Nanodevice-induced conformational and functional changes in a prototypical calcium sensor protein†
Valerio
Marino
a,
Alessandra
Astegno
b,
Marco
Pedroni
bc,
Fabio
Piccinelli
bc and
Daniele
Dell'Orco
*ad
aDepartment of Life Sciences and Reproduction, Section of Biological Chemistry, University of Verona, Verona, Italy. E-mail: daniele.dellorco@univr.it; Fax: +39-045-802-7170; Tel: +39-045-802-7637
bDepartment of Biotechnology, University of Verona, Verona, Italy
cSolid State Chemistry Laboratory, University of Verona, Verona, Italy
dCenter for BioMedical Computing, University of Verona, Verona, Italy
First published on 17th October 2013
Abstract
Calcium (Ca2+) plays a major role in a variety of cellular processes. Fine changes in its concentration are detected by calcium sensor proteins, which adopt specific conformations to regulate their molecular targets. Here, two distinct nanodevices were probed as biocompatible carriers of Ca2+-sensors and the structural and functional effects of protein–nanodevice interactions were investigated. The prototypical Ca2+-sensor recoverin (Rec) was incubated with 20–25 nm CaF2 nanoparticles (NPs) and 70–80 nm liposomes with lipid composition similar to that found in photoreceptor cells. Circular dichroism and fluorescence spectroscopy were used to characterize changes in the protein secondary and tertiary structure and in thermal stability upon interaction with the nanodevice, both in the presence and in the absence of free Ca2+. Variations in the hydrodynamic diameter of the complex were measured by dynamic light scattering and the residual capability of the protein to act as a Ca2+-sensor in the presence of NPs was estimated spectroscopically. The conformation, thermal stability and Ca2+-sensing capability of Rec were all significantly affected by the presence of NPs, while liposomes did not significantly perturb Rec conformation and function, allowing reversible binding. NP-bound Rec maintained an all-helical fold but showed lower thermal stability and high cooperativity of unfolding. Our analysis can be proficiently used to validate the biocompatibility of other nanodevices intended for biomedical applications involving Ca2+-sensors.
Introduction
Calcium (Ca2+) triggers and regulates a broad variety of cellular and physiological processes and acts as an important second messenger in many biochemical pathways.1 The detection of Ca2+ is performed by sensor proteins, which adopt specific conformations in response to fine changes in intracellular [Ca2+].2 Among Ca2+-sensor proteins, neuronal calcium sensors (NCS) are mostly localized in the neuronal tissue, although their expression has been detected also in other tissues.3,4 A prototypical NCS is recoverin (Rec), a sensor belonging to the EF-hand superfamily, which is involved in the regulation of the biochemical events occurring in photoreceptor cells upon light exposure. The post-translational myristoylation at the N-terminus allows Rec to act as a regulator of the signalling cascade as [Ca2+] drops during the phototransduction process.5,6 Specifically, the binding of Ca2+ triggers the protein structural transition from a “tense” (T), compact conformation in which the myristoyl group is sequestered in a hydrophobic pocket to a “relaxed” (R), more extended conformation, in which the myristoyl moiety is solvent-exposed (Fig. 1). Exposure of the myristic group is accompanied by an overall augmented hydrophobicity of the protein surface and provides an anchor that binds Rec to the photoreceptor disc membranes, thus permitting the regulation of the rhodopsin kinase activity.5,7 This switch mechanism constitutes a functional hallmark for myristoylated Rec (mRec) and the physico-chemical details have long been investigated.7,8 Interestingly, mRec binds two Ca2+ ions with moderate affinity (apparent KD ∼ 17–18 μM),9,10 while the non-myristoylated variant (nmRec) has two binding sites with significantly higher affinity (KD1 = 0.11 μM, KD2 = 6.9 μM).9 | ||
| Fig. 1 Three-dimensional structure of myristoylated recoverin in its Ca2+-free (left) and Ca2+-bound (right) forms, corresponding to the T and R states, respectively. The backbone is represented by gray cartoon tubes, the myristic moiety by green spheres, and the two Ca2+ ions by red spheres. The three Trp residues are represented by purple sticks and labeled, while the molecular surface is shown in transparency. Solvent-accessible surface areas (ASA) for each Trp in the absence and in the presence of Ca2+ are: W31 (49.4 Å2 to 58.6 Å2); W104 (0 Å2 to 12.6 Å2); W156 (2.6 Å2 to 33.3 Å2); structural data are from NMR average structures (left: PDB entry 1IKU; right: PDB entry 1JSA). | ||
It is well established that distortion in the cell Ca2+-homeostasis may lead to severe diseases. In particular, hereditary cone dystrophies represent a severe class of disorders that correlate with mutations in genes coding for NCS proteins, which can eventually lead to impaired vision and blindness.11–15 In order to rescue the physiological levels of second messengers including Ca2+, a novel approach could be the direct delivery of well-defined and precisely structured proteins into the cell to replace the dysfunctional protein, hence avoiding some of the potential problems of gene therapy. Ideally, the means of delivery should minimally perturb the proteins to be carried in order to keep them fully functional. For the sake of protein delivery, nanodevices (NDs) may offer unique opportunities in that the high surface to volume ratio would ensure high amounts of protein carried by devices of relatively small volumes. It is however fundamental to assess the effects that the ND exerts on the protein conformation and function upon interaction. Indeed, very recent lines of evidence suggest that nanoparticles (NPs) may lead to significant structural changes and even unfolding of proteins bound to the particle surface.16–19
Among the most promising nanoscale devices for nanomedicine purposes, lanthanide-doped upconverting NPs have raised considerable attention in the last few years because of their capacity to be multiphoton-excited with near infra-red (NIR) light and generate emission at higher energies spanning the UV to the NIR, a feature that can be exploited in a variety of bioimaging and biomedical goals.20–26 Particularly promising are CaF2 NPs,27 which can be synthesized by a single-step hydrothermal method and coated by citrate, thus ensuring appropriate dispersion in water environments.28 In particular, CaF2 NPs doped with luminescent lanthanide ions can be used as multifunctional nanoprobes for highly penetrating fluorescence, which can be exploited together with the fluorescence thermal sensitivity property.29 Moreover, they showed no toxicity in tests performed with HeLa cells, thus setting the basis for biocompatible carrier systems that can conveniently exploit the upconversion phenomenon.29
In this work, we explored the possibility to use CaF2 NPs and lipid nanovesicles as convenient carriers of Ca2+ sensors for biomedical purposes. We investigated the effects that the interaction with the ND exerted on the structural and functional properties of both mRec and nmRec and evaluated the residual capability of the protein to act as a Ca2+-sensor in the presence of the ND. Our results show that CaF2 NPs significantly alter the structural and functional properties of both mRec and nmRec, which significantly decrease their sensitivity to free Ca2+. The interaction between the protein and the particles is not mediated by the citrate coating NPs, thus suggesting a direct electrostatic interaction that alters the size of the protein as well as its secondary and tertiary structures. NP-bound mRec and nmRec showed a significantly lower thermal stability and the unfolding profile was found to be highly cooperative, independent of the presence of free Ca2+. Liposomes on the other hand did not significantly perturb protein secondary and tertiary structures, and the capability to work as a Ca2+-sensor in their presence was preserved.
The partial reversibility of binding to CaF2 NPs observed for mRec and the specific conformational features observed suggest that the interaction with the particle surface induces a structure that is significantly different from the respective equivalent in solution. While Ca2+-sensing is greatly altered, the novel conformation is completely folded and perhaps associated with novel functions to be explored in future studies.
Results and discussion
Ca2+-dependent structural changes in myristoylated and non-myristoylated Rec
Binding of two Ca2+ ions to mRec showed distinct spectroscopic features, which represent the hallmarks of the myristoyl-switch mechanism. Far UV-CD spectra showed the typical pattern of an all-α protein, with two minima at 208 and 222 nm (Fig. 2A). In line with previous findings,30 we observed that the binding of Ca2+ leads to a significant decrease of the minimum at 222 nm with substantially unaltered intensity at 208 nm, thus causing the θ222/θ208 ratio to switch from 0.77 in the apo-form to 0.87 in the Ca2+-bound form (Table 1). CD spectroscopy in the near UV region allows monitoring of the asymmetric environment of the aromatic residues, which was observed to significantly change upon Ca2+-binding in the case of mRec (Fig. 2B). | ||
| Fig. 2 Structural changes occurring in mRec and nmRec upon binding of Ca2+ and upon interaction with CaF2 NPs. All measurements were performed at T = 25 °C in 5 mM Tris–HCl pH 7.5, 150 mM KCl buffer. (a) Far-UV CD spectra of 6.3 μM mRec in the presence of equal amounts (46 μM) of saturating EGTA or Ca2+, both isolated and incubated with 1 mg mL−1 CaF2 NPs. (b) Near-UV CD spectra of 31.4 μM mRec in the presence of equal amounts (230 μM) of saturating EGTA or Ca2+, both isolated and incubated with 5 mg mL−1 CaF2 NPs. (c) Intrinsic Trp fluorescence emission spectrum (λexc = 290 nm) of 0.78 μM mRec in the presence of equal amounts (5.8 μM) of saturating EGTA or Ca2+, both isolated and incubated with 125 μg mL−1 CaF2 NPs. (d) Far-UV CD spectra of 5.8 μM nmRec in the presence of equal amounts (46 μM) of saturating EGTA or Ca2+, both isolated and incubated with 1 mg mL−1 CaF2 NPs. (e) Near-UV CD spectra of 29.3 μM nmRec in the presence of equal amounts (230 μM) of saturating EGTA or Ca2+, both isolated and incubated with 5 mg mL−1 CaF2 NPs. (f) Intrinsic Trp fluorescence emission spectrum (λexc = 290 nm) of 0.73 μM nmRec in the presence of equal amounts (5.8 μM) of saturating EGTA or Ca2+, both isolated and incubated with 125 μg mL−1 CaF2 NPs. The θ222/θ208 ratio for all forms and the wavelengths of maximal emission are reported in Table 1. | ||
| +EGTA | +Ca2+ | |||||||
|---|---|---|---|---|---|---|---|---|
| θ 222/θ208a (n) | T m (°C) | H c | λ max flud (nm) | θ 222/θ208 (n) | T m (°C) | H c | λ max flu (nm) | |
| a Ratio between ellipticity at 222 and 208 nm; n stands for number of replicates in each experiment. b Melting temperature obtained by fitting the experimental curve to a 4 parameter Hill sigmoid (see eqn (1) in Methods). c Hill coefficient. d Wavelength at which fluorescence emission is maximal upon excitation at λ = 290 nm. | ||||||||
| mRec | 0.77 (5) | 73.1 | 15.0 | 329 | 0.87 (5) | 78.8 | 10.7 | 336 |
| nmRec | 0.83 (5) | 73.8 | 7.8 | 338 | 0.88 (5) | 79.4 | 11.5 | 339 |
| NP + mRec | 0.91 (5) | 66.4 | 21.9 | 336 | 0.91 (5) | 65.4 | 23.9 | 336 |
| NP + nmRec | 0.90 (5) | 68.6 | 19.4 | 339 | 0.92 (5) | 68.1 | 19.2 | 339 |
| LP + mRec | 0.77 (5) | — | — | 330 | 0.92 (5) | — | — | 338 |
| LP + nmRec | 0.84 (5) | — | — | 339 | 0.92 (5) | — | — | 340 |
As observed in previous studies,31 metal binding caused the ellipticity to drastically decrease mostly in the tyrosine (Tyr) and tryptophan (Trp; W) bands, while only a minor change was observed in the phenylalanine (Phe) region. The fluorescence spectrum (Fig. 2C) arising mostly from the intrinsic fluorescence of three Trp residues (W31, W104 and W156) showed a 7 nm red-shift (329 nm to 336 nm) of the maximum wavelength (Table 1) and a moderate quenching of the signal, as already found in previous studies.32 This is in line with the increased solvent-exposure of hydrophobic residues upon occurrence of the myristoyl switch during the Ca2+-binding process, in particular structural data confirm that all three Trp residues (W31, W104 and W156) become significantly exposed (Fig. 1). The significant variations observed in mRec conformation upon saturation with Ca2+ were confirmed also by dynamic light scattering (DLS) measurements (Fig. S1A;† see also Table 2 and Fig. 4D and E), which revealed a ∼0.3 nm increase in the hydrodynamic diameter. The overall increase in mRec hydrodynamic size was postulated earlier7 and appears to be in line with the radius of gyration and solvent-accessible surface area as determined by comparing the average NMR structures of the apo8 and Ca2+-bound5 forms. Overall, our spectroscopic data are consistent with a significant conformational change in mRec secondary and tertiary structures upon Ca2+-binding, compatible with the myristoyl switch mechanism.
| + EGTA | + Ca2+ | |||
|---|---|---|---|---|
| d ± σ (nm) (n) | pdI | d ± σ (nm) (n) | pdI | |
| a Liposomes used in experiments with mRec. b Liposomes used in experiments with nmRec. | ||||
| mRec | 5.22 ± 0.02 (20) | 0.43 | 5.52 ± 0.02 (19) | 0.37 |
| nmRec | 5.57 ± 0.06 (12) | 0.51 | 5.3 ± 0.08 (13) | 0.49 |
| CaF2 NP | 25.4 ± 0.2 (15) | 0.08 | 27.61 ± 0.17 (15) | 0.14 |
| LPa (mRec) | 71.6 ± 0.7 (6) | 0.14 | 72.3 ± 0.7 (8) | 0.12 |
| LPb (nmRec) | 78.6 ± 0.5 (10) | 0.08 | 78.0 ± 0.5 (10) | 0.08 |
| NP + mRec | 28.0 ± 0.2 (19) | 0.13 | 29.29 ± 0.17 (18) | 0.13 |
| NP + nmRec | 28.9 ± 0.2 (21) | 0.17 | 32.7 ± 0.3 (20) | 0.16 |
| LP + mRec | 74.2 ± 2.5 (7) | 0.21 | 77.4 ± 0.6 (8) | 0.11 |
| LP + nmRec | 78.9 ± 0.8 (8) | 0.08 | 79.1 ± 0.6 (8) | 0.08 |
The non-myristoylated form of the same protein (nmRec) showed different spectroscopical features (Fig. 2). While the overall fold is clearly all-α, in this case both minima detected by far UV-CD spectroscopy (Fig. 2D) at 208 and 222 nm became more negative upon Ca2+-binding, leading to a less pronounced variation in the θ222/θ208 ratio, which switched from 0.83 in the apo-form to 0.88 in the Ca2+-bound form (Table 1). The overall different spectral shape of the Ca2+-bound form compared to mRec was previously observed in other NCS that do not undergo a myristoyl switch,12,30 and can be attributed to an increased compactness of the protein in the presence of Ca2+.12 Along the same line, the variation in the tertiary structure was less prominent for nmRec (Fig. 2E) compared to mRec (Fig. 2B), and the changes mostly concerned the Trp and Tyr regions. Differences were detected also in the Trp-fluorescence spectrum (Fig. 2F and Table 1), which showed a modest (1 nm) red-shift and an increase of intensity in the presence of saturating Ca2+, substantially in line with previous findings.32 Interestingly, DLS measurements showed the co-presence of two populations, a monomeric form with hydrodynamic diameter decreasing upon Ca2+-binding (∼−0.27 nm, see Table 2 and Fig. S1C†) and a higher order oligomer likely comprising 5–7 units that behaved independent of Ca2+. While intensity peaks corresponding to higher order oligomers were detected both for mRec and nmRec in DLS experiments, their effective weight on the photon correlation signal is modest due to the 1/d6 scaling of scattered light intensity. Indeed, according to number and volume distributions of the same molecular species only monomeric forms were detected (data not shown). Thus, we may conclude that mRec and nmRec behave in an opposite way upon binding of Ca2+, the latter decreasing its hydrodynamic size at variance with the first one. An opposite behaviour of the two Rec variants was similarly found in a very recent study using surface plasmon resonance.33
Effects of CaF2 nanoparticles on Rec structure and stability
The same spectroscopic characterization performed with isolated proteins was done in the presence of CaF2 NPs. Working with a suitable NP concentration is necessary to distinguish between spectroscopic signals arising from the unbound proteins and those relative to NP-bound ones. According to a simple geometric model of Rec–NP interaction (see Methods and ESI†) we calculated the maximum number of bound Rec for a ∼30 nm diameter NP to be ∼244, thus after fixing the NP concentration we kept the corresponding protein concentration below this theoretical maximum in all the experiments, working under conditions at which the signal arising from the protein was clearly detectable.Interestingly, the structural features of mRec significantly changed in the presence of NPs (Fig. 2). The presence of saturating free Ca2+ led to lower dichroism in the far UV-CD region (Fig. 2A), at odds with the isolated protein and likely indicative of a loss of protein compactness upon interaction with the NP. The spectrum however showed a very similar shape for NP-bound mRec independent of the presence of free Ca2+, as quantitatively demonstrated by the same θ222/θ208 ratio (0.91 in both cases, Table 1). This value is similar to that observed for the Ca2+-bound form of both mRec and nmRec (∼0.88, Table 1). Thus, the secondary-structural features in the presence of NPs are incompatible with those typical of the myristoyl switch, although under every tested condition the protein clearly showed a characteristic all-α folding. Along the same line, near UV-CD spectra in the presence of CaF2 NPs showed a tertiary structure hallmark significantly different from that of the isolated protein. In detail, while the Trp region was very similar to that of the apo mRec (Fig. 2B), Tyr and Phe bands showed a remarkable difference in their fine structure and increased signal. The tertiary structure appeared to be less affected by the presence of saturating free Ca2+ compared to the isolated protein and overall, the presence of CaF2 NPs yielded more prominent differences in the spectrum of Ca2+-bound forms.
Since the CaF2 NPs used in this study were coated with citrate, we further tested whether the interaction was mediated by citrate rather than occurring through the CaF2 matrix. The presence of K–citrate even at high concentration did not prevent the occurrence of the myristoyl switch (Fig. S2A†), moreover the θ222/θ208 ratio and the near-UV spectra (Fig. S2B and Table S1†) were all very similar to those of mRec in the absence of citrate (see Fig. 2A and B). The citrate coating therefore did not perturb the interaction of Rec with the NP, and was probably fully replaced by the protein milieu.
Very minor differences were detected in the intrinsic Trp fluorescence spectra (Fig. 2C) in the presence and in the absence of Ca2+. A hardly detectable blue shift of the maximum wavelength was observed upon saturation with Ca2+ and comparison with the unbound protein highlighted a quenching of the signal and a microenvironment of Trp residues similar to that of the Ca2+-bound isolated protein (λmax = 336 nm, Table 1). DLS experiments showed that mRec binds NPs both in the presence and in the absence of free Ca2+, and the changes in the hydrodynamic diameter of the NP–protein complex were 2.6 nm and 1.7 nm respectively (see Table 2, Fig. S1B,†4D and E).
Similar to mRec, incubating nmRec with CaF2 NPs led to significant structural changes. The secondary structure of NP-bound nmRec was comparable to the unbound condition (Fig. 2D) although a lower dichroism signal is likely indicative of decreased compactness. Similar θ222/θ208 ratios were observed in the absence (0.90) and in the presence (0.92) of Ca2+ (Table 1), thus indicating a very similar secondary structure compared to the myristoylated form when bound to the NP surface. Also similar to mRec, ellipticity in the near-UV range substantially increased in the presence of NPs (Fig. 2E) showing minor differences in the Trp bands and small but significant differences in the Phe and Tyr regions. The intrinsic Trp fluorescence spectrum (Fig. 2F) was virtually identical in the presence or in the absence of saturating Ca2+ and beside a very minor increase in fluorescence intensity in the presence of saturating Ca2+, no shift in the maximum wavelength was observed (Table 1). DLS experiments showed that also nmRec binds NPs both in the presence and in the absence of free Ca2+, and changes in the hydrodynamic diameter of the NP–protein complex were more significant compared to the myristoylated variant (3.5 nm and 5.1 nm, respectively; see Table 2, Fig. S1D,†4D and E).
Overall, these findings suggest that the interaction with CaF2 NPs causes dramatic changes in the secondary and tertiary structures of both mRec and nmRec. In particular, our data clearly show that the myristoyl switch does not occur in the presence of CaF2 NPs. It is worth noting that the interaction occurs under all the tested conditions, as proved by: (a) the spectroscopic evidence, which ensures the presence of only minimal amounts of unbound protein, and (b) the size determination by DLS, which shows that the thickness of the protein coating on the NP varies between 1.7 nm and 5.1 nm (Table 2), depending on the protein and the presence or absence of free Ca2+.
Further information as to the structural features of NP-bound Rec was obtained by limited proteolysis experiments. Limited proteolysis is a useful tool for detecting protein flexibility,34 since the sensitivity to protease digestion differs significantly between folded regions and unfolded regions, such as flexible loops. Even for proteins with known structures, limited proteolysis can provide important information about folding intermediates.35 The presence or absence of a bound ligand can affect the susceptibility of a protein segment to proteases, thus resulting in either increased or decreased accessibility. Limited proteolysis has been applied to investigate both the structural effects induced by the binding of small ligands such as Ca2+ ions36 as well as changes in protein structure and dynamics upon binding to nanoparticles.16 The proteolysis patterns of mRec and nmRec in the absence and in the presence of NPs are shown in Fig. 3A.
 | ||
Fig. 3 Analysis of limited proteolysis patterns and thermal denaturation profiles of mRec and nmRec in the presence and in the absence of CaF2 NPs. (a) Left: limited proteolysis of mRec in the presence of 2 mM CaCl2 or 2 mM EGTA: undigested (lanes 1 and 5), digested by TPCK–trypsin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (lanes 2 and 6), undigested in the presence of NPs (mRec–NP 200 100 (lanes 2 and 6), undigested in the presence of NPs (mRec–NP 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (lanes 3 and 7), digested by TPCK–trypsin 1 1) (lanes 3 and 7), digested by TPCK–trypsin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in the presence of NPs (mRec–NP 200 100 in the presence of NPs (mRec–NP 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (lanes 4 and 8). Lane (M) is the protein ladder. Right: identical analysis for nmRec. (b) Thermal denaturation profiles of ∼6 μM mRec (left) and nmRec (right) in the presence of equal amounts (46 μM) of EGTA or Ca2+, both with or without incubation with 1 mg mL−1 CaF2 NPs. Thermal denaturation was followed by monitoring the ellipticity signal at 208 nm over the 20–96 °C temperature range. Quantitative data obtained by fitting the experimental curve to a sigmoidal function are reported in Table 1. 1) (lanes 4 and 8). Lane (M) is the protein ladder. Right: identical analysis for nmRec. (b) Thermal denaturation profiles of ∼6 μM mRec (left) and nmRec (right) in the presence of equal amounts (46 μM) of EGTA or Ca2+, both with or without incubation with 1 mg mL−1 CaF2 NPs. Thermal denaturation was followed by monitoring the ellipticity signal at 208 nm over the 20–96 °C temperature range. Quantitative data obtained by fitting the experimental curve to a sigmoidal function are reported in Table 1. | ||
Tryptic treatment with 2 mM Ca2+ (lanes 1 and 2) and 2 mM EGTA (lanes 5 and 6) led to different digestion profiles for mRec and nmRec; such a treatment was more effective on nmRec. In particular, in the presence of 2 mM EGTA (lanes 5 and 6) nmRec became very sensitive to trypsin digestion, leading to the accumulation of a ∼15 kDa fragment, while mRec proteolysis was appreciably slowed down and no lower molecular mass fragments were detected by SDS-PAGE. This could indicate that exposed sites in the non-myristoylated protein are more protected in the myristoylated one. In the presence of NPs the electrophoretic patterns of both nmRec and mRec were characterized by the prevalence of a band with a very high molecular weight, most likely representing protein–NP complexes, with only a faint band corresponding to the full length Rec. Under these conditions the digestion profiles of nmRec and mRec are identical both with Ca2+ (lanes 3 and 4) and EGTA (lanes 7 and 8), supporting our previous conclusion that CaF2 NPs inhibit the occurrence of the myristoyl-switch of mRec under the investigated conditions. Proteins are probably “spread” on the surface of the NP, thus exposing hydrophobic residues in a Ca2+-independent manner. Moreover, while the presence of NPs seems to be ineffective in mRec proteolysis, it apparently protects nmRec from proteases in the presence of EGTA. In particular, for nmRec mixed with particles, only one protein band was visible with no accumulation of the 15 kDa tryptic fragment (lanes 6 versus lanes 8), indicating that sites exposed in the free protein are protected in the NP-bound protein.
The biophysical analysis was completed by the comparison of the thermal denaturation profiles of isolated proteins and NP–protein complexes in the presence and in the absence of Ca2+ (Fig. 3B), obtained by heating the system up to 96 °C. Thermal denaturation profiles of mRec (Fig. 3B) presented a typical sigmoidal shape which was satisfactorily fitted by a Hill function (see Methods and ESI†). The melting temperature increased by about 5.7 °C upon binding of Ca2+, indicative of the typical stabilizing effect of the cation. This value is in partial disagreement with the results reported by Permyakov et al.31, who measured by thermal scanning microcalorimetry an increase of 15 °C. The different technique employed for scanning as well as the overall conditions might partially explain such discrepancy. The melting process showed higher cooperativity in the apo-form (Table 1), and far UV-CD spectra recorded after cooling down the denatured sample to 25 °C showed a minimal residual structure both in the presence and in the absence of Ca2+, however the typical secondary structure was lost (results not shown). Very interestingly, incubation with CaF2 NPs led to significantly different thermal denaturation profiles (Fig. 3B). Thermal stability turned out to be independent of the presence of free Ca2+ and about 7 °C lower compared to the isolated protein in the absence of Ca2+, respectively 13 °C lower in the presence of Ca2+ (Fig. 3B and Table 1). Moreover, the unfolding process occurred with remarkably higher cooperativity (Table 1). A similar result was observed for nmRec (Fig. 3B and Table 1). Here as well Ca2+ increased the thermal stability of the unbound protein, but the presence of CaF2 NPs led to highly cooperative unfolding profiles which were substantially identical to one another, independent of the presence of free Ca2+. The NP–protein complex was slightly more stable (ΔTm ∼ 2–3 °C) in the case of nmRec compared to mRec (Table 1). The fact that for both mRec and nmRec the Hill coefficient was significantly higher in the presence of NPs suggests that the structure of the NP-bound protein is less “conformationally dynamic” compared to the equivalent in solution, and the thermal denaturation process indeed spans a narrower range of conformational states resulting in a highly cooperative transition.
Effects of CaF2 nanoparticles on Rec Ca2+-sensing
We further investigated whether the binding of Rec to CaF2 NPs was reversible, that is, if the protein could be released from the particle surface in the presence of saturating free Ca2+ or in its complete absence. Fig. 4A–C show the results of our DLS experiments, in which injections of small volumes of saturating Ca2+ and/or EGTA were performed in pre-incubated mixtures of protein and ND. Interestingly, the binding of mRec to the NP was found to be only partially reversible (Fig. 4A): injection of saturating Ca2+ followed by injection of saturating EGTA led to a modest change in hydrodynamic diameter (∼0.5 nm), which is however lower compared to the change induced in the NP–mRec complex by the presence of Ca2+ (∼1.3 nm). Unlike mRec, nmRec resulted to be more tightly bound to the NP's surface (Fig. 4B): in fact, the same procedure did not lead to a decrease of the hydrodynamic diameter after the second injection, but it rather caused a further increase of the overall size (+0.6 nm). We point out that from our DLS data it is not possible to clearly distinguish between variations in the hydrodynamic diameter solely due to changes in conformation of the bound protein and changes due to a shifted equilibrium between bound and unbound proteins. The significantly different behaviour observed by DLS for mRec (Fig. 4A) and nmRec (Fig. 4B) in contrast with the similar spectroscopic features of the NP-bound proteins (Fig. 3) suggests that the binding mechanism is strictly protein-dependent, and the myristoyl moiety plays a significant role in allowing binding/release of the NCS from the NP's surface.While informative as to the general behaviour of NP–Rec complexes in the presence of saturating Ca2+ as well as in its complete absence, in an “on–off” fashion, DLS experiments did not thoroughly probe the residual capability of mRec/nmRec to act as NCSs in the presence of the NP. We therefore performed Ca2+ titrations in the nM to mM range and monitored the intrinsic Trp fluorescence of the protein in the presence and in the absence of CaF2 NPs (Fig. 5). Fluorescence emission at the wavelength corresponding to the maximum intensity at low Ca2+ (Fλmax) from mRec showed a typical sigmoidal trend (Fig. 5A), and reached its half-maximal value (KappD) at 40.9 μM [Ca2+]. This value is higher compared to the apparent affinity for Ca2+ of mRec (∼18 μM (ref. 9 and 10)), which is reasonable since rather than directly studying the binding process we monitored the effect of Ca2+-binding on the protein conformation, ultimately responsible for the fluorescence signal.
The same reasoning applies to nmRec (Fig. 5B), for which KappD = 22.1 μM, higher than the apparent affinity for Ca2+ (KD1 = 0.11 μM, KD2 = 6.9 μM).9 It is worth noting that, while for mRec Fλmax decreased with increasing [Ca2+], in line with the augmented hydrophobicity of the Ca2+-bound protein, the opposite trend was observed for nmRec, thus Fλmax increased with increasing [Ca2+]. This is in line with previous reports.7,8,33
Incubating mRec and nmRec with CaF2 NPs resulted in very different trends for Fλmax. Neither form of the protein was able to respond normally to Ca2+ and remained “blocked” up to high concentrations ([Ca2+] > 200 μM; Fig. 5A and B). After the prolonged plateau, Fλmax monotonically increased in both cases in a similar manner, although at high [Ca2+] the change in Fλmax was more apparent for mRec than for nmRec (Fig. 5A and B). Therefore, in the presence of NPs, the Ca2+-sensing capability of both mRec and nmRec was highly compromised, suggesting that both proteins were blocked in a specific conformation up to relatively high [Ca2+], after which proteins did partially respond to Ca2+. The increase in fluorescence emission at high concentrations of Ca2+ observed for mRec might reflect the partial release of the protein from the NP surface observed in DLS experiments (Fig. 4A). Overall, this scenario appears fully compatible with the previous evidence from spectroscopic, DLS and limited proteolysis experiments.
Effects of ROS-like liposomes on Rec structure and stability
We tested whether completely different NDs exerted any structural and functional effects on Rec by creating nanovesicles with the same lipid composition as that found in rod-outer segment (ROS) discs within vertebrate photoreceptors. Far UV-CD spectra of mRec in the presence of ∼70 nm liposomes (Fig. S5A†) were very similar to those of the unbound protein (Fig. 2A), as shown by comparable θ222/θ208 ratios observed in the absence (0.77) and in the presence (0.92) of saturating Ca2+ (Table 1). Despite the noise due to the comparable size of the liposomes and the wavelength of the incident light, near UV-CD spectra (Fig. S5B†) were also very similar to those of the unbound protein, except for minor differences in the fine structure observed for the Tyr and Trp bands. Fluorescence spectra also showed high similarity with respect to the isolated protein, and a 8 nm red-shift accompanied by intensity quenching was observed in this case too (Fig. S5C†), although both maxima were slightly red-shifted. Interestingly, DLS experiments proved that mRec-bound liposomes in the presence of saturating Ca2+ (Fig. 4C and Table 2) led to a ∼5.2 nm increase in the size of the complex, which was almost completely reversible upon addition of saturating EGTA (Fig. 4C). In the absence of Ca2+ (i.e. 240 μM EGTA), however, the size of the complex did not significantly change within the experimental error (Table 2), compatible with a very low propensity of apo mRec to bind liposomes. The situation was slightly different for nmRec, as the presence of ROS-like liposomes did not significantly alter the secondary structure (Fig. S5D†), thus resulting in similar θ222/θ208 ratios as those of the unbound protein (0.84 in the absence and 0.92 in the presence of saturating Ca2+, Table 1), while slight changes in optical activity were observed in the near UV range (Fig. S5E†), where fine spectral structures appeared corresponding to all three aromatic bands, both in the presence and in the absence of saturating Ca2+. On the other hand, the behaviour comparable to that of the unbound protein was observed in the intrinsic Trp fluorescence emission (Fig. S5F†), the main differences being a 1 nm instead of 2 nm red-shift and a more prominent increase of intensity.The observed differences are not likely to be due to a stable binding between nmRec and liposomes, as DLS experiments could not detect any significant variation of the hydrodynamic size of the liposomes in the presence of proteins, independent of the presence of saturating EGTA or Ca2+ (Table 2 and Fig. S3†). They are likely due to excluded volume effects and a balance between long-range electrostatic effects induced by liposomes and transient contacts between the protein and the liposome surface.
Overall, these results show that ROS-like liposomes work as suitable “anchors” for Ca2+-bound mRec, which mimic biological membranes and unlike CaF2 NPs do not prevent the protein from functioning as a NCS via the myristoyl switch mechanism. This interpretation seems to be completely in line with previous findings.37 In this respect, liposomes could be used as suitable carriers for mRec in a biological context. On the other hand, nmRec did not show significant interactions with the liposomes under the tested conditions.
Free Ca2+-dependence of ND-Rec hydrodynamic size
In this work we investigated the interaction of Rec in both its myristoylated and non-myristoylated forms with two significantly different types of NDs, namely CaF2 NPs and liposomes with a ROS-like lipid composition. We found that the interaction generally depends on both the presence of post-translational modification in the protein and the presence or absence of free Ca2+ in solution. Fig. 4D–E summarize the results obtained by DLS (Table 2): mRec, whose size increased by ∼0.3 nm upon Ca2+ binding (Fig. 4D), apparently binds CaF2 NPs independent of the presence of free Ca2+ in solution as clearly shown by the increase in the hydrodynamic diameter of the complex observed both in the presence of Ca2+ and EGTA (Table 2). The increase in size though depends on the free Ca2+, being 2.6 nm in the absence of Ca2+, and 1.7 nm increase in the presence of free Ca2+ in solution. In contrast, nmRec decreased in size by ∼0.3 nm upon Ca2+ binding, however when it was incubated with CaF2 NPs, it increased the hydrodynamic diameter of the particles by 3.5 nm in the absence of free Ca2+ and by 5.1 nm in the presence of free Ca2+ in solution. Interestingly, free Ca2+ affects the hydrodynamic size of the protein–ND complex in a protein-specific way (Fig. 4E). High free Ca2+ led to significantly thicker coating of CaF2 NPs by nmRec compared to mRec, as clearly assessed by comparing the difference in the hydrodynamic diameter (Δd = dCa2+ − dEGTA) of the NP–protein complex in the presence of Ca2+ and in the presence of EGTA (Δd = 3.8 nm for nmRec and 1.3 nm for mRec; Fig. 4E). It is unclear whether this is due to a higher propensity of nmRec to form aggregates already in solution (see Fig. S1A†) or, more likely, to a not well-defined surface phenomenon involving the coating by multiple protein layers.A different situation was observed when proteins were incubated with ROS-like liposomes. While no significant interaction with nmRec was observed either in the presence or in the absence of free Ca2+ (Fig. 4E, S3† and Table 2), incubation with mRec at high free Ca2+ increased the hydrodynamic diameter of the liposomes by 3.2 nm (Fig. 4E). In the absence of free Ca2+, however, the hydrodynamic diameter showed high variance (Table 2 and Fig. 4E) indicative of a more polydisperse suspension (Table 2). Overall, this is compatible with a stabilizing effect of free Ca2+ on liposome–mRec interaction, which is reflected by the significantly lower polydispersity index (Table 2).
Conclusion
Our data show that both CaF2 NPs and liposomes interact with mRec and nmRec, however the interaction strongly depends on the specific ND. Fig. 6 schematically summarizes our findings on mRec. In the absence of free Ca2+ (Fig. 6, top left), most of the protein is present in solution in its apo-form (T), and does not interact with the vesicle. Under conditions of saturating Ca2+ however, the myristoyl switch mechanism occurs, thus leading to the Ca2+-bound form (R) and to the exposure of the myristic group, which allows the protein to stably bind to the liposome surface. The extent of binding is directly regulated by the fully preserved capability of mRec to detect Ca2+, and the association process can be reversed if the free Ca2+ is chelated (see Fig. 4).A completely different scenario applies to the molecular process in the presence of CaF2 NPs (Fig. 6, bottom). Upon incubation with particles, mRec sticks to the NP surface adopting a conformation (blue cartoons in Fig. 6) that differs from both the R and T ones. This tight interaction prevents mRec from functioning as a Ca2+ sensor, at least up to high Ca2+ concentrations (see Fig. 5). However, the NP-bound protein is not unfolded at all. It rather adopts a new, highly helical conformation that exposes hydrophobic residues in a manner that partly resembles the R-form, and the binding is partially reversible. A similar scheme applies to nmRec in the presence of CaF2 NPs, but the interaction with CaF2 NPs appears even tighter and irreversible. Moreover, no significant interaction occurs with liposomes.
In conclusion, liposomes could be used as suitable surface carriers of mRec in a biological context to preserve the protein structure and functional properties. On the other hand, CaF2 NPs seem to induce a different conformation to the protein, stabilizing a state that is greatly independent of the levels of free Ca2+ and suggesting a direct interaction between the Ca2+-binding motifs of the protein and the CaF2 matrix, which seems to be the case both for mRec and nmRec. The specific mode of interaction is currently unknown, and together with the novel biological functions, it will be the object of future studies.
Experimental
Protein expression and purification
Myristoylated and nonmyristoylated Rec were expressed essentially as reported,10,38 with some modifications elucidated in the ESI.†Nanoparticle synthesis, structural and spectroscopic characterization
The synthesis procedure of lanthanide doped CaF2 NPs was similar to the method developed by some of us.29 Details are provided in the ESI.†Preparation of liposomes
Liposomes were prepared as described previously10 and is reported in the ESI.†Limited proteolysis
26.5 μg of nmRec or mRec were mixed with nanoparticles (Rec–NP 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 5 mM Tris–HCl pH 7.5, 150 mM KCl, 1 mM DTT and 2 mM CaCl2 or 2 mM EGTA and incubated for 1 h at 25 °C. Particle–protein mixtures were treated with TPCK–trypsin 1
1) in 5 mM Tris–HCl pH 7.5, 150 mM KCl, 1 mM DTT and 2 mM CaCl2 or 2 mM EGTA and incubated for 1 h at 25 °C. Particle–protein mixtures were treated with TPCK–trypsin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (w/w). For each protein a control sample without any protease was studied in parallel. After 30 minutes the digestion reaction was stopped by rapid addition of reducing sample buffer and boiling for 5 min. Then, proteolysis pattern was visualized by SDS-PAGE on a 15% gel and Coomassie blue staining.
100 (w/w). For each protein a control sample without any protease was studied in parallel. After 30 minutes the digestion reaction was stopped by rapid addition of reducing sample buffer and boiling for 5 min. Then, proteolysis pattern was visualized by SDS-PAGE on a 15% gel and Coomassie blue staining.
Dynamic light scattering experiments
DLS measurements were performed with a Zetasizer Nano-S (Malvern Instruments) using a polystyrene low volume disposable sizing cuvette (ZEN0112). Viscosity and refractive index were set to be 0.8872 cP and 1.330 (default values for water), the temperature was set to 25 °C with 2 min equilibration time. The measurement angle was 173° backscatter and the analysis model was set to multiple narrow modes. For each measurement a minimum of 7 determinations were performed, each consisting of 13–15 repetitions. Buffers (5 mM Tris–HCl, 150 mM KCl pH = 7.5, adjusted with CaCl2 or EGTA), were filtered through a Jet Biofilm 0.22 mm membrane, while protein-only solutions were filtered through an Anotop 10 filter (Whatman, 0.02 μm).Circular dichroism spectroscopy and thermal denaturation profiles
Circular dichroism (CD) spectroscopy studies were performed with a Jasco V-710 spectropolarimeter equipped with a Peltier type thermostatted cell holder. Both near-UV (250–320 nm) and far-UV spectra (200–250 nm) were recorded at 25 °C at a scan rate of 50 nm min−1, a bandwidth of 1 nm and an integration time of 4 s. Five spectra accumulations were averaged for each sample, and the spectrum of the buffer (with or without NP or liposomes) was considered as a blank and subtracted. For recording far-UV spectra the protein concentration was 5–7 μM in 0.1 cm cuvettes, while for near-UV spectra it was 29–32 μM in 1 cm cuvettes. Far-UV spectra were recorded in the presence of either 45–50 μM EGTA or Ca2+ while near-UV spectra were recorded in the presence of either 230 μM EGTA or Ca2+.Thermal denaturation of Rec both isolated and in the presence of CaF2 NPs was monitored between 20 °C and 96 °C using the same conditions and concentration range as for the far-UV spectra measurements. The ellipticity signal at 208 nm (θ208) was recorded at a scan rate of 1°C min−1 and a response time of 4 s, using a 0.1 cm quartz cuvette. Thermally denatured samples were cooled down to 25 °C and, after 5–15 min, a far-UV CD spectrum was recorded to check for residual structures (results not shown).
The analysis of thermal denaturation curves was performed for each sample assuming a two-state transition process. By monitoring θ208 as a function of the temperature it was possible to quantitatively describe the fraction of folded and unfolded protein under different conditions. No complete unfolding was achieved for Rec under any experimental condition, as shown by the residual CD signal after thermal denaturation. Data were fitted according to two functions, one accounting for the variation of the Gibbs free energy (see ref. 12, results not shown), and another one consisting of a 4-parameter Hill sigmoid:
 | (1) |
Fluorescence spectroscopy and Ca2+-titrations
The emission fluorescence spectra were recorded between 300 and 380 nm at 25 °C in 1 cm quartz cuvette using a Jasco FP-750 spectrofluorimeter, after excitation at 290 nm, at a scan rate of 60 nm min−1. The excitation and emission bandwidth were set to 5 nm. The collected data were obtained by subtracting the signal of NPs or liposomes to an average of three accumulations. The protein concentration for all fluorescence spectra measurements was ∼1 μM in the presence of ∼6 μM EGTA or Ca2+.Ca2+ buffers used in fluorescence titration experiments were prepared as described previously.39 The solutions used for the fluorescence titration experiments were: (1) carefully decalcified 5 mM Tris–HCl 150 mM KCl pH = 7.5; (2) CaCl2 at variable concentrations; and (3) 1 mM EGTA, whose pH was adjusted to 7.5 by addition of KOH. Free Ca2+ concentration for each titration point was calculated according to the Ca–EGTA NIST software (http://www.maxchelator.stanford.edu/CaEGTA-NIST.htm), by fixing T = 25 °C, pH = 7.5 and ionic strength = 0.15 M. By mixing the three solutions, the final Ca2+ concentration for each point was varied in the 27 nM to 980 μM range. In each titration experiment the protein concentration was 0.8 μM while that of NPs was 135 μg mL−1. Ca2+ titration experiments were performed by monitoring the maximal Trp fluorescence emission (λex = 295 nm) at the lowest free Ca2+ concentration (27 nM), which is expected to change according to the increasing saturation of the protein by Ca2+. The corresponding λmax values in every case are reported in Fig. 6.
Estimation of the size-dependent protein coverage of the ND surface and concentration adjustments
The maximum number of proteins bound to each ND depends on the total surface accessible to proteins and on the fraction of the ND surface occupied by a bound protein. Based on the simplest geometric model of interaction and considering all the objects as rigid spheres, the maximal stoichiometric protein–NP ratio to ensure a minimal presence of unbound proteins in solution is: | (2) |
Also, assuming that the density of CaF2 NPs is equal to that of bulk CaF2 (ρ = 3.18 g cm−3), the molecular weight of CaF2 NPs (NP in eqn (3)) was calculated as follows:
 | (3) |
For example, in the presence of NPs of 30 nm diameter, NmaxRec = 244 and the molecular weight of CaF2 NPs is 27 × 106 g mol−1. For near UV CD spectra with a reasonable signal-to-noise ratio, approximately 30 μM (0.7 mg mL−1) Rec is required, so that 0.12 μM is the concentration of saturating CaF2 NPs (3.2 mg mL−1) that, under the maximal binding geometric assumption, guarantees to keep the amount of free Rec low.
Liposomes differ from NPs in terms of geometrical properties, as the former can be modeled as a spherical bilayer of thickness h whereas the latter can be modeled as a full sphere. The molecular weight of lipids making the ROS-like liposomes (MWROS = 756.725 Da) was calculated using a weighted mean of the molecular weight of each lipid (MWPC = 790.2 Da, MWPE = 748.1 Da, MWPS = 814.1 Da, MWCho = 386.7 Da). The number of lipid molecules in a unilamellar liposome is given by:
 | (4) |
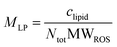 | (5) |
Thus, if 2 mg lipids are rehydrated in 2.6 mL aqueous buffer, the molar concentration MLP of liposomes expected is 20 nM. Knowing the MLP allows the determination of the stoichiometric relationship between liposomes and dissolved Rec, under the assumption of maximal geometric binding. The maximum number of Rec bound to 80 nm liposomes is NmaxRec = 1471 (see eqn (2)), therefore the maximum concentration of Rec bound to a suspension of 0.77 mg mL−1 liposomes is 29.4 μM. Since the hydrodynamic diameter of liposomes varied between 70 and 80 nm, in order to ensure stoichiometric excess of protein binding sites on ROS-like liposomes over free, unbound Rec, 0.77 mg mL−1 liposomes and 25 μM Rec concentration were chosen for DLS and near UV spectra measurements, whereas a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dilution was chosen for recording far UV spectra (0.15 mg mL−1 LP and 5 μM Rec) and a 1
5 dilution was chosen for recording far UV spectra (0.15 mg mL−1 LP and 5 μM Rec) and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 dilution was chosen for recording fluorescence spectra (0.18 μg mL−1 LP and 0.3 μM Rec).
80 dilution was chosen for recording fluorescence spectra (0.18 μg mL−1 LP and 0.3 μM Rec).
Acknowledgements
We would like to thank P. Dominici (University of Verona) for the encouragement in the initial phase of this work and for the continuous support. Plasmids for protein expression were a kind gift from K. W. Koch (University of Oldenburg). This work was supported by funds from the Italian Ministry for Research and Education via departmental funds (FUR2011DDO and FUR2012DDO).References
- M. J. Berridge, P. Lipp and M. D. Bootman, Nat. Rev. Mol. Cell Biol., 2000, 1, 11–21 CrossRef CAS PubMed.
- E. A. Permyakov and R. H. Kretsinger, Calcium Binding Proteins, Wiley, 2011 Search PubMed.
- R. D. Burgoyne, Nat. Rev. Neurosci., 2007, 8, 182–193 CrossRef CAS PubMed.
- P. P. Philippov and K. W. Koch, Neuronal Calcium Sensor Proteins, Nova Science Publishers, Inc., 2006 Search PubMed.
- J. B. Ames, R. Ishima, T. Tanaka, J. I. Gordon, L. Stryer and M. Ikura, Nature, 1997, 389, 198–202 CrossRef CAS PubMed.
- E. N. Pugh Jr and T. D. Lamb, Phototransduction in Vertebrate Rods and Cones: Molecular Mechanisms of Amplification, Recovery and Light Adaptation, Elsevier Science B.V., 2000 Search PubMed.
- D. Dell'Orco, M. Muller and K. W. Koch, Chem. Commun., 2010, 46, 7316–7318 RSC.
- T. Tanaka, J. B. Ames, T. S. Harvey, L. Stryer and M. Ikura, Nature, 1995, 376, 444–447 CrossRef CAS PubMed.
- J. B. Ames, T. Porumb, T. Tanaka, M. Ikura and L. Stryer, J. Biol. Chem., 1995, 270, 4526–4533 CrossRef CAS PubMed.
- I. I. Senin, T. Fischer, K. E. Komolov, D. V. Zinchenko, P. P. Philippov and K. W. Koch, J. Biol. Chem., 2002, 277, 50365–50372 CrossRef CAS PubMed.
- P. Behnen, D. Dell'Orco and K. W. Koch, Biol. Chem., 2010, 391, 631–637 CrossRef CAS PubMed.
- D. Dell'Orco, P. Behnen, S. Linse and K. W. Koch, Cell. Mol. Life Sci., 2010, 67, 973–984 CrossRef CAS PubMed.
- A. M. Dizhoor, S. G. Boikov and E. V. Olshevskaya, J. Biol. Chem., 1998, 273, 17311–17314 CrossRef CAS PubMed.
- F. Doonan, M. Donovan and T. G. Cotter, Invest. Ophthalmol. Visual Sci., 2005, 46, 3530–3538 Search PubMed.
- V. B. Kitiratschky, P. Behnen, U. Kellner, J. R. Heckenlively, E. Zrenner, H. Jagle, S. Kohl, B. Wissinger and K. W. Koch, Hum. Mutat., 2009, 30, E782–E796 CrossRef PubMed.
- R. Cukalevski, M. Lundqvist, C. Oslakovic, B. Dahlback, S. Linse and T. Cedervall, Langmuir, 2011, 27, 14360–14369 CrossRef CAS PubMed.
- H. Pan, M. Qin, W. Meng, Y. Cao and W. Wang, Langmuir, 2012, 28, 12779–12787 CrossRef CAS PubMed.
- E. Gabellieri, P. Cioni, E. Balestreri and E. Morelli, Eur. Biophys. J., 2011, 40, 1237–1245 CrossRef CAS PubMed.
- D. H. Tsai, F. W. Delrio, A. M. Keene, K. M. Tyner, R. I. Maccuspie, T. J. Cho, M. R. Zachariah and V. A. Hackley, Langmuir, 2011, 27(6), 2464–2477 CrossRef CAS PubMed.
- F. Vetrone, R. Naccache, A. Juarranz de la Fuente, F. Sanz-Rodriguez, A. Blazquez-Castro, E. M. Rodriguez, D. Jaque, J. G. Sole and J. A. Capobianco, Nanoscale, 2010, 2, 495–498 RSC.
- S. Jiang, Y. Zhang, K. M. Lim, E. K. Sim and L. Ye, Nanotechnology, 2009, 20, 155101 CrossRef PubMed.
- E. I. Altinoglu, T. J. Russin, J. M. Kaiser, B. M. Barth, P. C. Eklund, M. Kester and J. H. Adair, ACS Nano, 2008, 2, 2075–2084 CrossRef CAS PubMed.
- C. Vinegoni, D. Razansky, S. A. Hilderbrand, F. Shao, V. Ntziachristos and R. Weissleder, Opt. Lett., 2009, 34, 2566–2568 CrossRef CAS PubMed.
- S. A. Hilderbrand, F. Shao, C. Salthouse, U. Mahmood and R. Weissleder, Chem. Commun., 2009, 4188–4190 RSC.
- M. Wang, C. C. Mi, W. X. Wang, C. H. Liu, Y. F. Wu, Z. R. Xu, C. B. Mao and S. K. Xu, ACS Nano, 2009, 3, 1580–1586 CrossRef CAS PubMed.
- F. Wang, D. Banerjee, Y. Liu, X. Chen and X. Liu, Analyst, 2010, 135, 1839–1854 RSC.
- G. Wang, Q. Peng and Y. Li, J. Am. Chem. Soc., 2009, 131, 14200–14201 CrossRef CAS PubMed.
- M. Pedroni, F. Piccinelli, T. Passuello, M. Giarola, G. Mariotto, S. Polizzi, M. Bettinelli and A. Speghini, Nanoscale, 2011, 3, 1456–1460 RSC.
- N. N. Dong, M. Pedroni, F. Piccinelli, G. Conti, A. Sbarbati, J. E. Ramirez-Hernandez, L. M. Maestro, M. C. Iglesias-de la Cruz, F. Sanz-Rodriguez, A. Juarranz, F. Chen, F. Vetrone, J. A. Capobianco, J. G. Sole, M. Bettinelli, D. Jaque and A. Speghini, ACS Nano, 2011, 5, 8665–8671 CrossRef CAS PubMed.
- R. E. Hughes, P. S. Brzovic, A. M. Dizhoor, R. E. Klevit and J. B. Hurley, Protein Sci., 1998, 7, 2675–2680 CrossRef CAS PubMed.
- S. E. Permyakov, A. M. Cherskaya, L. A. Wasserman, T. I. Khokhlova, I. I. Senin, A. A. Zargarov, D. V. Zinchenko, E. Y. Zernii, V. M. Lipkin, P. P. Philippov, V. N. Uversky and E. A. Permyakov, J. Proteome Res., 2003, 2, 51–57 CrossRef CAS.
- S. Ray, S. Zozulya, G. A. Niemi, K. M. Flaherty, D. Brolley, A. M. Dizhoor, D. B. McKay, J. Hurley and L. Stryer, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 5705–5709 CrossRef CAS.
- D. Dell'Orco, S. Sulmann, S. Linse and K. W. Koch, Anal. Chem., 2012, 84, 2982–2989 CrossRef CAS PubMed.
- S. Dokudovskaya, R. Williams, D. Devos, A. Sali, B. T. Chait and M. P. Rout, Structure, 2006, 14, 653–660 CrossRef CAS PubMed.
- A. Fontana, P. P. de Laureto, B. Spolaore, E. Frare, P. Picotti and M. Zambonin, Acta Biochim. Pol., 2004, 51, 299–321 CAS.
- S. Pedigo and M. A. Shea, Biochemistry, 1995, 34, 1179–1196 CrossRef CAS.
- C. Lange and K. W. Koch, Biochemistry, 1997, 36, 12019–12026 CrossRef CAS PubMed.
- I. I. Senin, K. W. Koch, M. Akhtar and P. P. Philippov, Adv. Exp. Med. Biol., 2002, 514, 69–99 CrossRef CAS.
- R. Tsien and T. Pozzan, Methods Enzymol., 1989, 172, 230–262 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures, methods and tables. See DOI: 10.1039/c3nr04978g |
| This journal is © The Royal Society of Chemistry 2014 |



