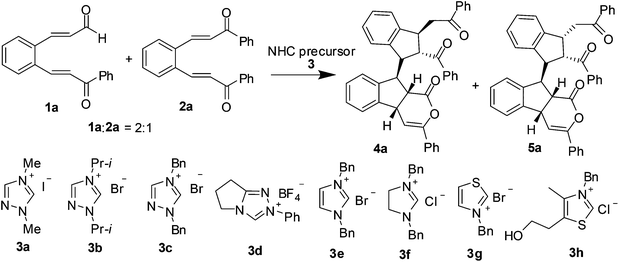
N-Heterocyclic carbene-catalyzed cascade reaction of 2-aroylvinylcinnamaldehydes with 2-aroylvinylchalcones: rapid assembly of six contiguous stereogenic centers with high diastereoselectivity†
Xing-Wen
Fan
and
Ying
Cheng
*
College of Chemistry, Beijing Normal University, Beijing 100875, China. E-mail: ycheng2@bnu.edu.cn; Fax: +86 1058805558; Tel: +86 1058805558
First published on 10th October 2013
Abstract
N-Heterocyclic carbene-catalyzed reaction of 2-aroylvinylcinnamaldehydes with 2-aroylvinylchalcones proceeded via a triple Michael addition and intramolecular lactonization cascade to produce novel 9-(2-aroyl-3-aroylmethyl-1-indanyl)-3-arylindeno[2,1-c]pyran-1-ones in good yields with high diastereoselectivity. This reaction constructed six contiguous stereogenic centers in a single reactive event. Among 32 possible diastereoisomers that contain six stereocenters, only two diastereoisomers were detected with diastereomeric ratios being 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–28
1–28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This work opens up a new avenue for the synthesis of complex indane derivatives, a type of compound that has been reported to possess various biological and pharmaceutical activities.
1. This work opens up a new avenue for the synthesis of complex indane derivatives, a type of compound that has been reported to possess various biological and pharmaceutical activities.
Introduction
The discovery and development of highly efficient and stereoselective methods to construct complex molecules with multiple stereogenic centers continue to be an important goal for organic chemists. Cascade reactions that allow the formation of multiple chemical bonds and stereogenic centers in one operation have been shown to be one of the most efficient strategies for the rapid generation of complexity of products,1 particularly in the total synthesis of natural products and biologically active compounds.2 In recent years, N-heterocyclic carbene-catalyzed cascade reactions have gained more and more attention.3 For example, the NHC-catalyzed dimerization of phthalaldehydes followed by cascade benzoin–aldol–hemiacetalization or benzoin–aldol–benzoin reaction affords isobenzofuran-spiro-indanones and indeno[2,1-a]indanones,4 while dimerization of o-aroylvinylarylaldehydes in the presence of NHC catalysts forms indene-spiro-indanones via Stetter–aldol–Michael cascades.5 In addition, cascade aza-benzoin–aldol or aza-benzoin–Michael reactions of phthalaldehyde or 2-formylcinnamates with imines have been reported to produce 2-aminoindanone derivatives.6 Furthermore, the NHC-catalyzed cascade [4 + 2] cycloaddition of α,β-unsaturated acid fluorides with silyl dienol ethers yields 1,3-cyclohexadienes,7 while the reactions of 2-propargyloxy-1-arylaldehydes with aldehydes produce chroman-4-one or benzofuran-3-one derivatives under different conditions.8 Last but not least, the NHC-promoted cascade reactions of formylcyclopropane-1,1-diesters with indole-2-carbaldehydes or salicylaldehydes lead to the formation of pyrrolo[1,2-a]indoles or coumarin.9Surprisingly, very few NHC-catalyzed reactions that comprise either 2-aroylvinylcinnamaldehydes or 2-aroylvinylchalcones as a reactant have been reported.10 For instance, catalyzed by N-heterocyclic carbenes, 2-aroylvinylcinnamaldehydes underwent a cascade intramolecular Michael addition and lactonization to form indeno[2,1-c]pyran-1-ones which were converted into indane-2-carboxylates or indane-2-carboxamides by the addition of alcohols or amines, respectively.10a,b The oxidative NHC-catalyzed cascade reaction of 2-aroylvinylcinnamaldehydes with β-diketones produced 9-(β-diketone)indeno[2,1-c]pyran-1-ones.10c On the other hand, NHC-catalyzed reactions between 2-aroylvinylchalcones and aldehydes or α,β-unsaturated aldehydes followed different pathways to afford trisubstituted indane derivatives10e or tetrahydrobenzo[f]isochromen-4-ones,10d respectively. For years, we have been studying NHC-catalyzed reactions.4,11 As a continuation of our study, we became interested in the reaction of 2-aroylvinylcinnamaldehydes with 2-aroylvinylchalcones. We envisioned that various possible reaction pathways between these two highly functionalized reactants would lead to complex multifunctional organic compounds. Herein, we report an NHC-catalyzed cascade reaction which assembled six contiguous stereocenters and three rings in one cascade process to produce good yields of functionalized 9-indanylindeno[2,1-c]pyran-1-ones in a highly diastereoselective manner.
Results and discussion
We commenced our study by investigating the reaction between 2-benzoylvinylcinnamaldehyde 1a and 2-benzoylvinylchalcone 2a. Initially, the reaction was catalyzed with 20 mol% of different triazole carbenes 3a′–3d′ which were generated from triazolium salts 3a–3d with DBU in THF at ambient temperature. In all cases, a pair of diastereoisomeric products, 9-(2-benzoyl-3-benzoylmethyl-1-indanyl)-3-phenylindeno[2,1-c]pyran-1-ones 4a and 5a were obtained. The highest yield of the major product 4a (77%) along with 18% yield of minor isomer 5a were obtained when 1,4-dibenzyltriazole carbene catalyst 3c′ was employed (Table 1, entries 1–4). However, under the same catalytic conditions, imidazole, imidazoline and thiazole carbenes 3e′–3h′ were found totally inefficient in promoting the reaction (Table 1, entries 5–8). To improve the chemical yield and diastereoselectivity, the reaction was further optimized by varying catalyst loading, temperature, solvent and the base that was used to generate a carbene catalyst. Under catalysis by 1,4-dibenzyltriazole carbene 3c′ in THF, either halving the catalyst loading to 10 mol%, or the changing the reaction temperature to 0 °C or around 60 °C, caused a reduction in the product yield (Table 1, entries 9–11). The use of other solvents including dichloromethane, toluene, acetonitrile and acetone, and the employment of other bases such as t-BuOK, (i-Pr)2NEt and Cs2CO3, gave decreased yields of products (Table 1, entries 12–18). Gratifyingly, the reaction that used NaH to generate carbene 3c′ significantly improved the diastereoselectivity, which afforded respectively products 4a and 5a in 86% and 4% yield (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1, entry 19).
1) (Table 1, entry 19).
| Entry | NHC precursor 3 | mol% of 3 | Baseb | Solvent | Temp. (°C) | Time (h) | Yieldc (%) | |
|---|---|---|---|---|---|---|---|---|
| 4a | 5a | |||||||
| a Reaction conditions: 1a (1 mmol), 2a (0.5 mmol), 4 Å MS 250 mg. b 20 mol% of DBU, t-BuOK, (i-Pr)2NEt or Cs2CO3, and 50 mol% NaH were used in the reaction. c Isolated yield. | ||||||||
| 1 | 3a | 20 | DBU | THF | rt | 6 | 58 | 25 |
| 2 | 3b | 20 | DBU | THF | rt | 16 | 22 | 12 |
| 3 | 3c | 20 | DBU | THF | rt | 2 | 77 | 18 |
| 4 | 3d | 20 | DBU | THF | rt | 16 | 19 | 11 |
| 5 | 3e | 20 | DBU | THF | rt | 12 | — | — |
| 6 | 3f | 20 | DBU | THF | rt | 12 | — | — |
| 7 | 3g | 20 | DBU | THF | rt | 12 | — | — |
| 8 | 3h | 20 | DBU | THF | rt | 12 | — | — |
| 9 | 3c | 10 | DBU | THF | rt | 10 | 57 | 14 |
| 10 | 3c | 20 | DBU | THF | 0 | 10 | 41 | 14 |
| 11 | 3c | 20 | DBU | THF | Reflux | 2 | 55 | 18 |
| 12 | 3c | 20 | DBU | DCM | rt | 10 | 9 | 21 |
| 13 | 3c | 20 | DBU | PhMe | rt | 10 | 52 | 15 |
| 14 | 3c | 20 | DBU | CH3CN | rt | 10 | 9 | 17 |
| 15 | 3c | 20 | DBU | CH3COCH3 | rt | 10 | 17 | 23 |
| 16 | 3c | 20 | t-BuOK | THF | rt | 6 | 51 | 5 |
| 17 | 3c | 20 | (i-Pr)2NEt | THF | rt | 16 | 25 | — |
| 18 | 3c | 20 | Cs2CO3 | THF | rt | 16 | 53 | 5 |
| 19 | 3c | 20 | NaH | THF | rt | 2 | 86 | 4 |
The generality of reaction was then investigated under the optimized conditions (Table 2). It was found that the reaction showed tolerance to the substituents on both 2-aroylvinylcinnamaldehydes 1 and 2-aroylvinylchalcones 2. To react with enone 2a, the aroyl group within enals 1 exhibits a smaller influence on the reaction than the substituent attached to the benzene ring of 1. For example, all cinnamaldehydes 1a–1f containing an o-methoxy-, m-methoxy-, p-methoxy-, p-methyl- or p-bromobenzoyl group reacted efficiently with 2-benzoylvinylchalcone 2a to give products 4a–4f in 63–86% yields (Table 2, entries 1–6), while the reactions of 4-methoxy-, 4-methyl-, and 4-fluoro-substituted 2-benzoylvinylcinnamaldehydes 1g–1i with 2-benzoylvinylchalcone 2a produced 4g–4i in slightly lower yields (52%–60%) (Table 2, entries 7–9). On the other hand, the aroyl substituents attached to bis(enone)s 2 show a larger influence on the reaction than the substituent on the benzene ring of 2. As indicated in Table 2, while p-methyl- and o-, m-, p-bromobenzoyl substituted bis(enone)s 2c–2f reacted efficiently with 2-benzoylvinylcinnamaldehyde 1a to afford products 4k–4n in 78–85% yields, the p-methoxybenzoyl substituted bis(enone) 2b gave a lower yield of 4j (54%) (Table 2, entries 10–14). When the 2-aroylvinylchalcones 2g–2i that were substituted with either an electron-donating methoxy or methyl group or an electron-withdrawing bromine atom on the benzene ring were used, their reaction with enal 1a proceeded smoothly to afford products 4o–4p in 69–86% yields. In addition to the major products 4, a trace amount of diastereoisomers 5 was detected in the crude products by 1H NMR (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ≈ 10
5 ≈ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–28
1–28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Besides the products 4 and 5, a small amount of by-products derived from the self-reaction of enals 1 was also detected in the reaction mixture by 1H NMR.
1). Besides the products 4 and 5, a small amount of by-products derived from the self-reaction of enals 1 was also detected in the reaction mixture by 1H NMR.
| Entry | 1 | Ar1 | X | 2 | Ar2 | Y | Time (h) | Yield of 4a (%) | Yield of 5 (%) | drc |
|---|---|---|---|---|---|---|---|---|---|---|
| a The minor isomers 5a, 5k and 5l were isolated and characterized. b Other minor products 5 were not isolated and their yields were determined by 1H NMR on the crude products. c Diastereomeric ratios were detected by 1H NMR on the crude products. | ||||||||||
| 1 | 1a | Ph | H | 2a | Ph | H | 2 | 4a: 86 | 5a: 4a | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 1b | o-MeOPh | H | 2a | Ph | H | 2 | 4b: 82 | 5b: 3b | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 1c | m-MeOPh | H | 2a | Ph | H | 2 | 4c: 86 | 5c: 8b | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 1d | p-MeOPh | H | 2a | Ph | H | 2 | 4d: 85 | 5d: 3b | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 1e | p-MePh | H | 2a | Ph | H | 2 | 4e: 80 | 5e: 8b | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 1f | p-BrPh | H | 2a | Ph | H | 3 | 4f: 63 | 5f: 4b | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 1g | Ph | MeO | 2a | Ph | H | 2 | 4g: 60 | 5g: 2b | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 1h | Ph | Me | 2a | Ph | H | 2 | 4h: 60 | 5h: 3b | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 1i | Ph | F | 2a | Ph | H | 2 | 4i: 52 | 5i: 3b | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 1a | Ph | H | 2b | p-MeOPh | H | 4 | 4j: 54 | 4j: 5b | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 1a | Ph | H | 2c | p-MePh | H | 4 | 4k: 78 | 5k: 4a | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 1a | Ph | H | 2d | p-BrPh | H | 2 | 4l: 85 | 5l: 4a | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 1a | Ph | H | 2e | m-BrPh | H | 2 | 4m: 78 | 5m: 3b | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 1a | Ph | H | 2f | o-BrPh | H | 2 | 4n: 80 | 5n: 7b | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 1a | Ph | H | 2g | Ph | MeO | 6 | 4o: 69 | 5o: 3b | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | 1a | Ph | H | 2h | Ph | Me | 4 | 4p: 81 | 5p: 7b | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17 | 1a | Ph | H | 2i | Ph | Br | 2 | 4q: 86 | 5q: 7b | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The structures of products were established on the basis of spectroscopic data, and the final unambiguous confirmation of the structures and their stereochemistry was obtained from the single crystal X-ray diffraction analysis of compounds 4l and 5l.12
A cascade reaction mechanism was proposed to account for the formation of 9-(2-aroyl-3-aroylmethyl-1-indanyl)indeno[2,1-c]pyran-1-ones from 2-aroylvinylcinnamaldehydes 1 and bis(enone)s 2. As illustrated in Scheme 1, the homoenolates 6 derived from the addition of carbene 3′ to enals 1 undergo an intermolecular Michael addition to enones 2 to form intermediates 7. An intramolecular Michael addition of 7 results in the formation of indane intermediates 8. The second intramolecular Michael addition provides the bis-indane derivatives 10. Intramolecular lactonization of 10 affords the final 9-(2-aroyl-3-aroylmethyl-1-indanyl)indeno[2,1-c]pyran-1-one products 4 and 5 (Scheme 1). It is interesting to note that six stereogenic carbon atoms are formed from the reaction cascade. In theory, products 4 would have a total of 32 diastereoisomers (26/2 = 32). Significantly, this NHC-catalyzed reaction produced only two diastereoisomers 4 and 5 with an excellent diastereoselectivity (dr ≈ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–28
1–28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The highly diastereoselective formation of major isomers 4 can be best explained by the steric effect of the substituents. It is the two stereocenters installed in the intermolecular Michael addition step of homoenolates 6 to enones 2 that effectively guide the stereochemistry of the other four stereocenters in the subsequent formation of the two indane rings. During the first intramolecular Michael addition of intermediates 7, all three substituents attached to the indane rings of 8 would be preferentially oriented in the trans-configuration to reduce the repulsion among the substituents. In the second intramolecular Michael addition of 9 followed by an intramolecular lactonization, while the two newly formed stereocenters of the second indane ring have been installed in the trans-configuration with the original stereocenter to reduce steric hindrance, the two fused stereocenters have been set in the cis-configuration to avoid fused-ring strain.
1). The highly diastereoselective formation of major isomers 4 can be best explained by the steric effect of the substituents. It is the two stereocenters installed in the intermolecular Michael addition step of homoenolates 6 to enones 2 that effectively guide the stereochemistry of the other four stereocenters in the subsequent formation of the two indane rings. During the first intramolecular Michael addition of intermediates 7, all three substituents attached to the indane rings of 8 would be preferentially oriented in the trans-configuration to reduce the repulsion among the substituents. In the second intramolecular Michael addition of 9 followed by an intramolecular lactonization, while the two newly formed stereocenters of the second indane ring have been installed in the trans-configuration with the original stereocenter to reduce steric hindrance, the two fused stereocenters have been set in the cis-configuration to avoid fused-ring strain.
 | ||
| Scheme 1 The proposed mechanism for the formation of 9-indanylindeno[2,1-c]pyran-1-ones from 2-aroylvinylcinnamaldehydes 1 and bis(enone)s 2. | ||
It is worth noting that indane including bis-indane is a framework that is found in a large number of bioactive and pharmaceutically important molecules.13 Moreover, the resulting novel and highly functionalized indeno[2,1-c]pyran-1-ones 4 would be useful in the synthesis of various indane derivatives through simple chemical transformations of indeno[2,1-c]pyran-1-one moiety10a,b and aroyl substituents.
Enantioselective synthesis of 9-(2-aroyl-3-aroylmethyl-1-indanyl)indeno[2,1-c]pyran-1-ones 4 was also attempted using chiral triazole carbenes. Unfortunately, the reaction of 2-benzoylvinylcinnamaldehyde 1a with 2-benzoylvinylchalcone 2a catalyzed by chiral triazole carbenes gave very low yields of product 4a, along with the intramolecular reaction product of enal 1a.10b The reason was most probably that the homoenolate intermediates 6 derived from the bulky chiral catalysts and enal 1 have huge steric hindrance which prohibits further nucleophilic attack on the 2-benzoylvinylchalcone 2a which also has large intramolecular steric hindrance. Thus, the asymmetric catalysis of the reaction between enals 1 and bis(enone)s 2 was not further studied.
Conclusion
In summary, we have developed an efficient NHC-catalyzed cascade reaction of 2-aroylvinylcinnamaldehydes with 2-aroylvinylchalcones to produce good yields of novel 9-indanylindeno[2,1-c]pyran-1-one derivatives with six contiguous stereocenters in a highly diastereoselective manner. The unique multifunctional 9-indanylindeno[2,1-c]pyran-1-ones are potentially amenable to further transformations. This work opens up new opportunities for the synthesis of complex indane derivatives with potential biological and pharmaceutical applications.Experimental section
Commercially available chemical reagents were used without further purification. Anhydrous THF was prepared by distillation over Na. Melting points are uncorrected. 1H NMR (400 or 500 MHz) and 13C NMR (100 or 125 MHz) spectra were recorded in the indicated solvents using a Bruker instrument. J values are reported in Hz. IR spectra were recorded using an AVATAR 360 FT-IR spectrometer. Mass spectra were recorded on a Surveyor MSQ Plus (ESI) instrument. Column chromatography was performed using 200–300 mesh silica gel. The 2-aroylvinylcinnamaldehydes 1,14 2-aroylvinylchalcones 210e and NHC precursor 3c15 were prepared according to reported methods.General procedure for the NHC-catalyzed reaction of 2-aroylvinylcinnamaldehydes 1 with 2-aroylvinylchalcones 2
Under nitrogen atmosphere, a mixture of 2-aroylvinylcinnamaldehydes 1 (0.5 mmol),14 2-aroylvinylchalcones 2 (1 mmol),10e 1,4-dibenzyl-1,2,4-triazolium salt 3c15 (0.1 mmol) and 4 Å molecular sieves (250 mg) in dry THF (10 mL) was stirred for 10 min at room temperature, and then NaH (0.25 mmol) was added. The reaction mixture was stirred at room temperature until the starting materials were consumed. The solvent was removed under vacuum and the residue was chromatographed on a silica gel column eluting with a mixture of petroleum ether and ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give a mixture of isomers 4 and 5. The major products 4 were isolated from the crude products by recrystallization or by chromatography on a silica gel column eluting with a mixture of petroleum ether and ethyl acetate (10
1) to give a mixture of isomers 4 and 5. The major products 4 were isolated from the crude products by recrystallization or by chromatography on a silica gel column eluting with a mixture of petroleum ether and ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21172021) and the Beijing Municipal Commission of Education.Notes and references
- (a) L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Acc. Chem. Res., 2012, 45, 1278–1293 CrossRef CAS PubMed; (b) H. Ohno, Asian J. Org. Chem., 2013, 2, 18–28 CrossRef CAS; (c) D. Enders, C. Grondal and M. R. M. Hüttl, Angew. Chem., Int. Ed., 2007, 46, 1570–1581 CrossRef CAS PubMed; (d) X. Yu and W. Wang, Org. Biomol. Chem., 2008, 6, 2037–2046 RSC; (e) B. Westermann, M. Ayaz and S. S. van Berkel, Angew. Chem., Int. Ed., 2010, 49, 846–849 CrossRef CAS PubMed; (f) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237–294 CrossRef CAS.
- (a) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167–178 CrossRef CAS PubMed; (b) K. C. Nicolaou and J. S. Chen, Chem. Soc. Rev., 2009, 38, 2993–3009 RSC; (c) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134–7186 CrossRef CAS PubMed.
- A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314–325 CrossRef CAS PubMed.
- Y. Cheng, J.-H. Peng, Y.-J. Li, X.-Y. Shi, M.-S. Tang and T.-Y. Tan, J. Org. Chem., 2011, 76, 1844–1851 CrossRef CAS PubMed.
- E. Sánchez-Larios, J. M. Holmes, C. L. Daschner and M. Gravel, Org. Lett., 2010, 12, 5772–5775 CrossRef PubMed.
- (a) F.-G. Sun and S. Ye, Org. Biomol. Chem., 2011, 9, 3632–3635 RSC; (b) K.-J. Wu, G.-Q. Li, Y. Li, L.-X. Dai and S.-L. You, Chem. Commun., 2011, 493–495 RSC.
- S. J. Ryan, L. Candish and D. W. Lupton, J. Am. Chem. Soc., 2011, 133, 4694–4697 CrossRef CAS PubMed.
- (a) A. T. Biju, N. E. Wurz and F. Glorius, J. Am. Chem. Soc., 2010, 132, 5970–5971 CrossRef CAS PubMed; (b) M. Padmanaban, A. T. Biju and F. Glorius, Org. Lett., 2011, 13, 5624–5627 CrossRef CAS PubMed.
- (a) L. Li, D. Du, J. Ren and Z. Wang, Eur. J. Org. Chem., 2011, 614–618 CrossRef CAS; (b) D. Du and Z. Wang, Eur. J. Org. Chem., 2008, 4949–4954 CrossRef CAS.
- (a) Y. Li, X.-Q. Wang, C. Zheng and S.-L. You, Chem. Commun., 2009, 5823–5825 RSC; (b) E. M. Phillips, M. Wadamoto, A. Chan and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 3107–3110 CrossRef CAS PubMed; (c) A. Biswas, S. De Sarkar, R. Frohlich and A. Studer, Org. Lett., 2011, 13, 4966–4969 CrossRef CAS PubMed; (d) X. Fang, K. Jiang, C. Xing, L. Hao and Y. R. Chi, Angew. Chem., Int. Ed., 2011, 50, 1910–1913 CrossRef CAS PubMed; (e) E. Sanchez-Larios and M. Gravel, J. Org. Chem., 2009, 74, 7536–7539 CrossRef CAS PubMed; (f) E. Sanchez-Larios, J. M. Holmes, C. L. Daschner and M. Gravel, Synthesis, 2011, 1896–1904 CAS.
- (a) X.-W. Fan and Y. Cheng, Org. Biomol. Chem., 2012, 10, 9079–9084 RSC; (b) Z.-X. Sun and Y. Cheng, Eur. J. Org. Chem., 2012, 4982–4987 CrossRef CAS; (c) Z.-X. Sun and Y. Cheng, Org. Biomol. Chem., 2012, 10, 4088–4094 RSC; (d) J. Qu and Y. Cheng, Tetrahedron, 2013, 69, 888–894 CrossRef CAS PubMed.
- CCDC 939299 and 939300† [4l and 5l] contain the supplementary crystallographic data for this paper.
- Some examples: (a) H. Sheridan and N. Frankish, Int. Pat, WO 2013014660, 2013 Search PubMed; (b) A. Akincioglu, Y. Akbaba, H. Gocer, S. Goksu, I. Gulcin and C. T. Supuran, Bioorg. Med. Chem., 2013, 21, 1379–1385 CrossRef CAS PubMed; (c) R. K. Pal, H. Yasmin, L. Nahar, B. K. Datta, A. K. A. Chowdhury, J. K. Kundu, S. C. Bachar and S. D. Sarker, Med. Chem., 2012, 8, 874–882 CrossRef CAS; (d) M. Seki, O. Tsuruta, Y. Aoyama, A. Soejima, H. Shimada and H. Nonaka, Chem. Pharm. Bull., 2012, 60, 488–498 CrossRef CAS; (e) H. Shiohara, T. Nakamura, N. Kikuchi, T. Ozawa, R. Nagano, A. Matsuzawa, H. Ohnota, T. Miyamoto, K. Ichikawa and K. Hashizume, Bioorg. Med. Chem., 2012, 20, 3622–3634 CrossRef CAS PubMed.
- J. W. Yang, M. T. H. Fonseca and B. List, J. Am. Chem. Soc., 2005, 127, 15036–15037 CrossRef CAS PubMed.
- J. Z. Vlahakis, C. Lazar, I. E. Crandall and W. A. Szarek, Bioorg. Med. Chem., 2010, 18, 6184–6196 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H NMR and 13C NMR spectra of compounds 4a–4q and 5a, 5k, 5l are available. CCDC 939299 and 939300. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ob41656a |
| This journal is © The Royal Society of Chemistry 2014 |