The exothermic reaction route of a self-heatable conductive ink for rapid processable printed electronics†
Dong-Youn
Shin
a,
Jin Wook
Han
b and
Sangki
Chun
*c
aDepartment of Graphic Arts Information Engineering, Pukyong National University, Yongdang-dong, Nam-gu, Busan, 608-739, Republic of Korea. E-mail: dongyoun.shin@gmail.com; Fax: +82-51-629-6388; Tel: +82-51-629-6394
bDepartment of Chemistry, College of Natural Sciences, Institute of Nanoscience and Technology, Hanyang University, Seoul, 133-791, Republic of Korea. E-mail: jwhan@hanyang.ac.kr; Fax: +82-2-2299-0762; Tel: +82-2-2220-0939
cInformation and Electronic Materials Institute, LG Chem Research Park, Daejeon, 305-380, Republic of Korea. E-mail: sangki@lgchem.com; Fax: +82-42-861-2056; Tel: +82-42-866-5992
First published on 28th October 2013
Abstract
We report the exothermic reaction route and new capability of a self-heatable conductive ink (Ag2O and silver 2,2-dimethyloctanoate) in order to achieve both a low sintering temperature and electrical resistivity within a short sintering time for flexible printed electronics and display appliances. Unlike conventional conductive ink, which requires a costly external heating instrument for rapid sintering, self-heatable conductive ink by itself is capable of generating heat as high as 312 °C when its exothermic reaction is triggered at a temperature of 180 °C. This intensive exothermic reaction is found to result from the recursive reaction of the 2,2-dimethyloctanoate anion, which is thermally dissociated from silver 2,2-dimethyloctanoate, with silver oxide microparticles. Through this recursive reaction, a massive number of silver atoms are supplied from silver oxide microparticles, and the nucleation of silver atoms and the fusion of silver nanoparticles become the major source of heat. This exothermic reaction eventually realizes the electrical resistivity of self-heatable conductive ink as low as 27.5 μΩ cm within just 40 s by combining chemical annealing, which makes it suitable for the roll-to-roll printable electronics such as a flexible touch screen panel.
1 Introduction
Low cost flexible electronics and display appliances, which are fabricated using various printing techniques, are now regarded as the forthcoming mainstay of the electronics and display industries beyond the era of silicon technologies,1,2 and promising outcomes have been demonstrated, such as active thin film transistors using inorganic3,4 or organic semiconducting materials,5–9 and extremely fine metal meshes for display applications.10 Furthermore, the concept of flexible electronics has even been extended to more radical concepts such as stretchable electronics,11–13 wearable electronics,14 and three-dimensional integrated circuits.15,16In any form of flexible electronics using printing techniques, highly conductive electrodes are the most vital requisite because they directly influence the electrical functionality of products. They must be formed at a low sintering temperature because many low cost flexible substrates have not only a glass transition temperature as low as 200 °C and below, but also a high coefficient of thermal expansion as high as 20 ppm °C−1 and above.17
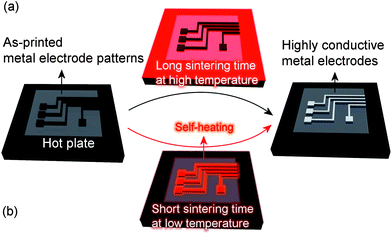
To decrease the sintering temperature, metal–organic compounds18,19 and metallic nanoparticles20,21 have been exploited as conductive inks. Because the metallic content of conductive inks based on a metal–organic compound is greatly limited by its low solubility in a solvent, however, conductive inks based on metallic nanoparticles have been the mainstay of the industry until now. Conductive inks based on metallic nanoparticles necessitate the use of a dispersant to prevent metallic nanoparticles from forming agglomerates. The use of a dispersant, however, adversely contributes to an increase in the sintering temperature and electrical resistivity of the sintered metallic parts due to the high thermal decomposition temperature of the used dispersant. For example, the thermal decomposition onset temperature of polyvinylpyrrolidone, which is widely used as a dispersant, is above 400 °C.22 Therefore, the benefit of using metallic nanoparticles in order to lower the sintering temperature could be severely compromised and either a high sintering temperature or a long sintering time has been required, as shown in Fig. 1(a)
 | ||
| Fig. 1 Formation of sintered metal electrodes with (a) conventional conductive ink with metallic nanoparticles and (b) self-heatable conductive ink. | ||
To satisfy these two contradictory requirements, i.e., a low sintering temperature but a short sintering time, a new concept of conductive ink based on silver oxide microparticles and a silver carboxylate has recently emerged.23–26 The silver carboxylate used in this novel conductive ink not only serves as a dispersant but also contributes to enhancing the electrical conductivity because it converts to a metallic state through its thermal decomposition. However, the most unique trait of this novel conductive ink lies in its intensive exothermic reaction, as shown in Fig. 1(b). Although conventional conductive inks require a costly external source of heat for rapid metallization, such as a laser,27,28 microwave,29,30 plasma,31,32 or pulsed light,33,34 this novel conductive ink has the ability to generate heat by itself for rapid sintering, which clearly differentiates it from conventional conductive inks. This self-heating capability eventually results in rapid metallization of the as-printed parts even without the use of a sophisticated heating instrument.
Until now, however, the exothermic reaction mechanism of a self-heatable conductive ink has not been fully understood. In this study, therefore, its exothermic reaction mechanism and sintering behaviours with self-heating capability were investigated with a view to further developing this attractive self-heatable conductive ink as a new generation of conductive ink for rapid processable printed electronics.
2 Experimental
2.1 Materials and sample preparation
Silver 2,2-dimethyloctanoate was synthesized as the silver carboxylate from 2,2-dimethyloctanoic acid. First, 0.2 mol of n-butyllithium 2.5 M solution in 80 ml of hexane was added drop-wise to 0.2 mol of diisopropylamine in 28 ml of tetrahydrofuran and stirred for 30 min at 0 °C under a nitrogen atmosphere. 0.1 mol of isobutyric acid in 9 ml of distilled water was added drop-wise to the mixture and stirred for 1 h at 0 °C. The temperature of the mixture gradually rose to room temperature in half an hour, and then decreased again to 0 °C. After that, 0.1 mol of 1-bromohexane in 14 ml of distilled water was added drop-wise to the mixture and stirred for 1 h at 0 °C. The mixture was left for 12 h at room temperature and poured into ice-cold 10% hydrochloric acid in 300 ml of distilled water. After it was extracted with ethyl acetate, the organic layer was washed with distilled water and dried over sodium sulphate. Finally, 2,2-dimethyloctanoic acid was obtained after double vacuum distillation at 80 °C under a vacuum of 0.3 Torr.For silver 2,2-dimethyloctanoate, an equimolar amount of silver nitrate in 200 ml of distilled water was added to 2,2-dimethyloctanoic acid in methanol and stirred for 1 h at room temperature. The precipitate was collected by filtration, washed with distilled water and methanol, and then dried in a vacuum oven overnight.
For the synthesis of a self-heatable conductive ink, silver oxide microparticles (D50 ≈ 1.89 μm, Kojundo Chemical Laboratory Co., Ltd., Japan), silver 2,2-dimethyloctanoate and α-terpineol (Kanto Chemical Co., Inc., Japan) were premixed for 2 min at a weight ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, and then degassed for 1 min using a planetary mixer (AR-250, Thinky Corp., Japan). The mixture was finely dispersed with a three roll mill (EXAKT 50, EXAKT Advanced Technologies GmbH, Germany).
1, respectively, and then degassed for 1 min using a planetary mixer (AR-250, Thinky Corp., Japan). The mixture was finely dispersed with a three roll mill (EXAKT 50, EXAKT Advanced Technologies GmbH, Germany).
The specimens were prepared with a four-sided applicator (PA-2020, BYK-Gardner GmbH, Germany) or a spin coater (SF-2J, BGK Rhabdos Co., Republic of Korea) on either PET films or glass cover slips (Cat. No. 2255033, Duran Group GmbH, Germany). The prepared specimens were thermally treated on a hot plate (HP2020300, DHSL Korea Co., Republic of Korea) at a specified temperature.
2.2 Physical characterizations
For the molecular structural verification of the synthesized silver 2,2-dimethyloctanoate, nuclear magnetic resonance, infrared and mass spectra were obtained using a Varian Utility Inova 500 NMR (Varian, Inc., USA), an FTS 3000 (Bio-Rad Laboratories, Inc., USA) and a Finnigan LCQ MS (Thermo Electron Corp., USA), respectively. An elemental analysis of the silver 2,2-dimethyloctanoate was conducted using the Flash 112 series CHNO (Thermo Electron Corp., USA). The results of the analysis of the 2,2-dimethyloctanoic acid and silver 2,2-dimethyloctanoate are summarized in the ESI.†For the thermal characterization of the silver 2,2-dimethyloctanoate, silver oxide and their mixture, a differential scanning calorimeter (DSC) (DSC 823e, Mettler-Toledo International Inc., Switzerland) and a thermogravimetric analyser (TGA/SDTA 851e, Mettler-Toledo International Inc., Switzerland) were used. The thermal images of the specimens during heat treatment were captured with a thermal imaging camera (T620, FLIR Systems, Inc., USA).
For the morphological and structural characterizations of the sintered specimens, a field emission scanning electron microscope (FE-SEM) (S-4800 UHR FE-SEM, Hitachi High-Technologies Corp., Japan) and a transmission electron microscope (TEM) (JEM-3011, Jeol, Ltd., Japan) were used.
For the characterization of the chemical composition and structure of the sintered specimens, X-ray diffraction patterns were obtained with a D4 Endeavor (Bruker Corp., USA). The organic substances were analysed with gas chromatography mass spectrometers (7890A/5975C, Agilent Technologies, Inc., USA/GCMS-QP2010 Ultra, Shimadzu Corp., Japan).
The electrical properties of the sintered specimens were characterized using a four-point probe (4P302, MS Tech Co., Republic of Korea) and a source meter (Model 2401, Keithley Instruments, Inc., USA) for the measurement of sheet resistance, and the thickness of the sintered specimens was measured using a digimatic micrometer (MDC-25MJ, Mitutoyo Corp., Japan).
3 Results and discussion
The self-heatable conductive ink is mainly composed of silver oxide microparticles and a silver carboxylate, i.e., silver 2,2-dimethyloctanoate in this study. When silver oxide microparticles are thermally treated in the presence of silver 2,2-dimethyloctanoate, the thermal reduction temperature for converting silver oxide to silver is dramatically reduced from 400 °C without silver 2,2-dimethyloctanoate, as shown in Fig. 2(a), to approximately 180 °C with an intensive exothermic reaction, as shown in Fig. 2(b).26 It has been reported that a reducing species with a functional group such as primary alcohol or aldehyde, which is a by-product of the thermally decomposed silver carboxylate, is responsible for the reduction of silver oxide to silver,35 and the exothermic reaction is induced by the combustion heat of the by-product.24,25 Therefore, in order to find the exact organic species which is responsible for the reduction of silver oxide to silver, as well as to find the source of heat, the gas chromatograms of silver 2,2-dimethyloctanoate were investigated, as shown in Fig. 3(a). | ||
| Fig. 2 DSC thermograms of (a) silver oxide and (b) self-heatable conductive ink (silver oxide + silver 2,2-dimethyloctanoate). | ||
 | ||
| Fig. 3 (a) Gas chromatograms and (b) DTG (black square) and DSC (red circle) thermograms of silver 2,2-dimethyloctanoate. | ||
The major by-products of thermally decomposed silver 2,2-dimethyloctanote were found to be 2,2-dimethyloctanoic acid, 2-methyl-2-octene, 2-methyl-1-octene and 2-octanone. However, no organic species with a reducing functional group such as primary alcohol or aldehyde was detected, except alkene, ketone and carboxylic forms, which do not have the direct capability to reduce silver oxide to silver, and hence no distinct source of the reduction reaction of silver oxide to silver was found, unlike the claims made in the relevant literature.24,25,35 Moreover, the thermally derived by-products of silver 2,2-dimethyloctanoate cannot be responsible for the intensive exothermic reaction of the self-heatable conductive ink at 180 °C because (1) they must be vividly formed at 240 °C, where the thermal decomposition rate of silver 2,2-dimethyloctanoate is the highest in accordance with its weight loss, and (2) their exothermic reactions are not only weak but also appear at 240 and 280 °C, as shown in the DTG and DSC thermograms of silver 2,2-dimethyloctanoate in Fig. 3(b).
In fact, the endothermic peak without the weight loss of silver 2,2-dimethyloctanoate at 180 °C indicates the solid-to-liquid phase transition of silver 2,2-dimethyloctanoate.36 Once a silver carboxylate turns to a liquid phase, the silver ion starts dissociating from the carboxylate anion, as shown in eqn (1).37,38
| C9H19COO−Ag+ ↔ C9H19COO− + Ag+ | (1) |
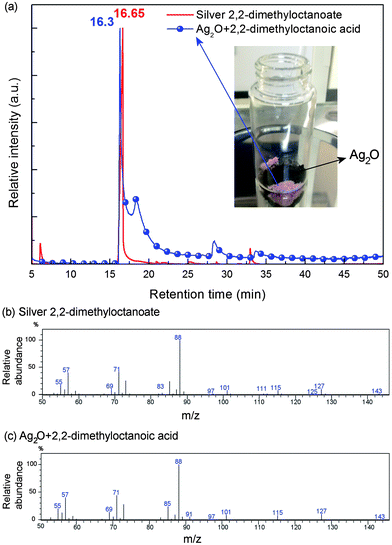
Therefore, if the 2,2-dimethyloctanoate anion in the molten silver 2,2-dimethyloctanoate is highly reactive enough to take a silver ion from silver oxide microparticles, then it can form back silver 2,2-dimethyloctanoate. To verify the reactivity of the 2,2-dimethyloctanoic anion with silver oxide, 2,2-dimethyloctanoic acid and silver oxide were mixed together at room temperature, as shown in the photo of Fig. 4(a), and the gas chromatograms and mass spectra of the product resulting from the reaction were compared with those of silver 2,2-dimethyloctanoate. As shown in Fig. 4(b) and (c), the mass spectra of silver 2,2-dimethyloctanoate and the product resulting from the reaction between the 2,2-dimethyloctanoic anion and silver oxide microparticles at the retention time of approximately 16 min in their gas chromatograms were found to be identical. This verifies the recursive reaction of the 2,2-dimethyloctanoate anion with silver oxide microparticles to form back silver 2,2-dimethyloctanoate, as shown in eqn (2).39 However, silver 2,2-dimethyloctanoate releases the silver ion again due to the applied heat, and becomes the 2,2-dimethyloctanoate anion again, as shown in eqn (1). In the meantime, the released silver ion receives an electron from an oxide anion in the silver oxide microparticles, as shown in eqn (3). This recursive reaction eventually dissociates the silver oxide microparticles and a massive number of silver atoms form silver nanoparticles, as shown in Fig. 5, where the size of the silver crystals calculated from the XRD patterns using the Scherrer equation is around 35 nm.26
| C9H19COO− + 1/2Ag2O → C9H19COO−Ag+ + 1/2O2− | (2) |
| 1/2O2− + Ag+ → 1/4O2 + Ag | (3) |
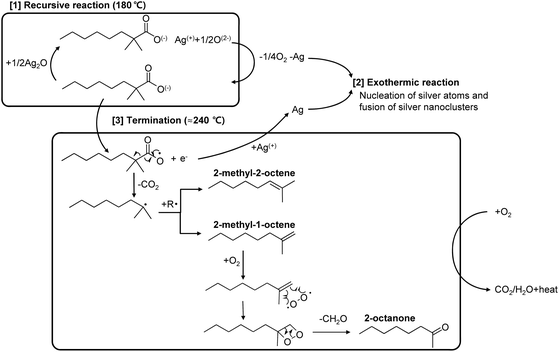
However, the source of the exothermic reaction cannot be explained solely with this recursive reaction of silver 2,2-dimethyloctanoate because the latter most likely undergoes no enthalpy change, just taking a silver ion from silver oxide microparticles and releasing it away. In contrast, the extraction of a silver ion from the silver oxide microparticles might be rather endothermic because the bond between silver and oxygen atoms has to be disintegrated. Therefore, the exothermic source can be postulated to be the nucleation of silver atoms, which are released through the recursive reaction of the 2,2-dimethyloctanoate anion with silver oxide microparticles, and the fusion of silver nanoparticles, as shown in Fig. 6.40
Unlike the exothermic reaction of silver nanoparticles due to the reduction of surface energy during densification and coarsening, this exothermic reaction additionally involves the massive nucleation of silver atoms, which originate from silver oxide microparticles through the recursive reaction of the 2,2-dimethyloctanoate anion. Since the standard enthalpy of formation, ΔH°f,298.15, for Ag2(g) is as high as 409.9 ± 3.5 kJ mol−1,41 the maximum temperature of the self-heatable conductive ink was found to reach as high as 312.2 °C, as shown in Fig. 7.
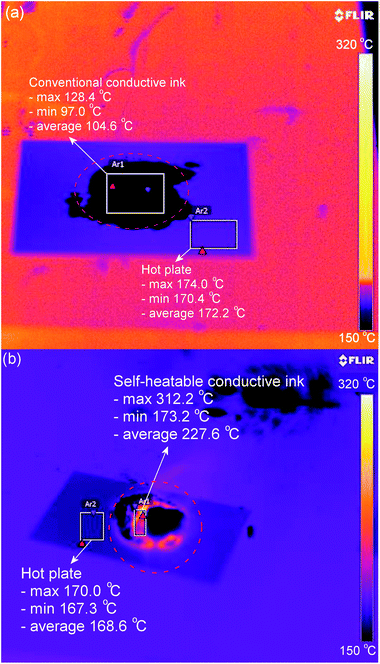 | ||
| Fig. 7 IR images of (a) conventional conductive ink and (b) self-heatable conductive ink during thermal treatment at around 180 °C. | ||
As the temperature increases above 180 °C, the 2,2-dimethyloctanoate anion starts forming a radical, C9H19˙, with the production of CO2 and an electron, as shown in eqn (4),42 and losing its weight, as can be seen in the DTG thermogram of silver 2,2-dimethyloctanoate in Fig. 3(b). This radical, C9H19˙, is further cleaved to form two alkenes, 2-methyl-1-octane and 2-methyl-2-octane, as shown in eqn (5), and 2-methyl-1-octane with O2 subsequently forms 2-octanone. The produced electrons reduce the remaining silver ions and CnH2n is decomposed into CO2 + H2O. These reactions become maximum at 240 °C and then finally end. The overall reaction scheme is summarized in Scheme 1.
| C9H19COO− → C9H19COO˙ + e− → C9H19˙ + CO2 + e− | (4) |
| C9H19˙ + R˙ → C9H18 + R | (5) |
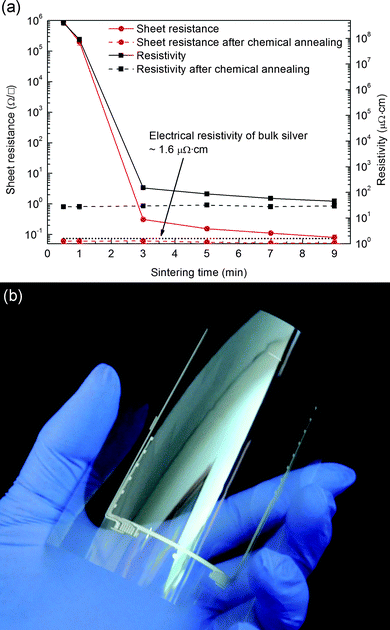
Because of the combined effect of self-heating and the production of silver nanoparticles through the nucleation of silver atoms, the electrical resistivity of the self-heatable conductive ink dropped very rapidly from 4.05 ± 0.90 × 108 μΩ cm in 30 s to 150.58 ± 20.88 μΩ cm in 180 s, and finally reached 45.72 ± 9.36 μΩ cm in 540 s, as shown in Fig. 8(a). This may be considered an encouraging experimental result because the electrical resistivity of the self-heatable conductive ink with silver oxide microparticles is almost comparable to that of a conventional conductive ink with silver nanoparticles.43,44
To further enhance the electrical resistivity of the self-heatable conductive ink at a shorter sintering time, chemical annealing could be an option. Silver nanoparticles, which were produced through the recursive and exothermic reactions of the self-heatable conductive ink, are known to form a well-developed network through self-catalytic addition when they are chemically annealed with an aqueous chloric solution at room temperature.26,45,46 When the bezel electrodes of a flexible touch screen panel, as shown in Fig. 8(b), were thermally treated at 180 °C for 30 s and then dipped into a 6 wt% of NaCl solution in DI water for 10 s, their electrical resistivity was greatly enhanced down to 27.48 ± 3.18 μΩ cm. (The 3D profile and surface roughness of the bezel electrodes screen-printed with self-heatable conductive ink are presented in the ESI.†)
There are several issues with the self-heatable conductive ink. First, the use of dispersants and organic binders is greatly limited due to the inherent trait of the self-heatable conductive ink which requires its constituents to readily react with each other. Since the use of dispersants and organic binders also adversely affects the electrical resistivity of a conductive ink with metallic nanoparticles, however, it is not the issue constrained to the self-heatable conductive ink solely. Second, the more significant issue with the self-heatable conductive ink is a residual solvent trapped in the as-printed part before the exothermic reaction. The vapour generated from the trapped solvent during the exothermic reaction renders the sintered part porous and hence both electrical resistivity and mechanical strength could be degenerated. Third, the used temperature to trigger the recursive reaction of silver 2,2-dimethyloctanoate with silver oxide was approximately 180 °C, which might be considered still high and hence it is advisable to choose a silver carboxylate with a lower temperature for the solid-to-liquid phase transition. Fourth, the intensive exothermic reaction temperature up to 312 °C might cause significant damage on a PET film. By controlling either the amount of self-heatable conductive ink printed on a PET film or the weight ratio of silver oxide to silver 2,2-dimethyloctanoate, the maximum exothermic reaction temperature can be adjusted to evade thermal damage on a PET film. It is also worth suggesting the use of silver oxide submicron particles, as the recursive and exothermic reactions of the self-heatable conductive ink would be significantly accelerated due to the enlarged specific surface area of the silver oxide submicron particles for the reaction with the 2,2-dimethyloctanoate anion. As a result, not only the exposure time of a PET film to a high exothermic reaction temperature could be minimized but also the resulting electrical resistivity of the self-heatable conductive ink would be expected to drop more rapidly.
4 Conclusions
In summary, this study investigated the exothermic reaction route of a self-heatable conductive ink (Ag2O and silver 2,2-dimethyloctanoate) and the opportunity it presents for flexible printed electronics due to its self-heating capability. It is found that the reduction and exothermic reactions of the self-heatable conductive ink result from (1) the recursive reaction of the carboxylate anion with silver oxide microparticles and (2) the nucleation of silver atoms and the fusion of silver nanoparticles, respectively. Especially due to the nucleation of silver atoms, an intensive exothermic reaction occurs unlike conventional conductive inks with silver nanoparticles and hence the resulting temperature reached up to 312.2 °C when the reaction of the self-heatable conductive ink was triggered at around 180 °C. Therefore, the resulting electrical resistivity of the self-heatable conductive ink was found to be comparable to that of conventional conductive inks with silver nanoparticles, even though silver oxide microparticles were used instead of metallic nanoparticles. For enhanced performance, subsequent chemical annealing with an aqueous chloric solution was found to be the best option. Silver nanoparticles, which were produced through the recursive and exothermic reactions of the self-heatable conductive ink, were chemically annealed at room temperature and the resulting electrical resistivity was as low as 27.48 ± 3.18 μΩ cm within 40 s. This unique trait of the self-heatable conductive ink will open a new window for rapid processability, as it is suitable for application in roll-to-roll production of flexible printed electronics appliances without the need for costly external heating instruments.Acknowledgements
This work was supported by a grant awarded under the New & Renewable Energy Technology Development Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) funded by the Korean Ministry of Knowledge Economy (Grant no. 20113020010060).Notes and references
- S. R. Forrest, Nature, 2004, 428, 911 CrossRef CAS PubMed.
- B. D. Gates, Science, 2009, 323, 1566 CrossRef CAS PubMed.
- T. Shimoda, Y. Matsuki, M. Furusawa, T. Aoki, I. Yudasaka, H. Tanaka, H. Iwasawa, D. Wang, M. Miyasaka and Y. Takeuchi, Nature, 2006, 440, 783 CrossRef CAS PubMed.
- A. J. Baca, M. A. Meitl, H. C. Ko, S. Mack, H.-S. Kim, J. Dong, P. M. Ferrera and J. A. Rogers, Adv. Funct. Mater., 2007, 17, 3051 CrossRef CAS.
- Z. Bao, Y. Feng, A. Dodabalapur, V. R. Raju and A. J. Lovinger, Chem. Mater., 1997, 9, 1299 CrossRef CAS.
- T. W. Kelley, P. F. Baude, C. Gerlach, D. E. Ender, D. Muyres, M. A. Haase, D. E. Vogel and S. D. Theiss, Chem. Mater., 2004, 16, 4413 CrossRef CAS.
- M. L. Chabinyc and A. Salleo, Chem. Mater., 2004, 16, 4509 CrossRef CAS.
- S. Gamerith, A. Klug, H. Scheiber, U. Scherf, E. Moderegger and E. J. W. List, Adv. Funct. Mater., 2007, 17, 3111 CrossRef CAS.
- C. W. Lee, S. K. R. Pillai, X. Luan, Y. Wang, C. M. Li and M. B. Chan-Park, Small, 2012, 8, 2941 CrossRef CAS PubMed.
- C. E. Hendricks, P. J. Smith, J. Perelaer, A. M. J. van den Berg and U. S. Schubert, Adv. Funct. Mater., 2008, 18, 1031 CrossRef.
- I. Osborne, M. Lavine and R. Coontz, Science, 2010, 327, 1595 CrossRef CAS PubMed.
- J. A. Rogers, T. Someya and Y. Huang, Science, 2010, 327, 1603 CrossRef CAS PubMed.
- X. Hu, P. Krull, B. de Graff, K. Dowling, J. A. Rogers and W. J. Arora, Adv. Mater., 2011, 23, 2933 CrossRef CAS PubMed.
- B. Hu, D. Li, O. Ala, P. Manandhar, Q. Fan, D. Kasilingam and P. D. Calvert, Adv. Funct. Mater., 2011, 21, 305 CrossRef CAS.
- J.-H. Ahn, H.-S. Kim, K. J. Lee, S. Jeon, S. J. Kang, Y. Sun, R. G. Nuzzo and J. A. Rogers, Science, 2006, 314, 1754 CrossRef CAS PubMed.
- A. C. Hübler, G. C. Schmidt, H. Kempa, K. Reuter, M. Hambsch and M. Bellmann, Org. Electron., 2011, 12, 419 CrossRef PubMed.
- M.-C. Choi, Y. Kim and C.-S. Ha, Prog. Polym. Sci., 2008, 33, 581 CrossRef CAS PubMed.
- A. L. Dearden, P. J. Smith, D.-Y. Shin, N. Reis, B. Derby and P. O'Brien, Macromol. Rapid Commun., 2005, 26, 315 CrossRef CAS.
- S. F. Jahn, T. Blaudeck, R. R. Baumann, A. Jakob, P. Ecorchard, T. Rüffer, H. Lang and P. Schmidt, Chem. Mater., 2010, 22, 3067 CrossRef CAS.
- A. Kamyshny, M. Ben-Moshe, S. Aviezer and S. Magdassi, Macromol. Rapid Commun., 2005, 26, 281 CrossRef CAS.
- S. Jeong, K. Woo, D. Kim, S. Lim, J. S. Kim, H. Shin, Y. Xia and J. Moon, Adv. Funct. Mater., 2008, 18, 679 CrossRef CAS.
- Y. K. Du, P. Yang, Z. G. Mou, N. P. Hua and L. Jiang, J. Appl. Polym. Sci., 2005, 99, 23 CrossRef.
- S. Chun, D. Grudinin, D. Lee, S.-H. Kim, G.-R. Yi and I. Hwang, Chem. Mater., 2009, 21, 343 CrossRef CAS.
- T. Morita, Y. Yasuda, E. Ide and A. Hirose, Mater. Trans., 2009, 50, 226 CrossRef CAS.
- Y. Yasuda, S. Terada, T. Morita and H. Kawaji, Jpn. J. Appl. Phys., 2012, 51, 025001 CrossRef.
- D.-Y. Shin, G.-R. Yi, D. Lee, J. Park, Y.-B. Lee, I. Hwang and S. Chun, Nanoscale, 2013, 5, 5043 RSC.
- N. R. Bieri, J. Chung, S. E. Haferl, D. Poulikakos and C. P. Grigoropoulos, Appl. Phys. Lett., 2003, 82, 3529 CrossRef CAS.
- J. Chung, S. Ko, N. R. Bieri, C. P. Grigoropoulos and D. Poulikakos, Appl. Phys. Lett., 2004, 84, 801 CrossRef CAS.
- J. Perelaer, B.-J. de Gans and U. S. Schubert, Adv. Mater., 2006, 18, 2101 CrossRef CAS.
- J. Perelaer, M. Klokkenburg, C. E. Hendriks and U. S. Schubert, Adv. Mater., 2009, 21, 4830 CrossRef CAS PubMed.
- I. Reinhold, C. E. Hendriks, R. Eckardt, J. M. Kranenburg, J. Perelaer, R. R. Baumann and U. S. Schubert, J. Mater. Chem., 2009, 19, 3384 RSC.
- S. Wünscher, S. Sumpf, A. Teichler, O. Pabst, J. Perelaer, E. Beckert and U. S. Schubert, J. Mater. Chem., 2012, 22, 24569 RSC.
- W.-S. Han, J.-M. Hong, H.-S. Kim and Y.-W. Song, Nanotechnology, 2011, 22, 395705 CrossRef PubMed.
- D. J. Lee, S. H. Park, S. Jang, H. S. Kim, J. H. Oh and Y. W. Song, J. Micromech. Microeng., 2011, 21, 125023 CrossRef.
- Printing Technology for Flexible Substrates, InterLingua Publishing, CA, USA, 2006 Search PubMed.
- D.-Y. Shin, M. Jung and S. Chun, J. Mater. Chem., 2012, 22, 11755 RSC.
- M. S. Akanni, H. D. Burrows and P. B. Begun, Thermochim. Acta, 1984, 81, 45 CrossRef CAS.
- M. S. Akanni, E. K. Okoh, H. D. Burrows and H. A. Ellis, Thermochim. Acta, 1992, 208, 1 CrossRef CAS.
- D.-Y. Shin and S. Chun, Mater. Res. Soc. Symp. Proc., 2013, 1567 DOI:10.1557/opl.2013.680.
- R. Patakfalvi and I. Dékány, J. Therm. Anal. Calorim., 2005, 79, 587 CrossRef CAS.
- Q. Ran, R. W. Schmude, Jr, K. A. Gingerich, D. W. Wilhite and J. E. Kingcade, Jr, J. Phys. Chem., 1993, 97, 8535 CrossRef CAS.
- A. R. Fiorucci, L. M. Sana, E. T. G. Cavalheiro and E. A. Neves, Thermochim. Acta, 2000, 356, 71 CrossRef CAS.
- H.-H. Lee, K.-S. Chou and K.-C. Huang, Nanotechnology, 2005, 16, 2436 CrossRef CAS PubMed.
- J. R. Greer and R. A. Street, Acta Mater., 2007, 55, 6345 CrossRef CAS PubMed.
- S. Magdassi, M. Grouchko, O. Berezin and A. Kamyshny, ACS Nano, 2010, 4, 1943 CrossRef CAS PubMed.
- M. Grouchko, A. Kamyshny, C. F. Mihailescu, D. F. Anghel and S. Magdassi, ACS Nano, 2011, 5, 3354 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR, IR, ACPI-MS and elemental analysis data of 2,2-dimethyloctanoic acid and silver 2,2-dimethyloctanoate, and the 3D profile and surface roughness of the bezel electrodes screen-printed with self-heatable conductive ink. See DOI: 10.1039/c3nr04645a |
| This journal is © The Royal Society of Chemistry 2014 |