) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713 CrossRef CAS PubMed; (c) M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520 CrossRef CAS PubMed.
- For an example of endo-cyclization via an asymmetric intramolecular oxy-Michael addition from phenol derivatives mediated by a bifunctional organocatalyst, see: M. M. Biddle, M. Lin and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 3830 CrossRef CAS PubMed.
- Although reactions from β-disubstituted α,β-unsaturated ketones were also examined, they were less reactive in this catalytic process. See ESI for details (Scheme S1†).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical and spectroscopic data for synthetic compounds, copies of NMR. CCDC 962820. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ob41938j |
| This journal is © The Royal Society of Chemistry 2014 |