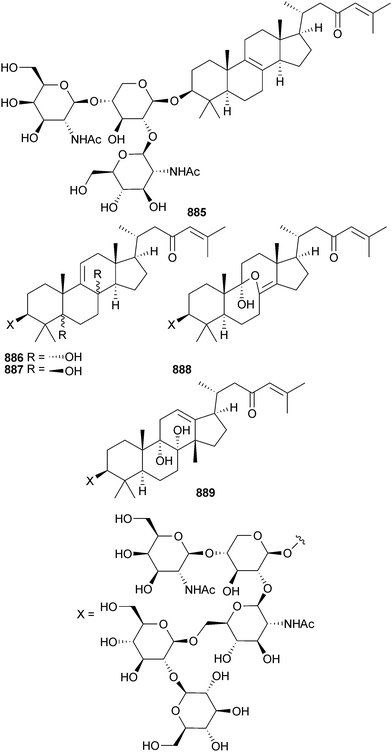
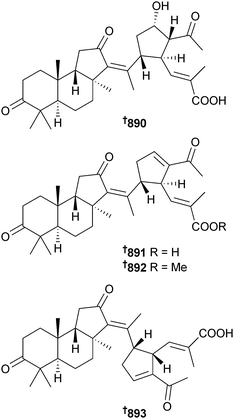
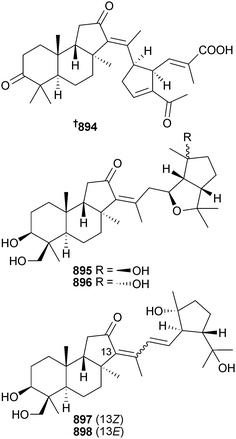
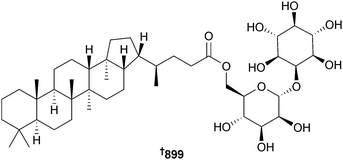
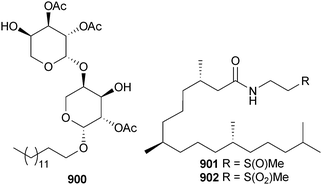
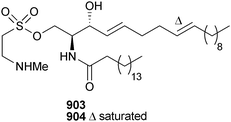
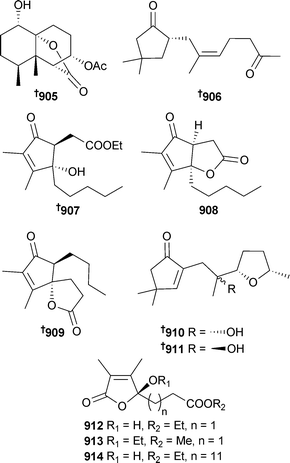
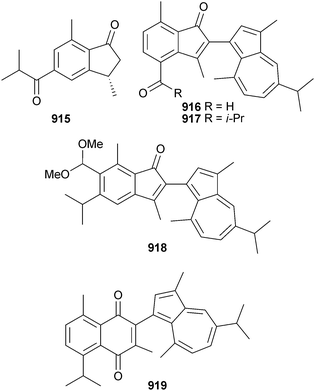
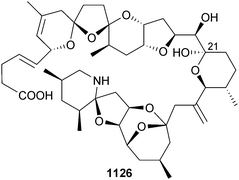
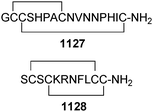
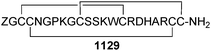
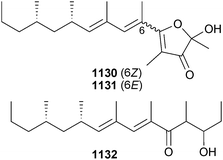
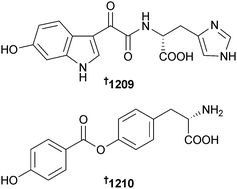
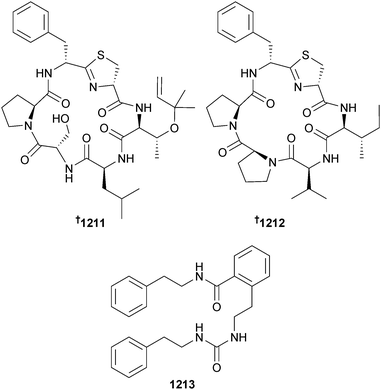
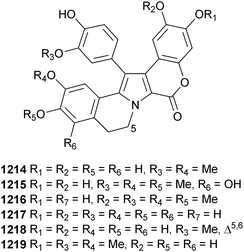
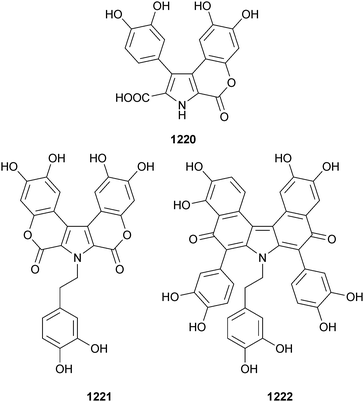
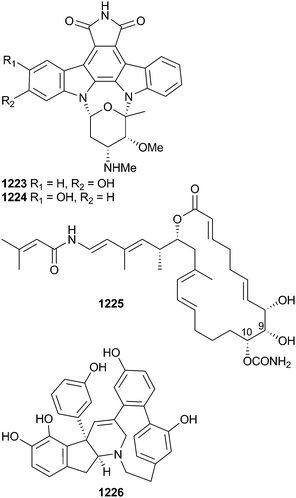
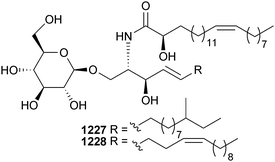
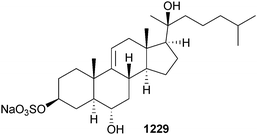
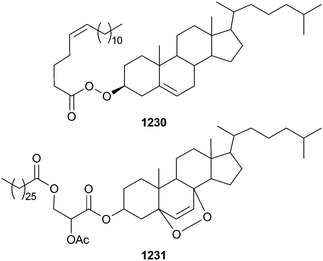
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Marine natural products
John W.
Blunt
*a,
Brent R.
Copp
b,
Robert A.
Keyzers
c,
Murray H. G.
Munro
a and
Michèle R.
Prinsep
d
aDepartment of Chemistry, University of Canterbury, Christchurch, New Zealand. E-mail: john.blunt@canterbury.ac.nz
bSchool of Chemical Sciences, University of Auckland, Auckland, New Zealand
cCentre for Biodiscovery, and School of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
dDepartment of Chemistry, University of Waikato, Hamilton, New Zealand
First published on 3rd January 2014
Abstract
Covering: 2012. Previous review: Nat. Prod. Rep., 2013, 30, 237–323.
This review covers the literature published in 2012 for marine natural products, with 1035 citations (673 for the period January to December 2012) referring to compounds isolated from marine microorganisms and phytoplankton, green, brown and red algae, sponges, cnidarians, bryozoans, molluscs, tunicates, echinoderms, mangroves and other intertidal plants and microorganisms. The emphasis is on new compounds (1241 for 2012), together with the relevant biological activities, source organisms and country of origin. Biosynthetic studies, first syntheses, and syntheses that lead to the revision of structures or stereochemistries, have been included.
1 Introduction
This review is of the marine natural product (MNP) literature for 2012 and describes 1241 new MNPs from 382 articles, an 8% increase in the number of compounds reported for 2011.1 As in previous reviews, the structures are shown only for new compounds, or for previously reported compounds where there has been a structural revision or a newly established stereochemistry. Previously reported compounds for which first syntheses or new bioactivities are described are referenced, but separate structures are generally not shown. Where the absolute configuration has been determined for all stereocentres in a compound, the identifying diagram number is distinguished by addition of the † symbol.2 Reviews
A selection of the many reviews on various aspects of MNP studies is listed here. A comprehensive review of MNPs reported in 2010 has appeared,2 as well as the highlights of compounds reported in 2011.3 The two volume ‘Handbook of Marine Natural Products’ has been published.4 The continuing series on ‘Marine Pharmaceuticals: The Preclinical Pipeline’ is now accessible from a web site.5,6 A survey of new drugs derived from all natural products over the past 30 years has been presented.7 Many classes or specific examples of marine-sourced compounds have been reviewed to varying extents, including conopeptides,8,9 didemnins,10 largazole,11 peptides,12–14 prenylated quinones and hydroquinones,15 arsenolipids,16 bryostatins,17 multisulfide-containing metabolites,18 fascaplysin,19 tunichromes,20 glycosides from sponges,21 sea anemone toxins,22 mycalamides and related compounds,23,24 and polyhydroxysterols.25 There were three reviews of general classes of compounds that included reference to marine compounds – sesquiterpenoids,26 cembrane diterpenes,27 and diketopiperazines.28 Reviews of natural products from various marine sources include those from cyanobacteria,29,30 microalgae,31,32 actinomycetes,33 gorgonians,34 ascidians,35,36 sea cucumbers,37 sponges,38–40 seaweeds41 and their endophytic fungi,42 the marine flora and fauna of Fiji,43 the sponges Aplysina aerophoba44 and Stylissa massa,45 and from the soft coral Nephthea.46,47 Particular types of bioactivity have been reviewed in papers on marine anticancer drugs,48 indoleamine 2,3-dioxygenase inhibitors,49 kinase inhibitors,50 protein tyrosine phosphatase inhibitors,51 compounds with reversal effects on cancer multidrug resistance,52 steroid nuclear receptor ligands from sponges,53 mammalian DNA polymerisation inhibitors from microorganisms54 and marine bioactives against inflammatory diseases.55 Topics in chemical ecology of cyanobacteria56 and sponges57 have been covered, while marine bioprospecting has been reviewed.41,58,59 The eighth in a companion series providing an overview of synthetic aspects of MNPs has appeared with coverage of publications from 2010.60 There have been a number of papers which, while not necessarily being reviews, are useful to include here as they describe advances in techniques or approaches to discovery that are relevant to MNP studies. These include papers on configurational assignments,61,62 marine proteomics,63 guiding principles for natural product drug discovery,64 mass spectometry-based metabolomics,65 techniques for bioactives discovery from marine fungi66 and a commentary on past and future aspects of MNP drug discovery.673 Marine microorganisms and phytoplankton
As a rich source of novel and bioactive marine microorganisms continue to be a major focus of many natural products research efforts, with a 10% increase in the number of compounds reported from the previous year following a 30% increase from 2010 to 2011. Unless otherwise stated, compounds described in this section were obtained from cultures of the named microorganisms.3.1 Marine-sourced bacteria (excluding from mangroves)
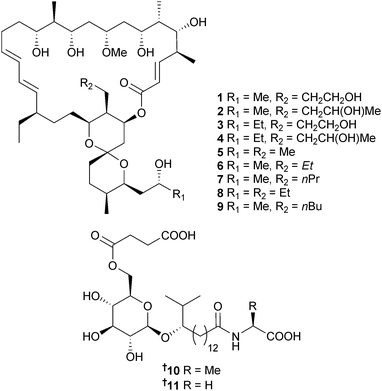
Actinoalloteichus sp. (sediment, Usa Bay, Kochi Prefecture, Japan) was the source of neomaclafungins A–I 1–9, 26-membered macrolides of the oligomycin subfamily, which all displayed significant activity against Trichophyton mentagrophytes (T. mentagrophytes).68 Glycolipopeptides eodoglucomide A 10 and B 11 were obtained from Bacillus licheniformis (sediment, Ieodo Reef, S. Korea) and are broad spectrum, moderately active antimicrobial agents, with ieodoglucomide B also cytotoxic to lung and stomach cancer cells.69
Bacillus mojavensis (pearl oyster Pinctada martensii, Weizhou Is., South China Sea) provided the antifungal iturinic lipopeptide mojavensin A 12,70 while the amicoumacin analogues lipoamicoumacin A–D 13–16 and a bacilosarcin analogue, bacisarcin C 17, were isolated from Bacillus subtilis (B. subtilis) (sediment, Red Sea) together with several known amicoumacins. Bacilosarcin B71 and amicoumacin A72 were cytotoxic to HeLa cells and are strongly antibacterial.73
The known synthetic toluhydroquinone derivatives, 5-bromotoluhydroquinone74 and 4-O-methyltoluhydroquinone75 were obtained for the first time from a natural source from Dothideomycete sp. (red alga Chondria crassicualis, Yokiji Is., S. Korea). Both compounds were moderate 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavengers.76 Of the erythrolic acids A–E 18–22, meroterpenoids with a modified terpene sidechain isolated from Erythrobacter sp. (sediment, Trinity Bay, Galveston, Texas), only erythrolic acid D exhibited modest activity towards non-small cell lung cancer cells.77
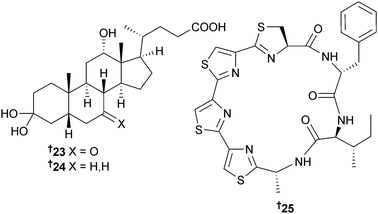
The cholic acid derivatives 23 and 24 were obtained from endophytic Hasllibacter halocynthiae (ascidian Halocynthia roretzi, Kyung-Po Beach, S. Korea)78 and Marinactinospora thermotolerans (deep sea sediment, South China Sea) was the source of the sequential tristhiazole-thiazoline-containing cyclic peptide marthiapeptide A 25, active against a panel of Gram-positive bacteria and strongly cytotoxic towards a panel of human tumour cell lines (HTCLs),79 while from M. rosaria (sediment, South China Sea) three additional fluostatins I–K 26–28 were isolated.
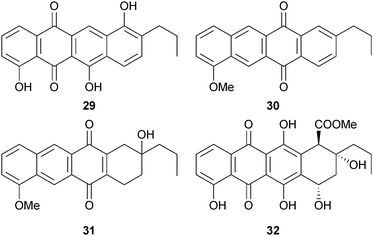
The antibiotic A201A, previously isolated from terrestrial Streptomyces capreolus,80 was obtained from the marine environment for the first time from M. thermotolerans (deep sea sediment, South China Sea), and the gene cluster responsible for biosynthesis identified.81 The known metabolites of terrestrial Streptomyces species, fluostatins C–F,82,83 and phenanthroviridone,84 were also obtained (first time from the marine environment).85 The anthracyclinones 29–32 were obtained from a Micromonospora sp. (tunicate Eudistoma vannamei, Taíba Beach, Ceará, Brazil) – 29 and 32 were moderately cytotoxic to HCT-8 cells.86
Peptidolipins B–F 33–37 are lipopeptides obtained from Nocardia sp. (ascidian Trididemnum orbiculatum, Florida Keys), but the location of the cyclopropyl group in the sidechain of peptidolipin F was not determined. Peptidolipins B and E exhibited moderate activity against methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA).87
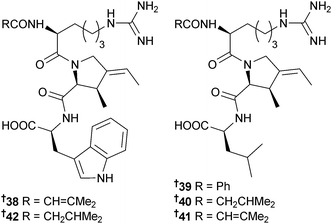
Upon assignment of the structure of the new lucentamycin analogue lucentamycin E 38 (Nocardiopsis lucentensis, sediment, Little San Salvador, Bahamas), the olefin geometries of the 3-methyl-4-ethylideneproline residues of lucentamycins A–D 39–42, other tripeptides isolated from the same organism,88 were reinvestigated and revised to (2S,3R,E)-3-methyl-4-ethylideneproline.89 Total synthesis of lucentamycin A was accomplished via a novel strategy from Garner's aldehyde and corroborated the revised configurations.90 Synthesis of the (E)-isomer of the proposed structure of lucentamycin A, via a stereoselective rhodium-catalysed reductive cyclisation process, and comparison of NMR data of synthetic with natural lucentamycin A indicated the need for the configurational revision.91Phaeobacter sp. (pulp mill effluent, coastal Southern USA) produces the known bacterial blue pigment indigoidine92 which inhibited growth of Vibrio fischeri and was isolated from the “marine” environment for the first time.93
Pseudoalteromones A 43 and B 44 were obtained from Pseudoalteromonas sp. (cultured-type octocoral Lobophytum crassum, Taiwan).94,95 The ubiquinone derivative pseudoalteromone A possessed a 9C nor-monoterpenoid moiety, was cytotoxic to MOLT-4 (human acute lymphoblastic leukaemia) cells and inhibited release of elastase by human neutrophils.
2-Methyl-3-butyl-prodiginine has previously been identified as a metabolite of the marine bacteria Hahella chejuensis96 and Pseudoalteromonas rubra97 (by mass spectrometry) and now has been fully characterised as a metabolite of Pseudoalteromonas sp. (seawater, Cape Muroto, Japan).98 Investigation of Salinispora pacifica (USDA Agricultural Research Service) resulted in the isolation of the complex metabolites (−)-lomaiviticin C–E 45–47 as growth inhibitors of several cancer cell lines. Lomaiviticin C was directly converted to the known (−)-lomaiviticin A,99 allowing the absolute configuration of (−)-lomaiviticin A to be established as 48.100
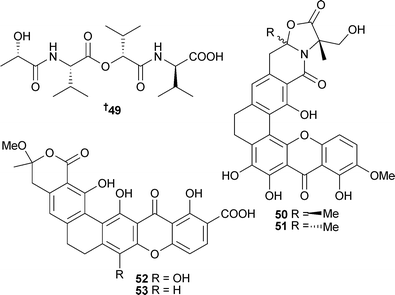
The peptide 49 was obtained from Streptomyces bacillaris (sediment, Galveston Bay, Texas).101 The polycyclic citreamicins A 50 and B 51, citreaglycon A 52 and dehydrocitreaglycon A 53 were isolated from S. caelestis (seawater, Red Sea, Jeddah, Saudi Arabia). All displayed broad spectrum antibacterial activity. Citreamicins A and B and citreaglycon A also inhibited MRSA while citreamicins A and B were also significantly cytotoxic to HeLa cells.102
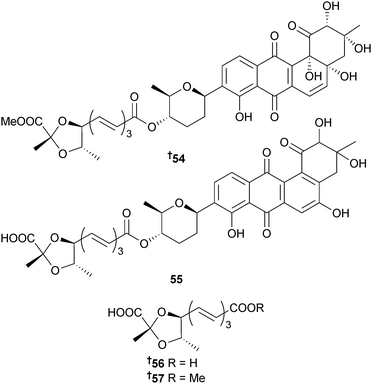
S. fradiae (sediment, source unspecified) yielded several capoamycin-type antibiotics, fradimycin A 54 and B 55 and fradic acid A 56 and B 57, of which fradimycins A and B inhibited Staphylococcus aureus (S. aureus) growth and both, as well as the known anthraquinone MK844-mF10,103 significantly inhibited growth of several cancer cell lines.104
Fradcarbazoles A–C 58–60, indolocarbazoles isolated from Streptomyces fradiae (sediment, Jiaozhou Bay, Shandong, China), possessed significant cytotoxicity against several HTCLs, in addition to inhibition of the kinase PKC-α.105
Culture of S. hygroscopicus (fish Halichoeres bleekeri106) led to the isolation of macrolides halichoblelide B 61 and C 62,107 both significantly cytotoxic to a panel of HTCLs.108 The C-glycoside angucyclines grincamycin B–F 63–67 were obtained from Streptomyces lusitanus (deep sea sediment, South China Sea). Of these, grincamycins B–E were moderately cytotoxic to several HTCLs and to B16 cells.109
S. spinoverrucosus (sediment, Trinity Bay, Galveston, Texas) produced the anthraquinones galvaquinone A–C 68–70. Galvaquinone B possessed epigenetic modulatory activity and moderate cytotoxicity against non-small-cell lung cancer (NSCLC) cells Calu-3 and H2887.110S-Methyl-2,4-dihydroxy-6-isopropyl-3,5-dimethylbenzothioate 71 was isolated from Streptomyces sp. (unidentified tunicate, Lyttelton Harbour, New Zealand) through use of an HPLC bioactivity profiling/microtitre plate technique in conjunction with microprobe NMR spectroscopy and was cytotoxic to P388 cells.111
JBIR-109–111 72–74 are trichostatin analogues isolated from a Streptomyces strain (unidentified sponge, Takara Is., Kagoshima, Japan).112
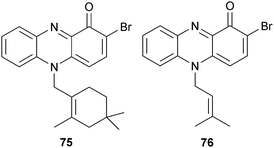
The phenazine derivatives 75 and 76 and lavanducyanin113 were isolated from a marine-derived Streptomyces sp. (source unspecified). All three compounds inhibited TNF-α-induced NFκB activity and LPS-induced nitric oxide (NO) production.114
A Streptomyces strain (sponge Dysidea tupha, Rovinj, Croatia) produced the lipopeptides cyclodysidin A–D 77–80115 while another Streptomyces sp. (sediment, Heron Is., Queensland, Australia) yielded heronamycin A 81, a benzothiazine ansamycin.116
Noteworthy in the totopotensamide A 82 and the aglycone totopotensamide B 83 structures [Streptomyces sp. (gastropod mollusk Lienardia totopotens, Mactan Is., Cebu, Philippines)] are the previously undescribed 2,3-diaminobutyric acid-containing macrolactam and 4-chloro-5,7-dihydroxy-6-methylphenylglycine components.117 Bahamaolide A 84, a strong inhibitor of Candida albicans isocitrate lyase, and bahamaolide B 85 are macrocyclic lactones from a Streptomyces sp. (sediment, North Cat Cay, Bahamas).118
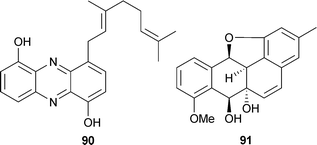
Spiroindimicins A–D 86–89 are spiro-bisindole alkaloids from a Streptomyces sp. (sediment, Bay of Bengal) obtained via a PCR-based screening approach. Spiroindimicins B–D displayed moderate cytotoxicity to a number of cancer cell lines.119 The phenazine geranylphenazinediol 90 isolated from a Streptomyces sp. (sediment, Kiel Fjord, Baltic Sea) was an inhibitor of acetylcholinesterase,120 while an angucyclinone derivative kiamycin 91 originating from a Streptomyces sp. (sediment, Qingdao, China) was an inhibitor of several HTCLs.121
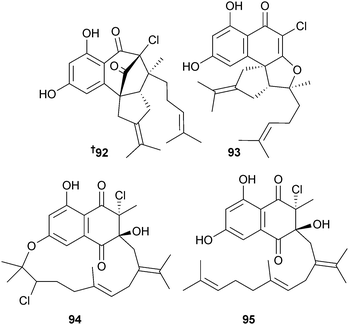
A Streptomyces sp. (sediment, Oceanside, California) was the source of the mixed polyketide-terpenoids merochlorin A–D 92–95. Merochlorins A122 and B123 were both potent inhibitors of MRSA. In vivo studies determined the genetic basis for biosynthesis of the merochlorins and implicated the involvement of rare bacterial vanadium-dependent haloperoxidase genes.122,123
The antibacterial indolosesquiterpenes dixiamycin A 96 and B 97, oxiamycin 98 and chloroxiamycin 99 were isolated from a Streptomyces sp. (sediment, South China Sea) along with the previously isolated xiamycin A.124 Dixiamycins A and B are the first examples of naturally occurring N–N-coupled atropo-diastereomers.125 The putative biosynthetic gene cluster for xiamycin A and oxiamycin was identified by a partial genome sequencing approach. Eighteen genes were proposed to be involved in the biosynthesis and indosespene was identified as a common precursor for the biosynthesis, which included an unusual oxidative cyclisation strategy.126
A histone deacetylase (HDAC)-based yeast assay employing a URA3 reporter gene was utilised in the isolation of streptosetin A 100 from a Streptomyces sp. (sediment, San Francisco Bay).127 The C-glycosylated benz[α]anthraquinone derivatives, urdamycinone E,128 urdamycinone G 101 and dehydroxyaquayamycin129 were obtained from a Streptomyces sp. (sponge Xestospongia sp., Sichang Is., Chonburi, Thailand) and along with urdamycin E128 were potent inhibitors of the P. falciparum K1 strain and inhibitors of Mycobacterium tuberculosis.130 Urdamycinone E128 and dehydroxyaquayamycin129 were obtained for the first time as natural products.130
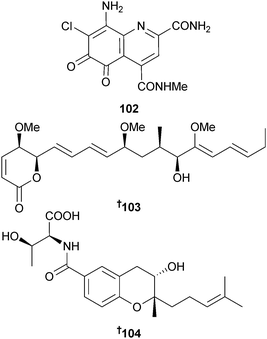
Streptomyces variabilis (sediment, Sweetings Cay, Bahamas) was the source of ammosamide D 102 (modest cytotoxicity to the MIA PaCa-2 pancreatic cancer cell line).131 The cytotoxic linear polyketide pterocidin was originally obtained from endophytic S. hygroscopicus associated with the bracken Pteridium aquilinum but due to a lack of material the absolute configuration could not be determined.132 The same authors have now isolated pterocidin from a marine Streptomyces strain (sediment, Otsuchi Bay, Japan) in sufficient quantities to determine the absolute configuration as 103.133 The absolute configuration of a threonine derivative obtained from Streptomyces xiamenensis (mangrove sediment, Fujian, China) as an inhibitor of the proliferation of WI26 (human lung fibroblast) cells was determined as 104.134 This threonine derivative had previously been reported in a Japanese patent as an inhibitor of asthma and rheumatoid arthritis.135
The hydroxyethylamine chromene derivatives ammonificin C 105 and D 106 were obtained from Thermovibrio ammonificans (hydrothermal vent, East Pacific Rise) and induced apoptosis in a cell-based screen.136
Vibrio nigripulchritudo (global marine Galathea 3 expedition)137 produced the siderophore nigribactin 107, a modulator of S. aureus virulence gene expression,138 while another Vibrio species (Gulf of Mexico) was the source of the amphiphilic siderophores ochrobactin-OH A–C, but only ochrobactin-OH B 108 was fully characterised.139 A third Vibrio sp. (source unspecified, Okinawa, Japan) provided the cyclic acyldepsipeptide kailuin F 109, as well as the known analogues kailuins B140 and E141 of which kailuin B displayed strong activity against the dinoflagellate Prorocentrum micans.142
3.2 Bacteria from mangroves
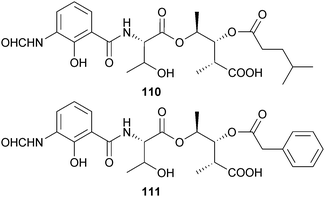
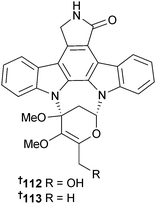
Of the two antimycins, B1 110 and B2 111 from Streptomyces lusitanus (mangrove sediment, Avicennia mariana, Fujian, China), only antimycin B2 had moderate activity against S. aureus and Laribacter hongkongensis.143 The indolocarbazoles streptocarbazole A 112 and B 113 were obtained from Streptomyces sp. (mangrove soil, species unspecified, Sanya, Hainan, China), but only streptocarbazole A was cytotoxic to HTCLs and arrested the cell cycle of HeLa cells at the G2/M phase.144The eudesmene-type sesquiterpenes kandenol A–E 114–118 were isolated from an endophytic Streptomyces sp. (mangrove stem Kandelia candel, Xiamen, Fujian, China) as weak to moderate inhibitors of B. subtilis and Mycobacterium vaccae growth.145
3.3 Marine-sourced fungi (excluding from mangroves)
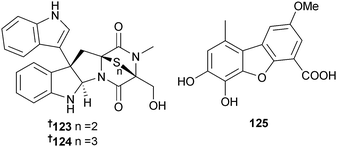
Cordyheptapeptides C–E 119–121 were isolated from Acremonium persicinum (sediment, South China Sea) with cytotoxicity against HTCLs noted for cordyheptapeptides C and E.146 Acremolin was isolated from Acremonium strictum (unidentified Choristida sponge, S. Korea) with the original structure incorporating a 1H-aziridine moiety.147 It was subsequently shown that the reported NMR spectroscopic data were incompatible with the proposed antiaromatic heterocycle, which would have been an extremely unstable compound. A plausible alternative structure, an isomeric substituted N2,3-ethenoguanine 122 has been suggested, which is consistent with all spectroscopic data.148Indole diketopiperazines luteoalbusin A 123 and B 124, isolated from Acrostalagmus luteoalbus (deep sea sediment, South China Sea), were potent cytotoxins against several HTCLs.149 Porric acid D 125, a dibenzofuran derivative from Alternaria sp. (Bohai Sea, Tianjin, China), was weakly inhibitory to S. aureus.150
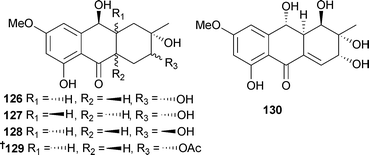
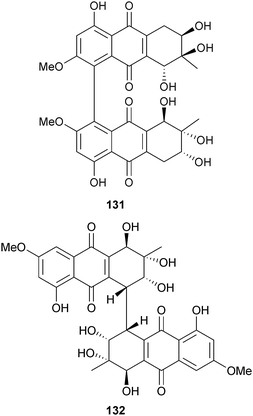
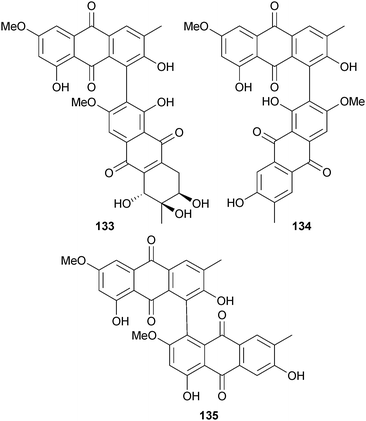
The hydroanthraquinone-derived tetrahydroaltersolanols C–F 126–129 and dihydroaltersolanol A 130, in addition to the alterporriol-type anthranoid dimers alterporriols N–R 131–135, were obtained from Alternaria sp. (soft coral Sarcophyton sp., Weizhou coral reef, South China Sea). Tetrahydroaltersolanol C and alterporriol Q were active against the porcine reproductive and respiratory syndrome virus while alterporriol P was cytotoxic to HTCLs.151
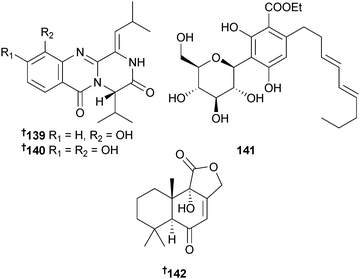
Aspergillus carneus (brown alga Laminaria sachalinensis, Kunachir Is., Russia) was the source of the prenylated indole alkaloids carneamide A–C 136–138, the quinazolinone derivatives carnequinazoline B 139 and C 140, the aryl C-glycoside carnemycin A 141 and a drimane sesquiterpenoid 142. New to the marine area was a synthetic compound152 (isolated as carnequinazoline A) and a known derivative of stromemycin153,154 (isolated as carnemycin B).155
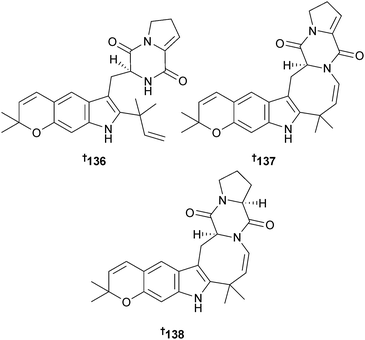
Two costaclavine alkaloids 143 and 144 were obtained from Aspergillus fumigatus (zoanthid Zoanthus sp., Amami Is., Kagoshima, Japan),156 while A. fumigatus (soft coral Sinularia sp., Kunashir Is., Kuril Islands) produced the spirocyclic diketopiperazine alkaloid spirotryprostatin F 145 which had stimulatory phytoregulatory activity.157 The same fungal strain was the source of the alkaloid fumiquinazoline K 146 and a nordammarane triterpenoid 147.158
From A. fumigatus (intertidal mud, Yingkou, China) several diketopiperazines were isolated: prenylcyclotryprostatin B 148, 20-hydroxycyclotryprostatin B 149, 9-hydroxyfumitremorgin C 150, 6-hydroxytryprostatin B 151 and spirogliotoxin 152. Prenylcyclotryprostatin B and 9-hydroxyfumitremorgin C were moderate inhibitors of human leukaemic monocyte lymphoma (U937) cells.159 20-Hydroxycyclotryprostatin B was also reported from two other sources in 2012, firstly from Aspergillus sydowii (gorgonian coral Verrucella umbraculum, Sanya, Hainan province, China) as cyclotryprostatin E160 and secondly, from a terrestrial A. fumigatus as 12β-hydroxy-13α-methoxyverruculogen TR-2.161 The indole alkaloid 153 is also noted as a natural product in the first report but no other references to it are given or can be found in extensive literature searches.160
The sesquiterpene pileotin A 154 was isolated from A. fumigatus (sea urchin Toxopneustes pileolus, source unspecified) along with oxalicine B 155, previously reported as a metabolite of Penicillium oxalicum in a PhD thesis162 but now reported in the literature. Oxalicine B displayed moderate cytotoxicity to P388 cells.163
Aspergillus insulicola (sediment, Hawaii) produced the tripeptide pre-sclerotiotide F 156164 and Aspergillus sp. (sponge Xestospongia testudinaria, Weizhou coral reef, South China Sea) the four bisabolane-type sesquiterpenoids, aspergiterpenoid A 157, (−)-sydonol 158, (−)-sydonic acid 159 and 160.165 Also isolated for the first time from a marine environment was the related (Z)-5-(hydroxymethyl)-2-(6′-methylhept-2′-en-2′-yl)phenol, a known metabolite of a fungal endophyte.166,167
An endophytic Aspergillus sp. (sponge Xestospongia testudinaria, Weizho Is., South China Sea) produced the phenolic bisabolane sesquiterpenoid dimers disydonol A–C 161–163. Only disydonols A and C were cytotoxic to HTCLs.168
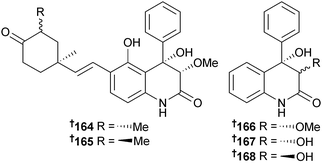
Aflaquinolones C–G 164–168 were obtained from an Aspergillus sp. (Dadaepo Beach, Busan, S. Korea); the same report also detailed the isolation of the related aflaquinolones A and B from a terrestrial Aspergillus sp.169
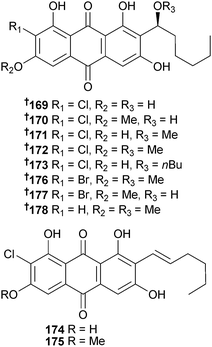
Investigation of Aspergillus sp. (sediment, South China Sea) resulted in the isolation of seven chlorinated anthraquinones 169–175. On addition of sodium bromide to the fermentation media, three additional metabolites were obtained: two brominated anthraquinones 176 and 177 and a nonhalogenated anthraquinone 178. 6-O-Methyl-7-chloroaveratin 170 inhibited growth of several HTCLs.170
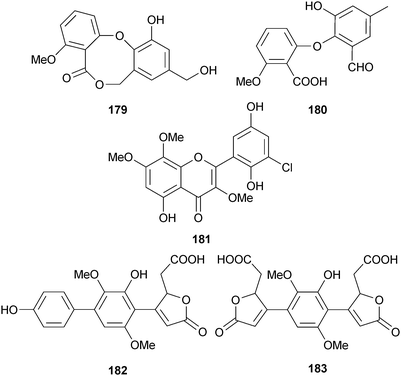
Aspergillus sp. (soil, Xiamen Beach, China) yielded barceloneic lactone B 179, barceloneic acid C 180 and 5′-hydroxychlorflavonin 181,171 in addition to two p-terphenyl derivatives, terphyl acid 182 and terphyl diacid 183.172
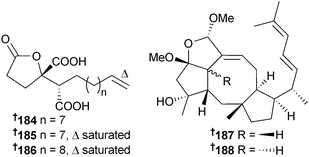
Three γ-butenolide derivatives spiculisporic acid B–D 184–186 were isolated from an endophytic Aspergillus sp. (sea urchin Anthocidaris crassispina, Qionghai, Hainan, China),173 while the sesterterpenes ophiobolin O 187 and 6-epi-ophiobolin O 188 were obtained from endophytic Aspergillus sp. (zoanthid Zoanthus sp., Ayamaru Cape, Amami Is., Japan) as moderate cytotoxins to P388 cells,174 with ophiobolin O also inducing apoptosis and cell cycle arrest of MCF-7 cells through activation of MAPK signaling pathways.175
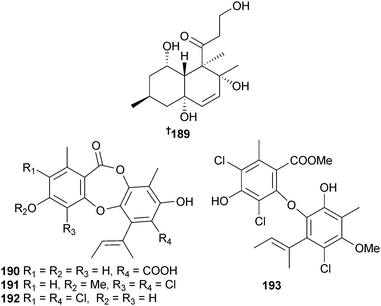
Decalin derivative decumbenone C 189 was isolated from Aspergillus sulphureus (sediment, location unspecified) as a potent cytotoxin against SK-MEL-5 human melanoma cells.176 Coincidentally, decumbenone C was also isolated from the terrestrial basidiomycete Craterellus odoratus and named craterellone C.177Aspergillus unguis (unidentified sponge, Tub-La-Mu Bay, Pang-nga, Thailand) was the source of the depsidones aspergillusidone A–C 190–192, a diaryl ether 193 and yielded the known synthetic intermediate 4-hydroxy-3-methyl-6-(1-methyl-1-propenyl)-2H-pyran-2-one178 (first isolation from a natural source). The fungal metabolites, nidulin,179 nornidulin180 and 2-chlorounguinol181 were also isolated, with nidulin and 2-chlorounguinol being first time MNP isolates. Aspergillusidones A–C, nidulin, nornidulin and 2-chlorounguinol all exhibited aromatase inhibition and aspergillusidones A and C showed radical scavenging activity in the xanthine/xanthine oxidase assay.182
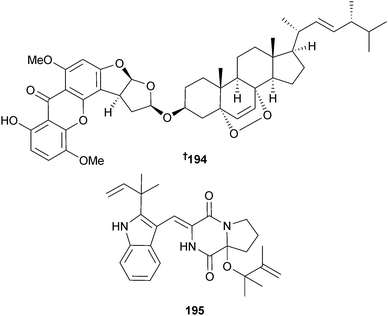
Asperversin A 194 and 9ξ-O-2(2,3-dimethylbut-3-enyl)brevianamide Q 195 were isolated from endophytic Aspergillus versicolor (brown alga Sargassum thunbergii, Pingtan Is., China), along with the known fungal metabolites brevianamide M,183 6,8-di-O-methylaverufin184 and 6-O-methylaverufin.185 Both brevianamide M183 and 6-O-methylaverufin185 were first time marine isolates.186
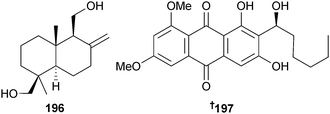
Endophytic A. versicolor (green alga Codium fragile, Dalian, China) produced the sesquiterpene albican-11,14-diol 196, potently toxic to brine shrimp and a strong inhibitor of E. coli and S. aureus.187 From another endophytic A. versicolor (brown alga Sargassum thunbergii, Qingdao, Shandong, China) the anthraquinone compound 6,8-di-O-methylaverantin 197 was obtained188 together with the known anthraquinones 6,8-di-O-methylversiconol189 and 6,8-di-O-methylnidurufin.190
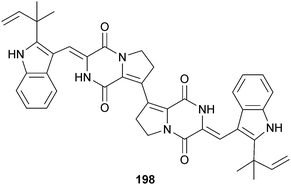
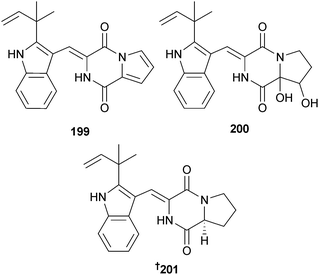
A. versicolor (sediment, Bohai Sea, China) was the source of the dimeric diketopiperazine brevianamide S 198 and the monomeric brevianamides T–V 199–201. Brevianamide S showed selective activity against the Bacille Calmette-Guérin (BCG) strain of Mycobacterium bovis suggestive of a new mechanism of action with potential as an antitubercular drug lead.191
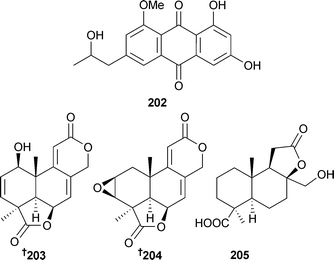
The anthraquinone isorhodoptilometrin-1-methyl ether 202 was isolated from endophytic A. versicolor (green alga Halimeda opuntia, Rass Mohamed, south Sinai, Egypt) and displayed moderate activity against B. subtilis, B. cereus and S. aureus.192 Examination of the endophytic A. wentii EN-48 (brown alga Sargassum sp., source unspecified) revealed three new tetranorlabdane diterpenoids asperolide A–C 203–205193 in addition to the terrestrial fungal metabolites, wentilactones A and B,194 LL-Z1271-β,195 and a known tetranorditerpenoid derivative.196 All of these known metabolites were first time marine isolates.193
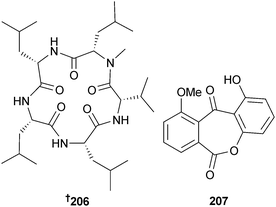
Application of OSMAC (one strain-many compounds) methodology to Asteromyces cruciatus (unidentified decaying green alga, La Jolla, USA) identified the pentapeptide lajollamide A 206 (absolute configuration determined by total synthesis).197Beauveria bassiana (unidentified sponge, Iriomote Is., Okinawa) produced 1-hydroxy-10-methoxy-dibenz[b,e]oxepin-6,11-dione 207,198 in addition to the known terrestrial metabolites chrysazin199 and globosuxanthone A,200 both first time marine isolates.
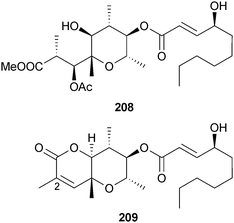
Botryotinia sp. (unidentified marine alga, Seongsan, Cheju, S. Korea) was the source of two botcinin derivatives 208 and 209, which were assumed to be methanol reaction products rather than natural products.201 The compound 209 was named botcinin G but this name had already been utilised for a metabolite of Botrytis cinerea202 and again for a different metabolite of B. cinerea203,204 which has a structure comparable to 209 but lacking the C-2–C-3 double bond. There is confusion in the chemical literature as one CAS Registry number appears to have been used for multiple structures.201–204
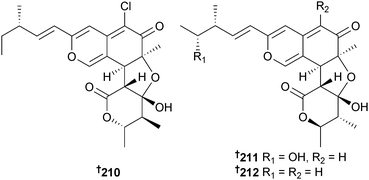
A strain of Chaetomium globosum (fish Mugil cephalus, Katsuura Bay, Japan), which has already yielded many chaetomugilin metabolites,205–208 was the source of three further analogues: chaetomugilins S 210, T 211 and U 212. Chaetomugilin S was a moderate growth inhibitor of HTCLs and P388 cells.209
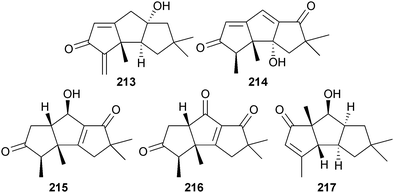
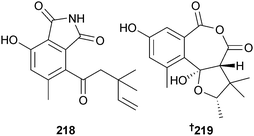
Chondrostereum sp. (soft coral Sarcophyton tortuosum, Hainan, South China Sea) yielded triquinane-type sesquiterpenoids chondrosterin A–E 213–217, of which chondrosterin A was cytotoxic to several cancer cell lines.210,211 Endophytic Coniothyrium cereale (green alga Enteromorpha sp., Fehmarn, Baltic Sea) produced the isoindole pseudoalkaloid conioimide 218 and the polyketide cereoanhydride 219. Conioimide exhibited selective inhibition of human leukocyte elastase. Biosynthetic feeding experiments with 13C-labelled acetate proved that the major and known C. cereale metabolite (−)-trypethelone212 was polyketide-derived and it is proposed as the precursor of cereoanhydride.213
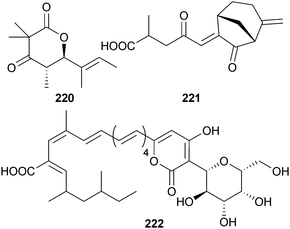
The lactone helicascolide C 220 was isolated from endophytic Daldinia eschscholzii (red alga Gracilaria sp., South Sulawesi coast, Indonesia) and was fungistatic against the phytopathogenic fungus Cladosporium cucumerinum.214 Both helicascolide C and helicascolide A,215 previously isolated from the fungus Helicascus kanaloanus, have been synthesised by acid catalysed acetonide deprotection, followed by a one-pot intramolecular lactonisation as the key step.216 The endophyte Emericellopsis minima (sponge Hyrtios erecta, Similan Islands, Thailand) was the source of the sesquiterpene 221,217 while an Epicoccum sp. (sponge Callyspongia sp., Sanya, China) provided the pyronepolyene C-glucoside iso-D8646-2-6 222 along with the known isomer D8646-2-6.218 Both pyrones significantly inhibited growth of the H1N1 virus along with weak NF-κB inhibition.219
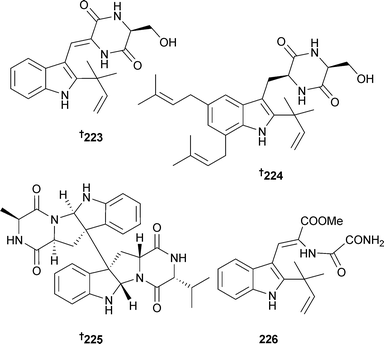
Endophytic Eurotium cristatum (brown alga Sargassum thunbergii, location unspecified) was the source of the indole alkaloids cristatumin A–D 223–226, of which cristatumin A and the co-isolated known fungal metabolite tardioxopiperazine A220 were active against E. coli and S. aureus, respectively, while cristatumin B and the co-isolated known fungal metabolite isoechinulin A221 were moderately toxic to brine shrimp.222
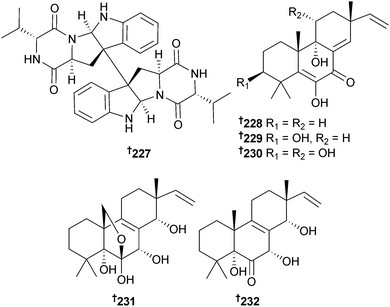
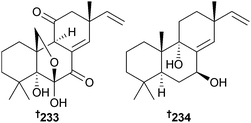
The diketopiperazine dimer eurocristatine 227 was isolated from endophytic Eurotium cristatum (sponge Mycale sp., Wonnapa Beach, Bangsaen, Thailand),223,224 while scopararanes C–G 228–232 are oxygenated pimarane diterpenes obtained from Eutypella scoparia (sediment, South China Sea). Scopararane C was moderately cytotoxic to MCF-7 cells, while scopararane D was moderately cytotoxic to both SF-268 and MCF-7 cells225 and was concurrently reported from another marine fungal species. This was Epicoccum sp. (sea cucumber Apostichopus japonicus, Yantai City, Shandong, China) and scopararane D was reported as a moderate cytotoxin, along with two further pimarane diterpenes, 233 and 234, of which 233 was strongly cytotoxic to KB and KBv200 cells.226
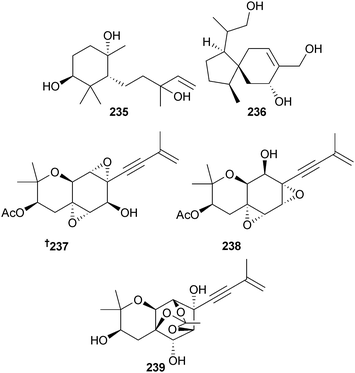
A monocyclofarnesane sesquiterpene 235 and an acorane sesquiterpene 236 were obtained from Eutypella scoparia (sediment, South China Sea),227 while Isaria felina (sediment, South China Sea, Vietnam) was the source of the chromene derivatives oxirapentyn B–D 237–239, in addition to oxirapentyn A, previously obtained from the terrestrial fungus Beauveria felina.228 This is the first isolation from a marine source.229
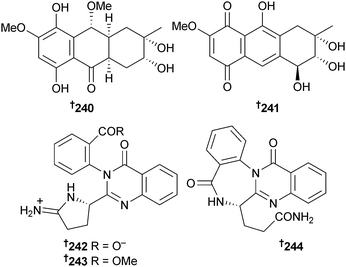
Two hydroanthraquinone analogues 4a-epi-9α-methoxydihydrodeoxybostrycin 240 and 10-deoxybostrycin 241 were isolated from endophytic Nigrospora sp. (unidentified sea anemone, Weizhou, South China Sea). 10-Deoxybostrycin was strongly active against B. cereus and A549 cells.230 An OSMAC approach to cultivation of Penicillium aurantiogriseum (mud, Bohai Sea, China) resulted in production of the auranomides A–C 242–244.231
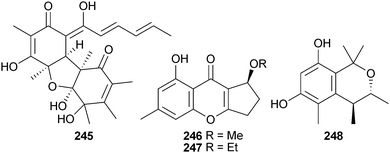
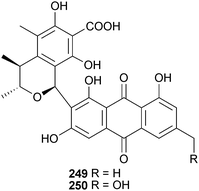
P. citrinum (unidentified sponge, Ishigaki Is., Okinawa, Japan) was the source of JBIR-124 245, a DPPH radical scavenger,232 while P. citrinum (gorgonian sea fan Annella sp., Similan Islands, Thailand) yielded several polyketides including the benzopyranones coniochaetone C 246 and D 247, an isochroman 248 and the anthraquinone-citrinin derivatives penicillanthranin A 249 and B 250. Penicillanthranin A exhibited moderate activity against S. aureus and MRSA.233
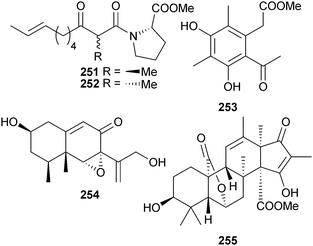
P. citrinum (sediment, Lanqi Is., Fujian, China) provided the epimeric tumonoic acids K 251 and L 252 along with methyl 2-(2-acetyl-3,5-dihydroxy-4,6-dimethylphenyl)acetate 253,234 while isophomenone 254 and 3-deacetylcitreohybridonol 255 were obtained from P. commune (sediment, South China Sea).235
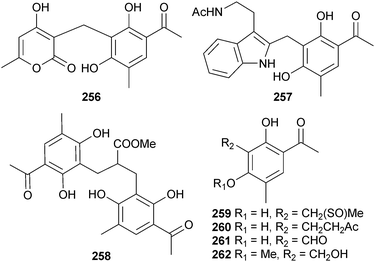
Communols A–G 256–262 are aromatic polyketides isolated from P. commune (gorgonian Muricella abnormalis, Danzhou, Hainan, China), of which communols A, F and G were moderately active against E. coli and Enterobacter aerogenes (E. aerogenes).236,237
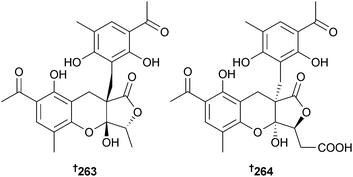
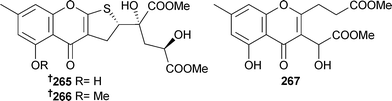
Penilactones A 263 and B 264 are highly oxygenated polyketides from P. crustosum (deep sea sediment, Prydz Bay, Antarctica).238 The dihydrothiophene-condensed chromones oxalicumone A 265 and B 266 and the chromone, oxalicumone C,239 were isolated from P. oxalicum (gorgonian Dichotella gemmacea, Sanya, South China Sea, China). Oxalicumone C 267 has previously been reported as a reaction product of chloromonilicum, a metabolite of the cherry rot fungus Monilinia fructicola,239 but this is the first notification as a natural product.240
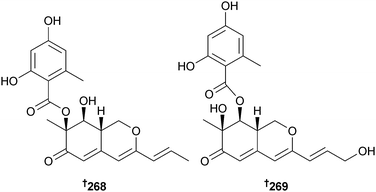
Pinophilins A 268 and B 269, hydrogenated azaphilones from P. pinophilum (seaweed Ulva fasciata, Kasai, Tokyo, Japan), along with the co-isolated metabolite Sch 725680,241 selectively inhibited the activities of mammalian DNA polymerases (pols), A (pol γ), B (pols α, δ, and ε), and Y (pols η, ι, and κ) families and the growth and proliferation of several HTCLs.242 Pinophilin A was simultaneously isolated as berkazophilone B from an extremophile P. rubrum (from an abandoned acidic, metal-sulfate contaminated open-pit copper mine) as a selective inhibitor of leukaemia cell lines.243 The absolute configuration was determined by total synthesis.244
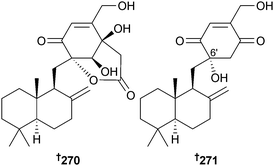
Random diethyl sulfate mutagenesis of P. purpurogenum (sediment, Bohai Bay, Tianjin, China) gave a mutant producing the drimenyl cyclohexenone derivatives purpurogemutantin 270 and purpurogemutantidin 271.245 From another Penicillium strain (deep sea sediment, northern South China Sea) penicilliumin A was isolated and determined to have the same structure as purpurogemutantidin but no assignment of configuration at C-6′ was made. Differences in optical rotations between the two compounds suggest they may be diastereoisomers.246
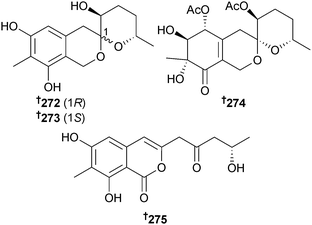
Peneciraistins A–D 272–275 were isolated from P. raistrickii (saline soil, Bohai Bay, Shandong, China). Peneciraistin C was moderately cytotoxic to A549 and MCF-7 cells whilst peneciraistins A, B and D exhibited radical scavenging activities against DPPH.247 Two further compounds were isolated and named peneciraistins E and F, with structures assigned as 3-indoleformic acid derivatives, however, these structures have subsequently been corrected to contain six-membered N-containing rings248 both of which are known compounds.249,250
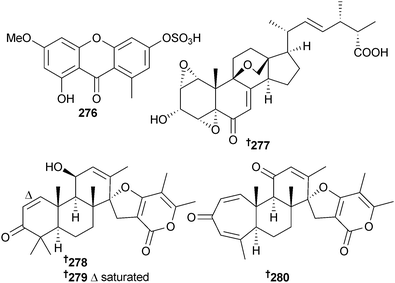
1-Hydroxy-3-methoxy-6-sulfo-8-methylxanthone 276 was isolated from P. sacculum (saltbush Atriplex sp., Dongying city, Shandong, China),251 whilst Penicillium sp. (deep sea sediment, East Pacific Ocean) provided the polyoxygenated sterol, sterolic acid 277 and the spiroditerpenoids brevione I–K 278–280. Brevione J and the known terrestrial fungal allelochemical brevione A252 (obtained here for the first time from a marine source) were cytotoxic (MCF-7 cells).253
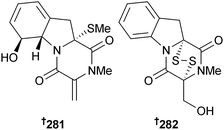
Penicillium sp. (deep sea sediment, Suruga Bay, Japan) yielded several gliotoxin-related compounds including 281 and 282. Of the other known compounds, bisdethiobis(methylthio)gliotoxin,254 5a,6-didehydrogliotoxin,255 gliotoxin256 and gliotoxin G257 inhibited histone methyltransferase (HMT), while 282 and gliotoxin G were cytotoxic to P388 cells.258
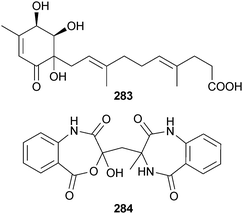
Penicillone A 283 and penicillactam 284 were isolated from a marine Penicillium sp. (source not given).259 A further compound, ethyl 2-(2,6-dihydroxybenzoyl)-3-hydroxy-5-(hydroxymethyl)benzoate,260 was isolated and although commercially available this was the first reported isolation as a natural product.259
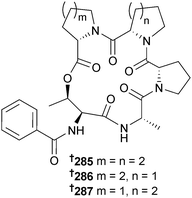
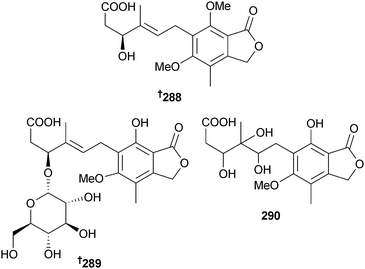
The depsipeptides JBIR-113, JBIR-114 and JBIR-115 285–287 were obtained from Penicillium sp. (unidentified marine sponge, Takarajima Is., Kagoshima, Japan)261 and another Penicillium sp. (sediment, South China Sea) was the source of the mycophenolic acid derivatives penicacid A–C 288–290 and the known terrestrial fungal metabolite 4′-hydroxy-mycophenolic acid,262 obtained for the first time from a marine source. All of the isolated metabolites inhibited inosine-monophosphate dehydrogenase (IMPDH) to varying degrees and penicacid A and 4′-hydroxy-mycophenolic acid also displayed immunosuppressive activity.263
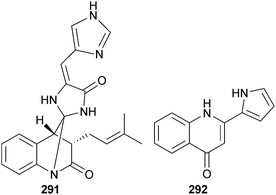
A halotolerant Penicillium sp. (source unspecified) yielded an alkaloid with a unique spiro-imidazolidinyl skeleton, penispirolloid A 291, that exhibited antifouling activity toward Bugula neritina larvae264 and Penicillium sp. (sediment, Jiaozhou Bay, China) was the source of the pyrrolyl 4-quinolinone alkaloid penicinoline E 292.265
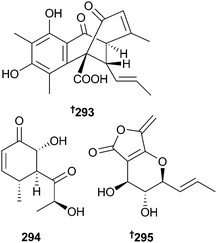
A sorbicillinoid derivative sorbiterrin A 293 with moderate acetylcholinesterase (AChE) inhibition, was obtained from P. terrestre (sediment, Jiaozhou Bay, China)266 and arthropsadiol C 294 and massarilactone H 295 are polyketides obtained from Phoma herbarum (sediment, Yellow Sea, China). Massarilactone H and the co-isolated known fungal metabolites massarilactone G,267 massarigenin C268 and enalin A269 displayed moderate neuraminidase inhibition. This was the first reported marine isolation of massarigenin C.270
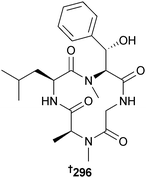
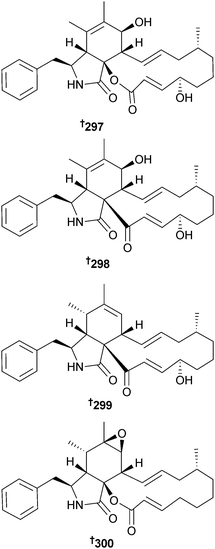
An endophytic Phoma sp. (giant jellyfish Nemopilema nomurai, southern coast, S. Korea) provided a cyclic tetrapeptide 296 which was a weak suppressor of NO production in RAW264.7 cells without notable cytotoxicity.271 The same strain of fungus also provided four cytochalasin derivatives: cytochalasin B2 297, deoxaphomin B 298, deoxaphomin C 299 and 20-deoxycytochalasin F 300. Cytochalasin B2 and deoxaphomin C were cytotoxic to several HTCLs.272,273
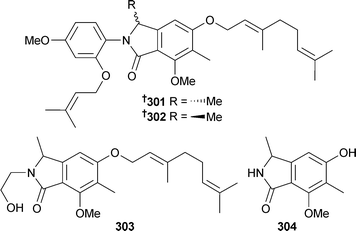
Stachylidium sp. (sponge Callyspongia cf. flammea, location unspecified) yielded phthalimidine derivatives, the enantiomeric marilines A1 301 and A2 302 and marilines B 303 and C 304. The various marilines were active in a wide range of bioassays.274
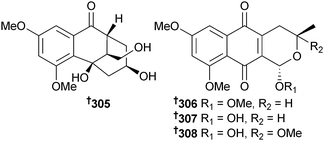
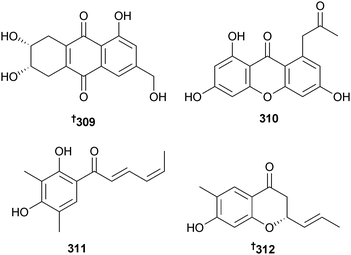
Torula herbarum (sea hare Notarchus leachii, Beihai, Guangxi, China) was the source of the heptaketide herbarone 305 and the ent-astropaquinones B 306 and C 307. O-Methylherbarin, a metabolite previously reported from T. herbarum,275 was also obtained and the absolute configuration determined as 308.276
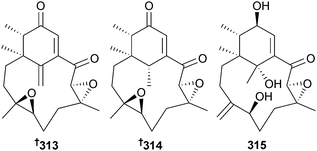
Trichoderma aureoviride (gorgonian sea fan Annella sp., Similan Islands, Thailand) provided trichodermaquinone 309 and trichodermaxanthone 310.277 An endophytic Trichoderma sp. (sea star Acanthaster planci, Hainan Sanya National Coral Reef Reserve, China) produced two sorbicillinoid analogues (4′Z)-sorbicillin 311 and 312 (moderately cytotoxic to several HTCLs),278 while three diterpenes phomactin K–L 313–315 were obtained from an unidentified fungus (brown alga Ishige okamurae, Tateishi, Kanagawa, Japan).279
3.4 Fungi from mangroves
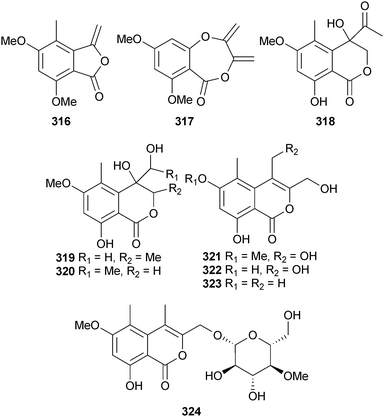
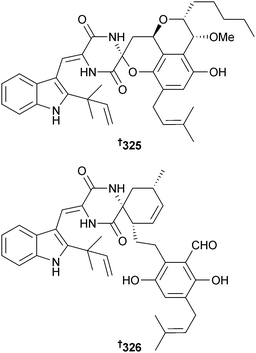
An endophytic Acremonium sp. (mangrove branch Rhizophora apiculata, Satun, Thailand) provided the phthalide derivative, acremonide 316 and the isocoumarin derivatives acremonone A–H 317–324.280The racemic spiroalkaloids effusin A and dihydrocryptoechinulin D (shown here as one of the enantiomers 325 and 326, respectively) were obtained from Aspergillus effusus (mangrove rhizosphere soil, Fujian, China). The racemates were subsequently each resolved and absolute configurations determined by solution time dependent density function theory (TDDFT) electronic CD (ECD) calculations. The racemate of dihydrocryptoechinulin D inhibited growth of P388 cells and the (12R,28S,31S)-enantiomer 326 showed selective, moderate inhibition of topoisomerase I.281
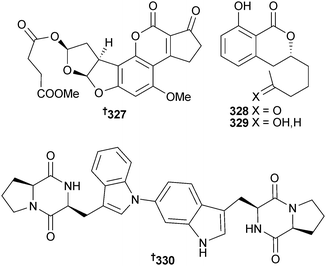
Aflatoxin B2b 327 was obtained from endogenous A. flavus (mangrove root Hibiscus tiliaceus, Wenchang, Hainan, China) and was moderately active against E. coli, B. subtilis and E. aerogenes.282 The dihydroisocoumarin derivatives aspergillumarin A 328 and B 329 were isolated from Aspergillus sp. (mangrove leaf Bruguiera gymnorrhiza, South China Sea),283 while aspergilazine A 330, a diketopiperazine dimer consisting of two diketopiperazine units with a rare N-1 to C-6 linkage, was obtained from A. taichungensis (mangrove root soil, Acrostichum aureum, source not given).284
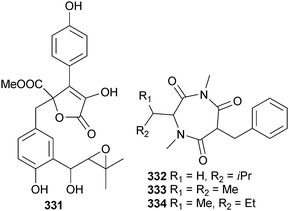
The butyrolactone 7′′-hydroxybutyrolactone III 331 and terretriones A–C 332–334 were isolated from A. terreus (sediment, unnamed mangrove, Guangxi Zhuang, China).285 Along with three known terrestrial fungal metabolites, verticillin D286 and pullularins A and C287 (isolated from a marine source for the first time), the endophytic Bionectria ochroleuca (mangrove leaf Sonneratia caseolaris, Hainan Is., China) produced the peptides pullularin E 335 and F 336. Pullularin E was characterised as the chloro-derivative 337 that it had rapidly converted to.288
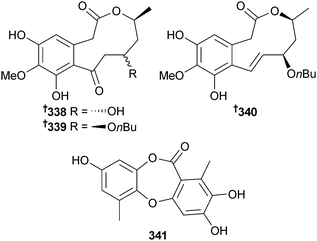
Corynespora cassiicola (mangrove leaf Laguncularia racemosa, Hainan Is., China) yielded xestodecalactones D–F 338–340 and corynesidone C 341 as well as the known terrestrial fungal metabolites corynesidone B289 and 6-(3-hydroxybutyl)-7-O-methylspinochrome B,290 both isolated for the first time as MNPs.291
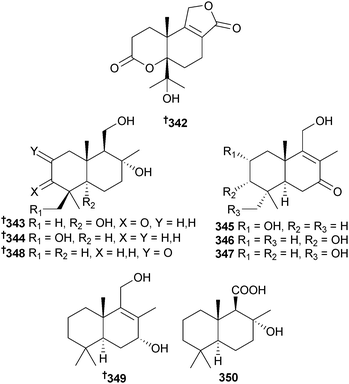
An endophytic Diaporthe sp. (mangrove leaves Rhizophora stylosa, Hainan Is., China) was the source of the sesquiterpenoid diaporol A 342 and drimane sesquiterpenoids diaporol B 343, C 344 and F–H 345–347.292 Three synthetic sesquiterpenoids diaporol D 348,293 E 349294 and I 350295 were isolated for the first time as natural products.292
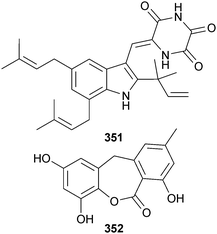
An endophytic strain of Eurotium rubrum, (semi-mangrove Hibiscus tiliaceus, Hainan Is., China) produced a diketopiperazine alkaloid 12-demethyl-12-oxo-eurotechinulin B 351 and an anthraquinone derivative 9-dehydroxyeurotinone 352,296 in addition to the known fungal metabolites variecolorin G297 and alkaloid E-7.297,298
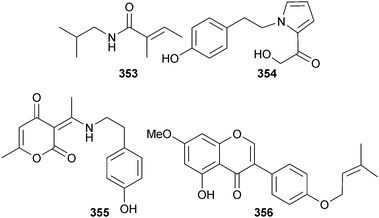
The alkaloids N-2-methylpropyl-2-methylbutenamide 353, fusarine 354 and fusamine 355 were discovered from endophytic Fusarium incarnatum (mangrove fruit Aegiceras corniculatum, Xiamen, Fujian, China)299 along with the known synthetic compounds, 2-acetyl-1,2,3,4-tetrahydro-β-carboline300 and 3-(1-aminoethylidene)-6-methyl-2H-pyran-2,4(3H)-dione,301 reported as first time MNPs. Fusarine was concurrently isolated from the Chinese plant Alhagi sparsifolia.302 The isoflavone derivative 356 was obtained from an endophytic Fusarium sp. (mangrove leaves Kandelia candel, Dong Zai, Hainan, China).303
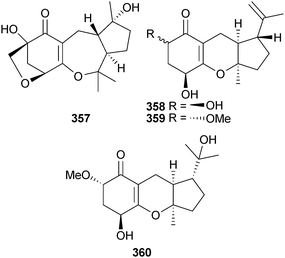
Endophytic Guignardia sp. (mangrove leaves Scyphiphora hydrophyllacea, Wenchang, Hainan, China) was the source of the meroterpenes guignardone F–I 357–360.304 It should be noted that the planar structure of guignardone G was the same as that of coibanol A, recently obtained from a plant endophyte, Pycnoporus sanguineus from Panama.305 The co-isolated guignardone B306 was obtained from a marine source for the first time.
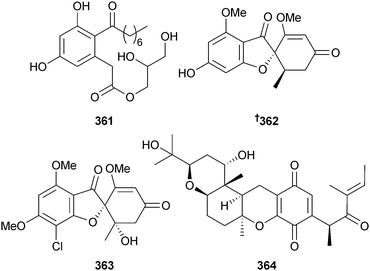
Use of epigenetic modifiers to activate secondary metabolite genes in Leucostoma persoonii (mangrove branch Rhizopora mangle, Florida Everglades) induced production of a new cytosporone analogue cytosporone R 361, in addition to known cytosporones. Of the isolated compounds, cytosporone E,307 a first time MNP, inhibited the malaria parasite P. falciparum and was moderately inhibitory towards MRSA.308 Endophytic Nigrospora sp. (semi-mangrove stem Pongamia pinnata, Guangxi Zhuang, China) yielded griseofulvin derivatives 362 and 363, 2,3-didehydro-19α-hydroxy-14-epicochlioquinone B 364, in addition to griseophenone C309 (obtained for the first time from a marine source).310
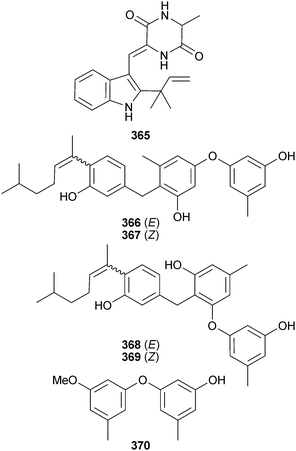
The diketopiperazine 365 (named chrysogenazine in an earlier patent application by the same authors),311 was obtained from endophytic Penicillium chrysogenum (mangrove leaves Porteresia coarctata, Chorao Is., Goa, India) and displayed antibacterial activity comparable to that of streptomycin.312 Endophytic P. expansum (mangrove Excoecaria agallocha, source unspecified) was the source of the polyphenols expansol C–F 366–369 and a new diphenyl ether derivative 3-O-methyldiorcinol 370.313
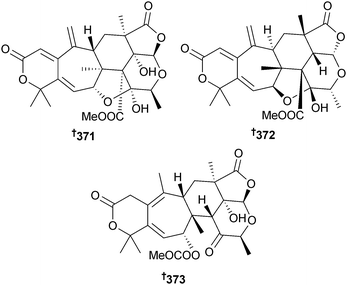
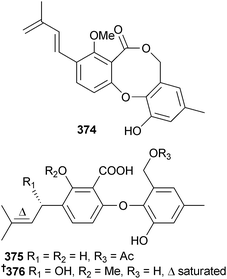
A Penicillium sp. (mangrove rhizospheric soil Bruguiera gymnorrhiza, Hainan Is., China) produced meroterpenoid derivatives 4,25-dehydrominiolutelide B 371, 4,25-dehydro-22-deoxyminiolutelide B 372 and isominiolutelide A 373 from static culture and diphenyl ether derivatives Δ1′3′-1′-dehydroxypenicillide 374, 7-O-acetylsecopenicillide C 375 and hydroxytenellic acid B 376 from shaken culture.314 A number of known compounds were also isolated from the shaken culture: penicillide,315 secopenicillide C,316 dehydroisopenicillide317 and 3′-O-methyldehydroisopenicillide318 (all obtained from a marine source for the first time).
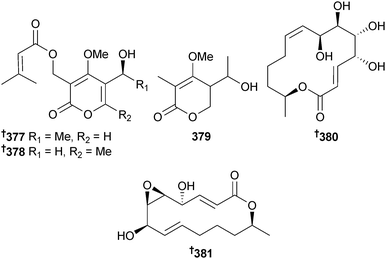
The α-pyrones pestalotiopyrone A–C 377–379 were isolated from Pestalotiopsis sp. (mangrove twigs Rhizophora apiculata, Trang province, Thailand) and the seiricuprolides pestalotioprolide A 380 and B 381 (isolated as the diacetate) were obtained from Pestalotiopsis sp. (mangrove twigs Rhizophora mucronata, Satun province, Thailand).319
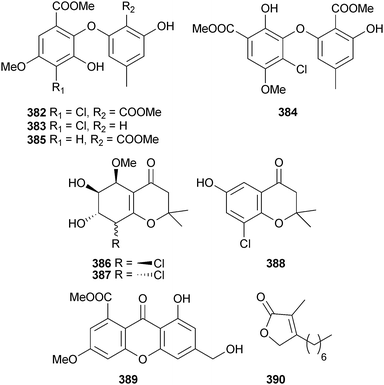
An endophytic Pestalotiopsis sp. (mangrove branch Rhizopora apiculata, Satun, Thailand) provided the diphenyl ether derivatives pestalotether A–D 382–385, the chromones pestalochromone A–C 386–388, pestaloxanthone 389 and the butenolide pestalolide 390 (modestly active against C. albicans and Cryptococcus neoformans).320
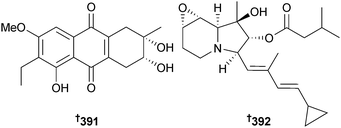
The tetrahydroanthraquinone derivative 391 was isolated from Phomopsis sp. (mangrove leaf Rhizopora apiculata, Songkhla, Thailand) and possessed weak cytotoxicity against MCF-7 cells in addition to antibacterial activity against S. aureus and MRSA.321Saccharopolyspora sp. (mangrove soil, Ishigaki Is., Japan) yielded the cyclizidine analogue JBIR-102 392, along with cyclizidine322 itself (first time from a marine source). Both were cytotoxic to human malignant pleural mesothelioma (MPM) ACC-MESO-1 cells.323
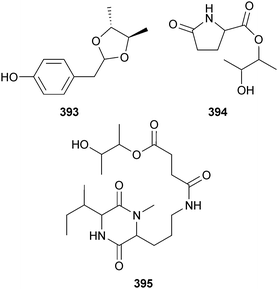
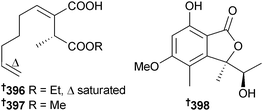
Trichoderma atroviride (mangrove root soil Ceriops tagal, South Sea, China) yielded three new metabolites 393, 394 and atroviridetide 395.324 The succinic acid derivatives xylacinic acid A 396 and B 397 were isolated from Xylaria cubensis (mangrove branch Bruguiera parviflora, Suratthani, Thailand),325 while Xylaria sp. (mangrove leaf Acanthus ilicifolius, Yangjiang, China) produced the lactone 398 in addition to (S)-8-hydroxy-6-methoxy-4,5-dimethyl-3-methylene-isochromen-1-one, which although claimed as new, was previously isolated from a terrestrial Leptosphaeria sp.326 The current isolation is however the first from the marine environment.327
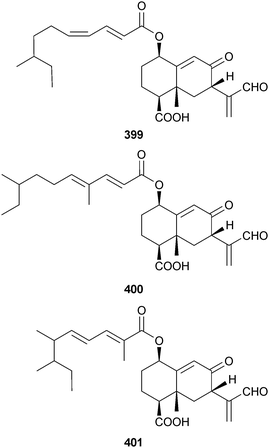
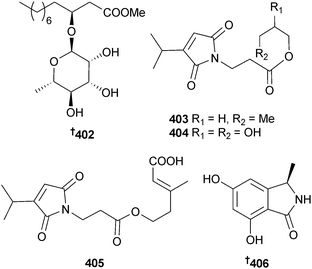
An endophytic Xylaria sp. (unidentified mangrove, South China Sea coast) was the source of the eremophilane sesquiterpenes 399–401328 and the known fungal metabolite 07H239-A.329 A fatty acid glucoside 402 with moderate inhibitory properties against S. aureus and MRSA was obtained from an unidentified endophytic fungus (mangrove leaves Scyphiphora hydrophyllacea, Wenchang, Hainan, China),330 while investigation of an unidentified endophytic fungus (mangrove leaves Avicennia marina, Oman) yielded farinomaleins C–E 403–405 and an isoindoline congener 406,331 in addition to the known farinomalein B.332
3.5 Cyanobacteria
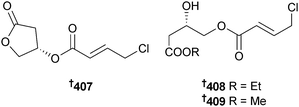
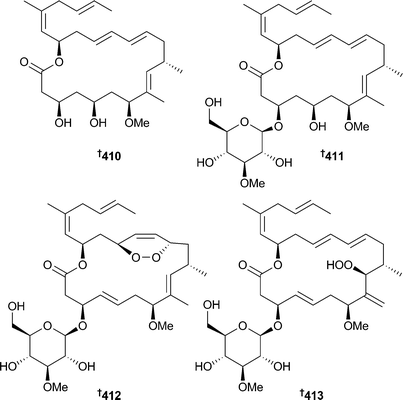
Cyanobacteria continue to be a valuable source of new compounds, although the number of new metabolites isolated in the past year is somewhat less than in 2011. Leptolyngbya crossbyana (Hōnaunau reef, Hawaii) was the source of honaucins A–C 407–409, chlorinated metabolites that were potent inhibitors of bacterial quorum-sensing (inhibited bioluminescence in Vibrio harveyi) and inhibited NO production and expression of several pro-inflammatory cytokines in RAW264.7 cells. Honaucin A was synthesised, along with some analogues, to determine the structural features required for activity and some of the analogues had improved potency in the assays.333Investigation of Lyngbya sp., (Tokunoshima Is., Japan) identified the macrolide biselyngbyolide A 410, which had potent apoptosis-inducing activity against HeLa S3 and HL-60 cells,334 while biselyngbyasides B–D 411–413, analogues of biselyngbyaside335 and biselyngbyolide A334 were obtained from another Lyngbya sp. (Tokunoshima Is., Japan). Biselyngbyaside B inhibited growth and induced apoptosis in HeLa S3 and HL-60 cells with increased cytosolic Ca2+ concentration in the HeLa S3 cells.336
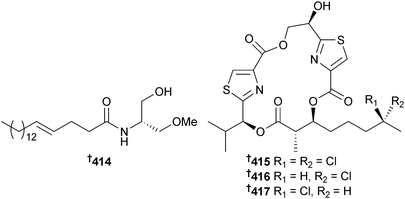
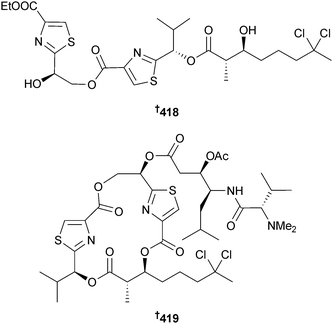
Serinolamide B 414, a fatty acid amide obtained from a Lyngbya sp. (Piti Bomb Holes, Guam) along with malyngamide B337 are cannabinomimetics, decreasing forskolin-induced cAMP accumulation.338 The lipopeptides lyngbyabellin K 415 and L 416, 7-epi-lyngbyabellin L 417 and lyngbyabellin M 418 and N 419 were isolated from Moorea bouillonii (Strawn Is., Palmyra Atoll, Central Pacific Ocean). The possibility that the linear lyngbyabellin M was an artefact of isolation could not be discounted. Lyngbyabellin N, which contains the unusual N,N-dimethylvaline terminus, was strongly cytotoxic to HCT116 cells.339
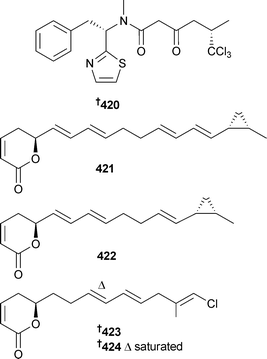
The biosynthetic gene cluster for the chlorinated molluscicide barbamide,340 obtained from the marine species Moorea producens, was heterologously expressed in the terrestrial actinobacterium Streptomyces venezuelae and resulted in the production of a new barbamide analogue 4-O-demethylbarbamide 420, which also functioned as a potent molluscicide against the marine snail Biomphalaria glabrata.341 An Oscillatoria sp. (Coiba National Park, Panama) was the source of the unsaturated polyketide lactone derivatives coibacin A–D 421–424 which had selective antileishmanial activity and potent anti-inflammatory activity.342
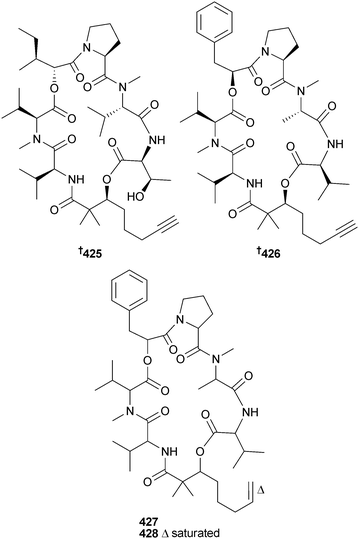
The viequeamides, 2,2-dimethyl-3-hydroxy-7-octynoic acid (Dhoya)-containing cyclic depsipeptides, were obtained from the “button” cyanobacterium Rivularia sp. (Playa de la Chiva, Vieques Is., Puerto Rico). Of these, viequeamide A 425 was highly toxic to H460 human lung cancer cells. Viequeamides B–F could not be separated but the absolute configuration of the major component of the mixture, viequeamide B 426 was determined, along with planar structures for viequeamides C 427 and D 428.343
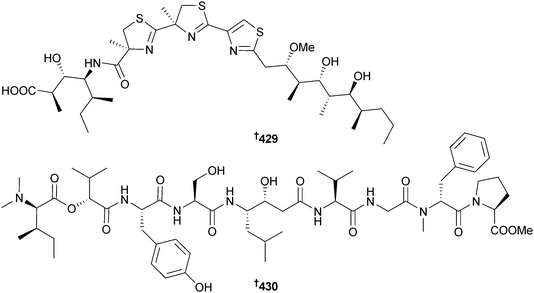
Hoiamide D 429, with two consecutive thiazolines and a thiazole as well as a modified isoleucine residue, was obtained independently in both its carboxylic and conjugate base forms from two Symploca sp. (Kolaio Is., Papua New Guinea; Kape Point, Papua New Guinea), respectively. The carboxylate anion inhibited p53/MDM2 protein binding.344 Symplocin A 430, an N,N-dimethyl-terminated peptide, was isolated from Symploca sp. (San Salvador Is., Bahamas) as a potent inhibitor of the protease enzyme cathepsin E. Determination of the absolute configuration of the terminal N,N-dimethylisoleucine and valic acid residues employed a new methodology; chiral-phase HPLC assignment of the corresponding 2-naphthacyl esters, a procedure that may be generally applicable to other N-terminal blocked peptides.345
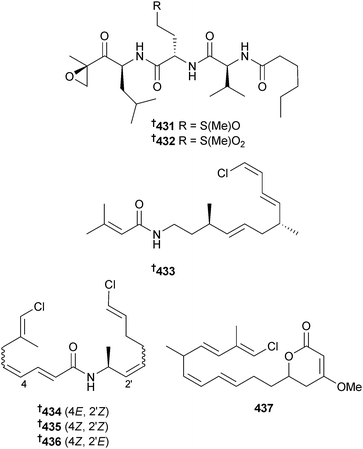
A collection of Symploca sp. (CARMABI, Curaçao) was the source of carmaphycins A 431 and B 432, peptidic proteasome inhibitors containing a leucine-derived α,β-epoxyketone connected respectively to methionine sulfoxide or methionine sulfone. Covergent and flexible total syntheses of each were achieved. Both carmaphycins A and B strongly inhibited the β5 subunit (chymotrypsin-like activity) of the S. cerevisiae 20S proteasome and displayed strong cytotoxicity to lung and colon cancer cells, as well as potent antiproliferative effects to HTCLs.346 Collections of cyanobacteria from Curaçao and Papua New Guinea each yielded metabolites containing a chlorovinyl group. The lipoamide janthielamide A 433 was obtained from “tropical marine Symploca” (Jan Thiel Bay, Curaçao) whilst further lipoamides kimbeamide A–C 434–436 and a ketide-extended pyranone kimbelactone A 437 were isolated from a consortium of “tropical marine Symploca” and Moorea producens (Kime Bay, New Britain, Papua New Guinea). Of these compounds, janthielamide A and kimbeamide A displayed moderate sodium channel blocking activity in murine Neuro-2a cells and janthielamide A was also an antagonist of veratridine-induced sodium influx in murine cerebrocortical neurons.347
3.6 Dinoflagellates
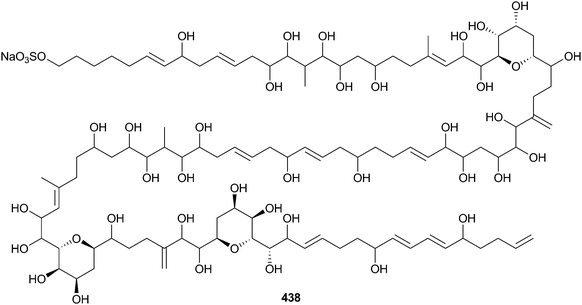
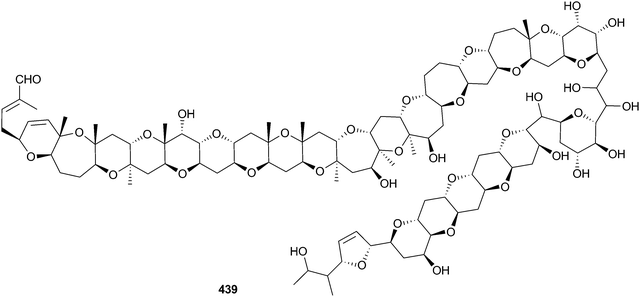
Amphidinium sp. (red alga Digenea simplex, Okinawa, Japan) was the source of the polyol amdigenol A 438,348 while the polyether brevisulcenal F 439 was obtained from Karenia brevisulcata (Wellington, New Zealand)349 and exhibited mouse lethality and toxicity to P388 cells.350The structure of ovatoxin-a 440, the major toxin of benthic Ostreopsis ovata (seawater, Adriatic and Tyrrhenian coasts, Italy), reported in a preliminary communication,351 has now been fully characterised using NMR-based analysis.352 Preliminary in vivo assessment of the activity of ovatoxin-a in mice indicated that it was lethal over a very short time period and also caused limb paralysis.353 A new palytoxin congener, ovatoxin-f was detected in a North Western Adriatic strain of Ostreopsis cf. ovata (Portonovo, Italy) but was not fully characterised.354
3.7 Ciliates
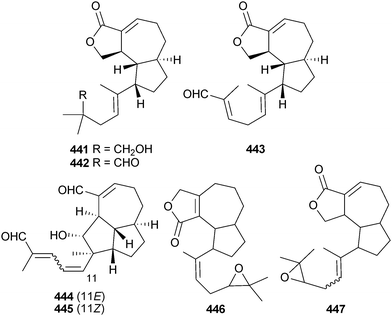
A number of diterpenes have been obtained from various strains of the ciliate Euplotes rariseta. One strain (Omaha Bay, New Zealand) provided the irregular diterpenoids omaholidenol 441 and omaholidenal 442, while ubatubaolidenal 443, ubatubadial A 444 and ubatubadial B 445 were obtained from a different strain (Ubatuba, Brazil). Euplotes quinquecarinatus (Mughsayl, Oman) yielded epoxyfocardolide 446 and Euplotes parkei (Margarita Is., Venezuela) the prenyl epoxyrarisetenolide 447.3553.8 Synthetic aspects
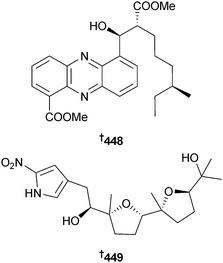
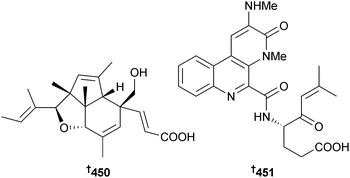
Asymmetric synthesis of streptophenazine G356 has been achieved by alkylation and aldol reactions using chiral oxazolidinones as the key steps and resulted in revision of the published structure to 448,357 consistent with the structural revision of streptophenazine A reported previously via synthesis.358 (−)-Heronapyrrole C, a farnesylated 2-nitropyrrole from an Australian Streptomyces sp.,359 has been synthesised in eight steps from commercially available starting materials and is suggested to be the enantiomer of the natural product. The absolute configuration of naturally occurring heronapyrrole C is proposed as 449.360Total synthesis of the tricyclic polypropionate indoxamycin B, originally isolated from a marine-derived actinomycete,361 has resulted in a stereochemical reassignment of the natural product to 450.362 A benzonaphthyridine alkaloid originally isolated from mangrove sediment derived Streptomyces albogriseolus363 was synthesised and the absolute configuration determined as 451.364
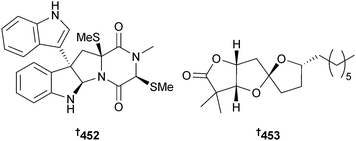
A synthesis of apiosporic acid, originally isolated from Apiospora montagnei (fungal endophyte of the North Sea alga Polysiphonia violacea),365 has confirmed the absolute configuration as originally drawn.366 (+)-Gliocladin B, a diketopiperazine metabolite of the fungus Gliocladium sp.,367 has been synthesised in an enantioselective manner utilising a new regioselective Friedel–Crafts-based strategy that determined the absolute configuration as 452.368 Cephalosporolide H is a lactone from the marine fungus Penicillium sp.369 Mismatch of spectral data from an earlier synthesis of the proposed structure with those of the natural compound indicated that the structure may need to be revised.370 A stereocontrolled synthesis of the reported structure of cephalosporolide H and three diastereoisomers, via a zinc-chelation strategy for controlling the stereochemistry of oxygenated 5,5-spiroketals, led to a suggested structure for cephalosporolide H as 453. This awaits comparison with an authentic sample for confirmation.371
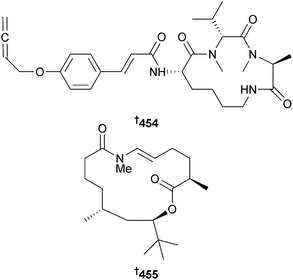
Xyloallenoide A, an N-cinnamoylcyclopeptide isolated from the endophytic mangrove fungus Xylaria sp.,372 has been synthesised and the absolute configuration determined as 454.373 Palmyrolide A is a neuroprotective macrolide obtained from an assemblage of the cyanobacteria Leptolyngbya and Oscillatoria spp.374 Synthesis of (+)-ent-palmyrolide A has been achieved via a macrocyclisation reaction that established the absolute configuration of the natural product as 455.375,376 (−)-Palmyrolide A was subsequently synthesised via a protecting-group-free method.376
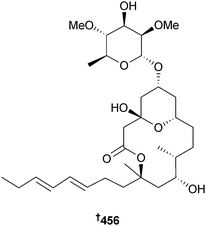
The aglycon of the originally assigned structure of lyngbouilloside, a glycosidic macrolide obtained from a Papua New Guinean Lyngbya sp.,377 has been synthesised from commercially available 3-methylbut-3-enol. A mismatch of the NMR spectroscopic data against the original data strongly indicates that the configuration at C-11 of lyngbouilloside should be revised to 456.378 This is in accord with results of an earlier synthesis of the macrocyclic core379 and also with the stereochemistry of lyngbyaloside B,380 a metabolite obtained from a different species of Lyngbya, but with the same macrocyclic core as lyngbouilloside.378
Veraguamide A is a cyclic hexadepsipeptide isolated from the cyanobacteria Symploca cf. hydnoides (Guam)381 and Oscillatoria margaritifera (Panama).382 Total synthesis of the proposed structure from three key fragments revealed that although the synthetic compound had a similar optical rotation to that reported for the natural product, there were significant differences in the NMR spectral data, particularly associated with the two N-methyl valine residues. The originally assigned structure may need revision.383 A convergent total synthesis of lodopyridone, the unusual alkaloid originally isolated from Saccharomonospora sp., (sediment, La Jolla Submarine Canyon, USA),384 was achieved in nine linear steps via cross-coupling of an iodopyridone fragment with a (quinolinethiazolyl)stannane.385 Marinoquinoline A, a pyrroloquinoline alkaloid obtained from the gliding bacterium Rapidithrix thailandica,386,387 has been synthesised from commercially available starting materials in six linear steps.388 Synthesis of tirandamycin C, a tetramic acid originally isolated from a Streptomyces sp.,389 has been completed390 and the total synthesis of maremycin B, originally isolated from a marine Streptomyces sp.,391 has been accomplished starting from L-isoleucine and S-methyl-L-cysteine.392 Salinipyrone A, a polyketide of the Palauan actinomycete Salinispora pacifica,393 has been prepared in 14% yield via an eight step procedure from a vinylketene silyl N,O-acetal.394 Macrosphelide M, originally isolated from the fungus Periconia byssoides associated with the sea hare Aplysia kurodai,395 has been synthesised from diacetone glucose.396 As a positional isomer of macrosphelide E, this isomer has also been synthesised as part of a library of all 16 diastereomers of the natural products macrosphelides A and E.397 (±)-Penostatin B, originally isolated from Penicillium sp. associated with the green alga Enteromorpha intestinalis,398 has been synthesised utilising a diastereoselective Pauson–Khand reaction and a relay ring-closing metathesis.399 7a(S)-p-Hydroxyphenopyrrozin, sourced originally from the fungus Chromocleista sp. (sediment, Gulf of Mexico),400 has been synthesised in an enantiospecific manner from L-proline utilising base-mediated cyclisation and oxygenation as key steps.401 An improved method for the sulfenylation of 2,5-diketopiperazines utilising alkali metal hexamethyldisilazide bases has been employed in the synthesis of gliotoxin G,258 a metabolite of the marine fungus Penicillium sp.402 Balticolid, a 12-membered macrolide, originally isolated from an Ascomycetous fungus separated from driftwood,403 has been synthesised utilising a Hoveyda–Grubbs II catalyst assisted ring-closing metathesis.404 Also reported are the syntheses of phomolides G and H, nonenolides originally obtained from an endophytic Phomopsis sp. of the mangrove Kandelia candel,405 from (R)-epichlorohydrin.406 7′,8′-Dihydroaigialospirol, a metabolite of the mangrove fungus Aigialus parvus,407 was prepared in a highly convergent synthesis.408 Apratoxin D, a cytotoxic cyclodepsipeptide obtained from Lyngbya majuscula and Lyngbya sordida from Papua New Guinea,409 has been synthesised by a procedure which utilised an Evans syn-aldol and a Paterson anti-aldol reaction amongst the key asymmetric transformations employed.410 The linear depsipeptide grassystatin A, originally isolated from Lyngbya cf. confervoides,411 has been synthesised by a [4 + 6] strategy.412 Total synthesis of gambieric acid A, a polycyclic ether metabolite of the dinoflagellate Gambierdiscus toxicus,413 has been accomplished,414 which reinforced the previously established revised stereostructure.415 Amphidinolide F, originally obtained from the dinoflagellate Amphidinium sp.,416 has been synthesised in 34 steps which included silver-catalysed dihydrofuran formation.417
3.9 Assorted bioactivities
The dibenzodiazepine alkaloid diazepinomicin, isolated as a metabolite of a Micromonospora sp. associated with the ascidian Didemnum proliferum,418 has broad-spectrum antitumour activity. It has now been shown to be a potent antioxidant and an inhibitor of the proteases rhodesain and cathepsin L.419 Prodigiosin,420 a tripyrrole red pigment with immunosuppressive and anticancer activities was found to exhibit selectivity for cells overexpressing the gene ErbB-2, so could show potential in human breast cancer therapy.421Streptomyces praecox (brown alga Undaria pinnatifida, S. Korean coast) produced the known (6S,3S)-6-benzyl-3-methyl-2,5-diketopiperazine (bmDKP)422 and (6S,3S)-6-isobutyl-3-methyl-2,5-diketopiperazine423 which both exhibited antifouling activity (inhibited zoospore settlement).424 Several known alkaloids were obtained from Bacillus pumilus (black coral Antipathes sp., Otoque Is., Panama), of which 3-hydroxyacetylindole425 and N-acetyl-β-oxotryptamine426 were inhibitors of growth of Trypanosoma cruzi with moderate cytotoxicity to Vero cells.427 Several known bacterial metabolites, including 2-undecen-1′-yl-4-quinolone,428 3-hexyl-6-pentyl-4-hydroxyl-2H-pyran-2-one429 and 6-heptyl-3-hexyl-4-hydroxyl-2H-pyran-2-one430 were isolated from Alteromonas sp. (seawater, Masan Bay, S. Korea) as potent algicides.431 The diterpene lobocompactol was originally isolated from the soft coral Lobophytum compactum432 but has now been obtained from Streptomyces cinnabarinus (seaweed, S. Korean Coast), where increased production was induced by co-culture with Alteromonas sp. Lobocompactol exhibited significant antifouling activity against the macroalga Ulva pertusa and the diatom Navicula annexa and inhibited growth of fouling bacteria.4333.10 Biosynthesis
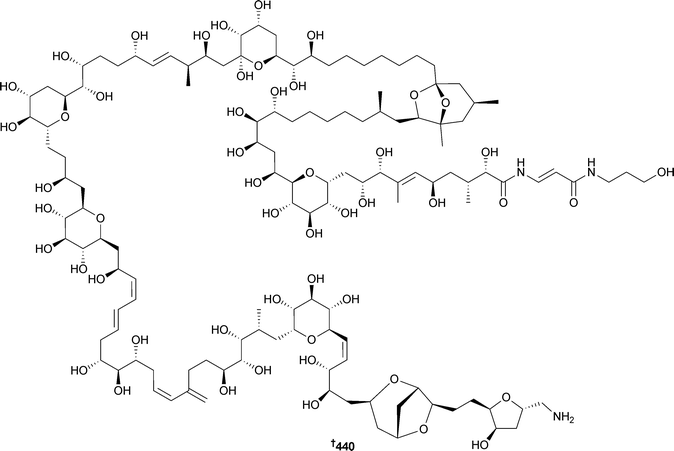
A series of genetic experiments involving the discovery and heterologous expression of the biosynthetic genes for marinopyrroles, 1,3′-bipyrrole metabolites of a Streptomyces sp.,434,435 has indicated that two flavin-dependent halogenases catalyse the unprecedented N,C-bipyrrole homocoupling reaction.436 Haterumalides are antitumour halogenated macrolides obtained from terrestrial plant-associated bacteria,437,438 and subsequently from a marine sponge439 and an ascidian.440 The biosynthetic gene cluster for the potent oocydin A (haterumalide NA) was identified by genome sequencing, comparative genomics and chemical analysis and found to be organised into three transcriptional units encoding trans-acyltransferase polyketide synthases.441 Several Streptomyces species389,442,443 have yielded tirandamycins, metabolites that target bacterial RNA polymerase. Inactivation of the enzyme TrdE, a putative glycoside hydrolase within the tirandamycin biosynthetic cluster, leads to accumulation of pre-tirandamycin. In vitro and site-directed mutagenesis studies demonstrated that TrdE catalyses the installation of the Δ11,12 double bond during tirandamycin biosynthesis in an atypical manner.444 Caerulomycins and collismycins are two groups of bacterial metabolites which contain a 2,2′-bipyridine core. Caerulomycins have been isolated from both terrestrial and marine445 sources, whilst collismycins are terrestrial in origin. Cloning of the caerulomycin biosynthetic gene cluster enabled mining of a highly conserved gene cluster encoding collismycin biosynthesis in a Streptomyces strain previously unknown as a 2,2′-bipyridine producer. In vitro and in vivo experiments indicated that caerulomycins and collismycins share a common paradigm with an atypical hybrid PKS/NPRS system responsible for the 2,2′-bipyridine core formation.446 Treatment of a strain of Penicillium purpurogenum (sediment, Bohai Bay, Tianjin, China) with the antibiotic gentamicin produced a mutant in which previously silent gene clusters were activated to produce antitumour compounds of four different chemical types: janthinone,447 fructigenine A,448 aspterric acid methyl ester449 and citrinin,450 demonstrating the potential of this approach to elicit dormant fungal metabolic potential.451 Haloroquinone is a protein kinase B inhibitor and antitumour polyketide obtained from the fungus, Halorosellinia sp.452 Feeding experiments on Halorosellinia sp. with [2-13C]malonate and [1,2,3-13C3]malonate indicated that fifteen carbon atoms of the haloroquinone skeleton were derived from malonate, eight from the methylene group and seven from the carboxyl group, thus determining its origin via a polyketide pathway using malonyl-CoA as both the starter and the extender unit.453 The biosynthesis of two N-acylated dihydropyrroles, (8E)-1-(2,3-dihydro-1H-pyrrol-1-yl)-2-methyldec-8-ene-1,3-dione and 1-(2,3-dihydro-1H-pyrrol-1-yl)-2-methyldecane-1,3-dione, originally isolated from terrestrial Penicillium brevicompactum454 but subsequently also obtained from a marine-derived Penicillium citrinum,455 has been investigated in the latter species. Feeding experiments utilising 13C-labelled precursors established that the biosynthesis of both metabolites involves the incorporation of acetate, methionine and ornithine.456 Reduction of emodin, a metabolite of both marine457 and terrestrial458 fungal origin, by sodium dithionite, resulted in the formation of two tautomeric forms of emodin hydroquinone. Subsequent conversion by the short-chain dehydrogenase/reductase (SDR) enzyme MdpC into the corresponding 3-hydroxy-3,4-dihydroanthracen-1(2H)-one implies that deoxygenation is the first step in the biosynthesis of the marine459 and terrestrial460 fungal metabolite monodictyphenone.461 NotB, the enzyme which catalyses the indole 2,3-oxidation of the Aspergillus metabolite notoamide E462 to notoamides C462 and D462 through an apparent pinacol-like rearrangement, has been characterised in vitro. Precursor incorporation experiments utilising [13C]2-[15N]2 quadruply labelled notoamide S,463 demonstrated that notoamide S is a pivotal branching point in notoamide biosynthesis.464,465 Stable-isotope labelling experiments on the endophyte Fusarium incarnatum (mangrove embryo Aegiceras corniculatum, unspecified source) indicated that the Fusarium processes coriolic acid, didehydrocoriolic acid and an epoxy fatty acid derived from linoleic acid by a process involving Δ15-desaturation and 13-lipoxygenation.466 The epoxy fatty acid was isolated in minute quantities as an inseparable mixture of diastereoisomers.466 The ladder-frame polyether yessotoxin (YTX) is produced by the dinoflagellate Protoceratium reticulatum. Culture of P. reticulatum under an 18O2 atmosphere and with supplementation of the culture media with [18O2]-acetate, followed by collision-induced dissociation tandem mass spectrometry (CID MS/MS) of the labelled yessotoxin, indicated that the ether oxygens were labelled from 18O2 and the hydroxy oxygen on C-32 was derived from [18O2]-acetate. This supports the proposed biosynthetic mechanism of marine ladder-frame polyethers that a polyene precursor is oxidised by a monooxygenase after acetate condensation.467 The biosynthetic origin of the okadaic acid water-soluble ester derivative DTX5c468 was investigated by addition of sodium [1-13C]- and [2-13C]-acetate to artificial cultures of the dinoflagellate Prorocentrum belizeanum and indicated that the polyketide backbone is interrupted by three “m-m” sequences in the ester side chain.469 The finding in 2011 that the ascidian-derived didemnins are produced by an α–proteobacterium of the genus Tistrella470 has been followed by another significant paper in 2012 reporting that T. mobilis (Red Sea and other sources) also produced didemnins. A putative didemnin biosynthetic gene cluster has been isolated from the genome of the Red Sea species. This locus encodes a 13-module hybrid NPRS–PKS enzyme complex for the synthesis of the acyl glutamine didemnins X and Y, precursors to didemnin B. Mass spectrometry of T. mobilis bacterial colonies captured the time-dependent extracellular conversion of didemnins X and Y to didemnin B.471 The discovery of the didemnin biosynthetic gene cluster potentially obviates the supply problems that presently hinder the development of the didemnins as therapeutic agents as well as paving the way for the engineering of new didemnin congeners.4 Green algae
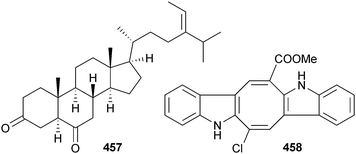
There were just two new metabolites reported from publications on green algae in 2012. Research into Tydemania expeditionis (Yellow Sea, China) defined the new ketosteroid 457 along with three known sterols and established modest activity against prostate cancer cells.472 The other new metabolite characterised was the weakly antifungal chloro-bisindole 458 from Caulerpa racemosa (Zhanjiang coastline, China) isolated along with caulerpin and two related caulerpin derivatives.473Included in the green algal literature for 2012 were a number of papers, that while not reporting new compounds, are worthy of comment. These include the finding that separate isoprenoid-24-alkyl sterol pathways have evolved in fungi and green algae which converge to yield ergosterol,474 the role of caulerpin as a potential antiviral drug against HSV Type 1,475 tetrapyrrolic pigments as photosensitisers for photodynamic therapy476 and a careful evaluation of the antioxidant activity of a wide range of Hawaiian brown, red and green algae.477 Also of interest was the identification of 208 volatile compounds from the green alga, Capsosiphon fulvescens, used by Koreans for centuries for its unique taste and flavour properties.478
5 Brown algae
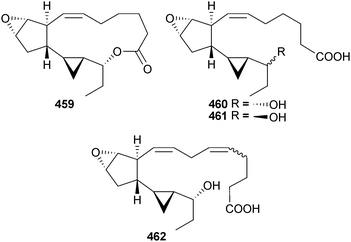
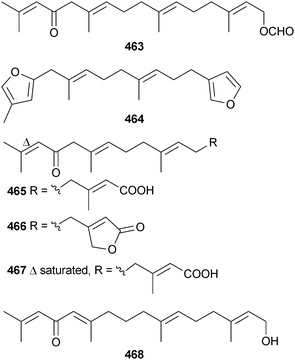
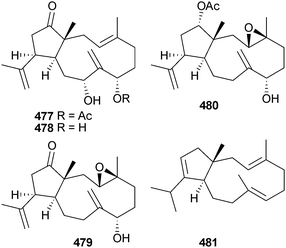
The chemistry of the Ochrophyta in 2012 was again dominated by terpenoids and phenolics and, as for the Chlorophyta, the number of new compounds characterised was relatively low. Further investigation into the chemistry of Cymathere triplicata (Deception Pass, Washington, USA) led to the characterisation of the unusual polycyclic oxylipins cymatherelactone 459 and cymatherols A–C 460–462 (isolated as the methyl esters).479,480 A plausible biogenesis for 459–461 was offered.Two studies on Bifucaria bifurcata (Roscoff, France) defined the metabolic makeup of this alga with the isolation of the sesquiterpenoids formyleleganolone 463, bibifuran 464 and four new eleganolone derivatives 465–468.481,482 Also isolated were eleganolone,483 five previously described eleganolone derivatives484–488 and 16-hydroxygeranylgeraniol489 (isolated for the first time from this species) which collectively can be correlated in a hypothetical metabolic grid.482
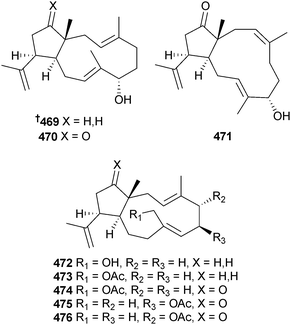
In a follow-up study the structures and relative configurations of a further 13 dolabellane diterpenoids 469–481 from the alga Dilophus spiralis (Elafonissos Is., Greece) were described along with the antibacterial properties of 469–477 and 481. The absolute configuration of 469 was determined and extended by implication across the series.490
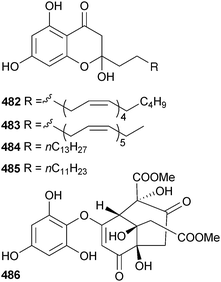
Investigation of the kinase and antibacterial activity of Zonaria spiralis (North Walkerville, Victoria, Australia) led to the isolation of the hemiketal spiralisones A–D, 482–485. These are phloroglucinol-derived lipids and were isolated as racemates. The relative instability of the spiralisones, the zero optical rotation and the isolation of co-occuring chromones corresponding to dehydration of spiralisones B and D led to a biogenetic scheme, biomimetic syntheses of spiralisones A and D and raised the possibility that previously reported algal chromones could be artefacts of isolation.491 Radical scavenging activity led to the isolation of sargussumol 486 from Sargassum micracanthum (Wando County, Jeonnam province, S. Korea).492 Sargussumol was the phenolic rather than the methyl ether (sargussumketone) previously isolated from S. kjellmanianum.493
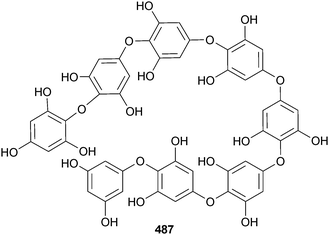
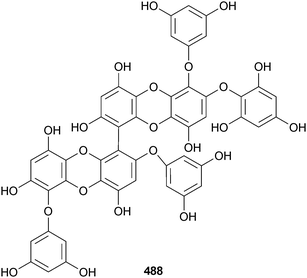
From a survey of 15 algae from the Sea of Japan for α-amylase activity, the phlorotannin DDBT was found in Sargassum patens (Noto Peninsula, Japan). DDBT was a potent competitive inhibitor of α-amylase and also inhibited α-glucosidase suggesting a potential role as a natural nutraceutical to prevent diabetes.494 DDBT was claimed as a new compound but had earlier been found in Cystophora congesta.495 The phlorotannin octaphlorethol A 487 from the S. Korean brown alga Ishige foliacea (Jeju Is.) mediated glucose uptake and as such has potential as an antidiabetic,496 while another Jeju Is. brown alga, Ecklonia cava, was the source of 2,7′′-phloroglucinol-6,6′-bieckol 488, a new antioxidant.497
The absolute configuration of dilospirane B (Dilophus spiralis, Elafonissos Is., Greece)498 has been assigned as 489 following conformational analysis and TDDFT calculations on the preponderant conformers (∼92% of the total population) of the (11R)-enantiomer. This afforded a negative Cotton Effect comparable to that observed experimentally.499
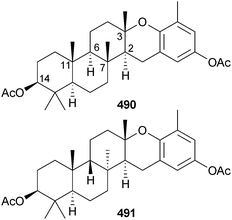
In light of the confusion that has surrounded the absolute configurations of the meroditerpenoids taondiol500,501 and epitaondiol502 vibrational CD (VCD) approaches have been used to clarify the situation.503 The diacetates were used in the study rather than the free alcohols which often show intermolecular solute–solute associations that complicate comparison of experimental and DFT calculated spectra. The calculations showed that the absolute configuration of taondiol diacetate 490 is (2S,3S,6R,7R,10R,11R,14S) and that of epitaondiol diacetate 491 is (2S,3S,6S,7S,10R,11R,14S) with single crystal X-ray analyses to support the relative configurations.
A stereoselective synthesis of the trans-hydrindane core of dictyoxetane504 has been reported,505 as has the development of “borono-sclareolide”, a terpenyl radical precursor that can be produced on the multi-gram scale allowing rapid access to a wide variety of meroterpenoids. This was demonstrated by the synthesis of 10 meroterpenoids, the majority being of marine origin.506 The C40 allenic-carotenoid fucoxanthin has been synthesised by a stereocontrolled route that also led to a longer chain (C42) analogue.507 The taxonomic implications of the re-isolation of five known diterpenoids of the dictyol series from Dictyota guineensis (Penha Beach, Brazil)508 and of a known meroditerpenoid509 from Cystoseira nodicaulis (Penmarc'h, Brittany, France) were considered.510 The incidence of betaines in species of the Laminariales was studied511 and two papers have been published on the biological properties of sargachromanol G from Sargassum siliquastrum512 covering anti-inflammatory properties513 and the expression of osteoclastogenic factors.514 The tyrosinase inhibitory activity of dieckol from Eklonia cava515 was examined in silico against mushroom tyrosinase and an effective binding site defined suggesting that dieckol has potential for further development as a pharmaceutical or cosmetic agent.516 A particularly interesting techniques paper was the application of electrochemical methods to guide the isolation of antioxidants from crude samples. This was illustrated with the isolation of four known antioxidants from Sargassum elegans (Noordhoeck, Port Elizabeth, South Africa).517
6 Red algae
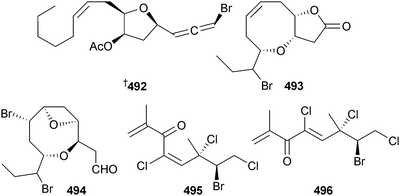
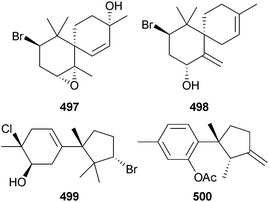
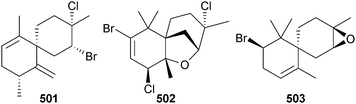
The 46 new compounds reported from red algae in 2012 is similar to the number reported from the previous year (42). Three bromoallenes, including the new dihydroitomanallene B 492, were obtained from Laurencia nangii (Sabah, Malaysia).518 Desepilaurallene 493 is a C12-acetogenin extracted from L. okamurai (Rongcheng, China), along with four new sesquiterpenes described later in this section.519 A different collection of L. okamurai (Weihai, Shandong Province, China) also contained a mixture of a C12–acetogenin okamuragenin 494 and five new sesquiterpenes (see later).520 An on-line/off-line HPLC–NMR study of an extract from Plocamium angustum (Pt. Lonsdale, Vic., Australia)521 revealed the known halogenated monoterpene plocamenone, but with the revised structure 495 shown here, and the new isomeric compound isoplocamenone with the structure 496 previously ascribed to plocamenone.522The brominated chamigrenes 497 and 498 and cuparene 499, together with the known sesquiterpene 500, but new as a natural product, were isolated from the same L. okamurai that yielded the C12-acetogenin 493 described earlier. The sesquiterpene 500 had potent antibacterial activity.519 In a separate paper, but resulting from this same collection of L. okamurai, three additional halogenated chamigrenes laurokamin A–C 501–503 were characterised.523
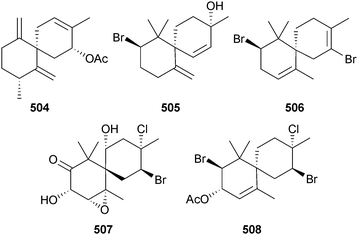
Four new chamigranes laurecomin A–D 504–507 and the sesquiterpene 508 were isolated from L. composita (Pingtan Is., China). Laurecomin B was the only compound showing antifungal activity.524
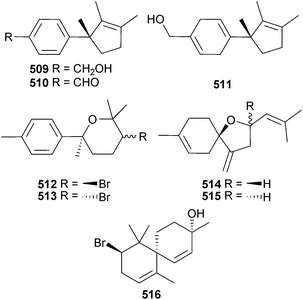
A Red Sea (Jeddah, KSA) collection of L. obtusa yielded three new laurene sesquiterpenes 509–511 all of which had strong antibacterial activity, while 510 also had significant antifungal and antitumour activities.525 Four new bisabolanes okamurene A–D 512–515 and the new chamigrane okamurane E 516 were obtained from the earlier-described L. okamurai.520
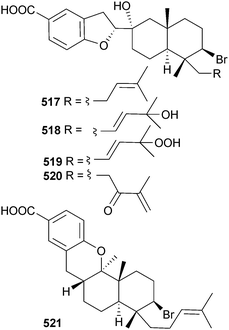
The structure proposed for the sesquiterpene aldingenin B (L. aldingensis)526 has been synthesised, but discrepancies between the spectroscopic data of the synthetic and natural material suggest that the claimed structure is incorrect.527 From a Fijian (Mango Bay Resort, Viti Levu) collection of Callophycus sp., five new compounds of the diterpene–benzoate class, bromophycoic acids A–E 517–521, were isolated. These compounds displayed a range of activities in antitumour, antimalarial and antibacterial assays.528
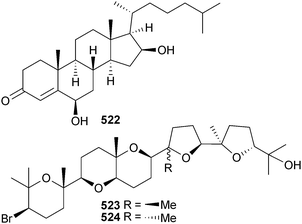
The antigenotoxic ketosteroid 522 was obtained from an extract of Jania adhaerens (Al-Shoaiba coast, Red Sea).529 The two new cytotoxic oxasqualenoids saiyacenol A 523 and B 524 were obtained from Laurencia viridis (Callao Salvaje, Tenerife, Canary Is.).530 These compounds share a significant part of their structures with aplysiol B, and with the definition of the stereocentre configurations in the saiyacenols well established, it was possible to confirm with more certainty the recently proposed structural revision for aplysiol B.531
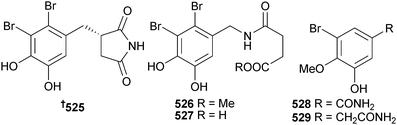
Bromophenols are frequently reported from red algae, and an additional 13 were described in 2012. The potently radical scavenging bromophenols 525–529 were obtained from Rhodomela confervoides (Dalian, Liaoning Province, China).532
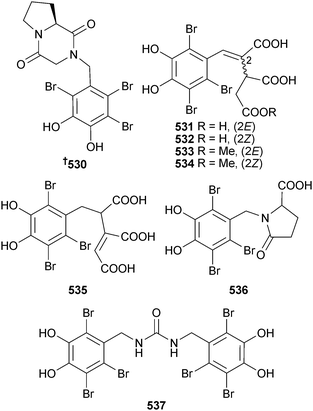
A collection of Symphyocladia latiuscula (Qingdao, Shandong Province, China) provided a bromophenol coupled to a diketopiperazine 530.533 In another paper, this same collection of S. latiuscula was reported as having produced the symphyocladins A–G 531–537. Symphyocladin G showed modest antifungal activity.534
Several papers described new biological activities for red algal metabolites, including anti-HSV-1 and HSV-2 glycolipids from Osmundaria obtusiloba,535 antitumour effects of elatol from Laurencia microcladia,536 antinociceptive and anti-inflammatory extracts from Bryothamnion triquetrum537 and anti-human rhinoviral activities of polybromocatechols from Neorhodomela aculeata.538 Surface enhanced Raman spectroscopy combined with transposed Orthogonal Partial Least Squares (T-OPLS) provided chemical images of the antibacterial surface-active 1,1,3,3-tetrabromo-heptan-2-one on Bonnemaisonia hamifera at concentration levels of sub-femtograms per μm2.539
7 Sponges
The number of new compounds reported from marine sponges in 2012 (355) has increased by approximately 20% compared with 2011.1 Pachastrissamine (Pachastrissa sp.),540 also known as jaspine B, requires dual inhibition of both Forkhead box 03 (Fox03) and cyclin-dependent kinase 2 (Cdk2) to prevent melanoma cell growth.541 Niphatenones A 538 and B 539 were isolated from Niphates digitalis (Pennville, Commonwealth of Dominica) and synthesised to permit bioactivity profiling. The enantiomers proved to be more active as androgen receptor inhibitors.542Leucettamols A and B (Leucetta microrhaphis)543 act as non-electrophilic activators of transient receptor potential (TRP) ion channels and have potential as pain modulators.544 A S. Korean Spirastrella abata yielded three sulfated sphingolipids 540–542 and one phosphorylated glycerol ether 543.545
Myrmekioside E 544 is an acetylated glycolipid from Myrmekioderma dendyi (Epi Is., Vanuatu Archipelago) with moderate potency against lung tumour cells.546 A Micronesian Xestospongia cave sponge was the source of three polyacetylenes 545–547 with low μM activity against Pseudomonas aeruginosa (P. aeruginosa),547 while an extract of Haliclona fulva (Procida Is., Gulf of Naples, Italy) contained the nine acetylenes fulvyne A–I 548–556.548
The structures of plakotenin 557 (Plakortis sp.),549 homoplakotenin 558 and norplakotenin 559 (Plakortis lita)550 have been revised following total synthesis and intensive spectroscopic characterisation.551,552 Two comprehensive synthetic, chemical and spectroscopic investigations of various plakortolide congeners (Plakinastrella clathrata) have confirmed that the originally reported plakortolides E (Plakortis sp.)553 and I (Plakortis simplex)554 are actually seco-plakortolide E 560 and plakortolide E 561, respectively, and that a related but previously unnamed variant from Plakinastrella sp.555 is plakortolide I 562.556,557Warning: it was shown that optical rotation alone is not a reliable predictor of absolute configuration in this structural class.556
Further investigation of P. clathrata (Gneerings Reef, Mooloolaba, Australia) yielded plakortolides T–W 563–566, plakortoperoxides A–D 567–570, carboxyplakortolides 1 571 and 2 572, and a series of other related metabolites 573–579 with 569–579 isolated as mixtures of diastereomers.558
Manadoperoxides E–K 580–586 and peroxyplakoric ester C 587 are potent antiprotozoals (Trypanosoma brucei rhodesiense) from Plakortis lita (Bunaken, Sulawesi, Indonesia).559 Mycalamide E 588 and congeners (Mycale hentscheli, Pelorus Sound, New Zealand) are potent protein synthesis inhibitors that are not actively transported by drug efflux pumps in yeast.560
Investigations of Plakortis simplex (Yongxing Is., South China Sea) revealed the moderately antifungal woodylides A–C 589–591,561 and the less active simplexolides A–E 592–596 and plakorfuran A 597.562
A Fijian Plakinastrella mamillaris contained plakilactones A–F 598–603 and gracilioether D 604. Plakilactone C has potential as an antidiabetic and anti-arthrosclerosis agent due to its covalent binding to the peroxisome proliferator-activated receptor γ (PPARγ), inhibiting adipocyte gene transcription.563 Further investigation of the same sponge sample yielded gracilioethers E–J 605–610 with only gracilioether H being antimalarial (P. falciparum), highlighting the importance of the peroxide functionality for activity.564
Tedarenes A 611 and B 612 are atropisomeric compounds from Tedania ignis (Sweetings Cay, Bahamas) with tedarene A inhibiting NO-production in LPS-induced macrophages.565 The published structure of a dimethoxy binaphthyl compound from Lendenfeldia sp. has been shown to be incorrect,566 and is likely a brominated biphenyl.567
A Malaysian Petrosia alfiani yielded 14-hydroxymethylxestoquinone 613, 15-hydroxymethylxestoquinone 614 and 14,15-dihydroxymethylxestoquinone 615, all of which activated the hypoxia-inducible factor-1 (HIF-1). Moreover, 613 enhanced respiration and decreased mitochondrial membrane potential suggesting its mode of action uncouples mitochondrial respiration.568
Xestosaprol N 616 has been reported from a Xestospongia sp. (Weno Is., Chuuk State, Federated States of Micronesia),569 while the antifungal aurantoside K 617 was isolated from a species of Melophlus (Cicia, Lau group of Fiji).570 Iotrochamides A 618 and B 619 (Iotrochota sp., Curacao Is., Queensland) were both moderately selective against Trypanosoma brucei.571
The total synthesis of (−)-irciniastatin B (Ircinia ramosa)572 has been achieved.573 The accepted structure of cyclocinamide A (Psammocinia sp.), originally reported in 1997 and revised in 2008,574,575 has been disproven by synthesis leaving the structure of the natural product as a mystery.576 Namalide 620 is a potent carboxypeptidase A inhibitor isolated from Siliquariaspongia mirabilis (Nama Is., Chuuk Lagoon, Federated States of Micronesia). The structure of 620 was confirmed by solid-phase synthesis.577 The Lithistid sponge Discodermia calyx (Shikine-Jima Is., Japan) provided the moderately antiproliferative calyxamides A 621 and B 622.
The presence of metagenomic DNA from the filamentous bacterium Candidatus Enthotheonella sp. that produces similar cyclopeptides in other sponges suggested that the calyxamides are produced by a symbiont.578 The proline-rich octapeptide stylissamide X 623 was reported from Stylissa sp. (Biak, Indonesia), and inhibited HeLa cell migration at sub-inhibitory concentrations.579
Droplet counter-current chromatography (DCCC) was used to isolate perthamides G–K 624–628 from a Theonella swinhoei (Solomon (Malaita) Is.). All of the perthamides isolated had some anti-inflammatory activity, suggesting structure activity relationships (SAR) were linked to the γ-methylproline, 3-amino-2-hydroxy-6-methylheptanoic acid and 2-amino-2-(2,4-dioxooxazolidin-5-yl)acetic acid side-chains.580
The sponge Neamphius huxleyi (Milln Reef, Cape Grafton, Queensland) yielded neamphamides B–D 629–631 as potent and non-selective cytotoxins.581 An earlier report had used the name neamphamide B to describe 632 from Neamphius sp. (Okinawa, Japan), which inhibited the growth of Mycobacterium smegmatis.582 Neither report detailed the full stereochemical assignment of the isolated material, but they are different compounds as 632 is claimed to contain a D-Asn while 629 has L-Asn at the same position.
The sponge Pipestela candelabra (Guadalcanal, Solomon Is.) contained the mixed NRPS-PKS pipestelides A–C 633–635, which are related to the well known jaspamides.583
The absolute configuration of both leiodermatolide (Leiodermatium sp.)584 and callipeltoside B (Callipelta sp.)585 were confirmed as originally drawn by total synthesis,586,587 while salarin C (Fascaplysinopsis sp.)588 was a potent activator of apoptosis.589 Spirastrellolides A 636 and B 637 were isolated from an Epipolasis sponge (Nagannu Is., East China Sea) as the free acids, although the methyl esters had been prepared previously to aid isolation of the natural products (Spirastrella coccinea).590–592 The free acids are more cytotoxic than their methyl esters.593
The first total synthesis of halichondrin B (Halichondria okadai)594 has been achieved.595N-Methylnorsalsolinol 638 is a radical scavenging antioxidant from Xestospongia sp. (Bougainville Reef, Queensland),596 while purpuroines A–J 639–648 are halogenated zwitterionic amino acid derivatives with antimicrobial and kinase inhibitory activities isolated from Iotrochota purpurea (Sanya, Hainan, China).597
Fuscain (Phacellis fusca)598 has been synthesised.599 The structures of haliclamines G 649 and H 650 have been reported from Haliclona viscosa (Kongsfjorden, Svalbard, Norway) and confirmed by synthesis.600
A Petrosid sponge collected from the Coral Sea (Queensland, Australia) yielded the potent antimalarials 22-hydroxyingamine A 651 and dihydroingenamine D 652 with activities in the low ng mL−1 range against various strains of P. falciparum.601
(−)-8,15-Diisocyano-11(20)-amphilectene (Hymeniacidon amphilecta)602 and 7,15-diisocyano-11(20)-amphilectene603 (Hymeniacidon sp.) are potent inhibitors (nM range) of thromboxane B2 suggesting potential as neurological anti-inflammatory agents,604 while bengamides A and B, originally isolated from a Jaspidae sponge,605 are potent immune modulators inhibiting NFκB at non-toxic concentrations (nM range) in RAW264.7 cells. The isolation of bengamides from a myxobacterial source (Myxococcus virescens) suggests a possible microbial, not sponge, origin for this class of compound.606
Acantholactone 653 was isolated from an Indonesian Acanthostrongylophora sp. but the bioactivity profile could not be established due to a paucity of material.607 The densanins A 654 and B 655 were reported from Haliclona densaspicula (Keomun Is., S. Korea) and inhibited NO production in LPS-stimulated BV2 monoglial cells.608
Renieramycin V 656 (Xestospongia sp.) is the first compound of the class to be conjugated to a sterol,609 while pre-treatment of another Xestospongia sp. (Puerto Galera, Philippines) with KCN unsurprisingly yielded the cyano-derivatives renieramycin W–Y 657–659.610
The sponge Axinella polypoides (Calvi, Corsica, France) was the source of a new betaine 660.611 The asymmetric total synthesis of nakinadine B (Amphimedon sp.)612 has been accomplished, although the original report did not contain an optical rotation so the absolute configuration of the natural product cannot be confirmed.613
Sponges continue to be a rich source of 3-alkylpyridinium alkaloids. Investigation of a Haliclona sp. (Sagyeri, Jeju Is., S. Korea) revealed cyclostellettamines N 661 and Q 662, along with eight unnamed congeners 663–670.614 While cyclostellettamines N and Q have been reported as synthetic intermediates previously,615,616 this is their first report as natural products.
An Arctic Haliclona viscosa (Blomstrandhalvøya, Svalbard, Norway) yielded viscosalines B1671, B2, 672, E1673 and E2674, with the structures established from NMR and comprehensive mass spectrometric fragmentation studies using different ionisation sources. The structures were confirmed by total synthesis.617
The proposed use of polymeric 3-alkylpyridinium alkaloids (Reniera sarai) as therapeutic adjuvants may be unwise due to their cardiotoxicity.618Mycale fibrexilis (Hainan Is., China) was the source of the 6-bromo-1H-indole-3-carboxamide 675,619 while a Thai Smenospongia sponge (PP Is., Krabi Province) provided a series of brominated indole alkaloids 676–684, isolated from a natural source for the first time.620
The synthesis of keramamine C 685 (Amphimedon sp.)621 established the absolute configuration.622
Hyrtioreticulins A–E 686–690 were reported from Hyrtios reticulatus (N. Sulawesi, Indonesia) with hyrtioreticulins A and B inhibiting the formation of the E1-ubiquitin-activating enzyme and having promise as anti-cancer proteasome modulators.623
Heterologous expression of a biosynthetic gene cluster isolated from metagenomic DNA extracted from Halichondria okadai led to the isolation of the yellow pigment halichrome A 691.624 Two brominated β-carbolines 692 and 693 have been isolated from a species of Penares collected by dredging at 95 m in the South China Sea.625
A Suberites sponge (Unten Port, Okinawa) was the source of nakijinamines A 694, B 695, F–I 696–699 and 6-bromoconicamin 700. Nakijinamines A, B, F and I were isolated as racemates.626
Suberitines A–D 701–704 were isolated from Aaptos suberitoides (Xisha Is., South China Sea), and are low micromolar inhibitors of P388 cells. They are thought to occur naturally via radical dimerisation.627
The bromopyrrole 705 was reported from Agelas mauritiana (Paracel (Xisha) Is.),628 while phorbatopsins A–C 706–708 are anti-oxidant aminoimidazolines from Phorbas topsenti (Marseille, France).629 Two new stevesines 709 and 710 and two debromolatonduines 711 and 712 have been isolated from an Indonesian Stylissa sp. (Derawan Is., Berau)630
The original latonduines (Stylissa carteri)631 are extremely potent correctors of ΔF508 protein misfolding by inhibition of the poly(ADP-ribose) polymerase (PARP) family, with latonduine A inhibiting PARP-3 in particular at the 400 pM level, whilst being totally inactive in all other assays tested. This makes the latonduines an important new lead for treatment of cystic fibrosis.632 The bromopyrrole ageladine A (Agelas nakamurai)633 has been used as an inherently fluorescent, pH-sensitive imaging molecule for transparent animals such as jellyfish.634
The pyrroloiminoquinone makaluvone (Zyzzya sp.)635 has been synthesised,636 while the related tsitsikammamine C 713 (Zyzzya sp., Rodda Reef, Queensland) was a potent antimalarial, inhibiting both chloroquine sensitive and resistant P. falciparum at the very low nM level.637
Eleven cytotoxic crambescins including the new congeners 714–721 have been reported from Crambe crambe (Villefranche-Sur-Mer, France).638
The structure of merobatzelladine B (Monanchora sp.)639 has been confirmed by synthesis,640 while the related monanchomycalins A 722 and B 723 were potent cytotoxins (low nM level) isolated from a dredged Monanchora pulchra (Sea of Okhotsk).641
Members of the kealiinine class (Leucetta chagosensis)642 have been synthesised independently by two different groups, but with discrepancies in spectral data to those of the natural products noted. These discrepancies are likely due to different tautomeric forms.643,644 An Ianthella sp. collected by trawling (Bass Strait, Australia) yielded ianthellidones A–H 724–731 and lamellarins O1 732 and O2 733. The ianthellidones A–H were isolated as racemates.645 The same sponge sample was also the source of the related dictyodendrins F–J 734–738, with all but variant G being modest β-secretase (BACE) inhibitors making them potential anti-Alzheimer's agents.646
Didebromonagelamide A 739 was isolated from a Caribbean Stylissa caribica (Bahamas). Cell-free enzyme preparations from the same sponge and also Agelas sceptrin were able to catalyse the synthesis of benzosceptrin C (Agelas sp.)647 and nagelamide H (Agelas sp.)648 from oroidin (Agelas oroides).649,650
The “meta-biosynthetic” conversion of oroidin to the sceptrin and nagelamide families likely goes through a series of single electron transfer (SET) and radical processes.651 The biomimetic SET-based syntheses of bromo- and dibromoageliferin (Agelas sp.)652 were achieved.653 An Axinella sponge (Great Australian Bight) yielded three new massadines 740–742,654 while Axinella donnani (Mauritius) was the source of donnazoles A 743 and B 744, oxidised analogues of the postulated “pre-axinellamine”, the key intermediate to all dimeric pyrrole-aminoimidazole alkaloids.655
Cavernicolin-1 and -2 (Aplysina cavernicola)656 have been synthesised starting from 3,5-dibromoverongiaquinol (Aplysina sp.),657,658 while ceratinines A–E 745–749 were reported from Pseudoceratina arabica (Hurghada, Egypt).659 Ianthellamide A 750 is a modestly potent and selective inhibitor of kynurenine-3-hydroxylase isolated from Ianthella quadrangulata (Harrier Point, Orpheus Is., Queensland).660
Tyrokeradines A–D 751–754 are bromotyrosine-derived alkaloids from an Okinawan Verongid sponge.661 Examination of Suberea ianthelliformis (Manta Ray Bommie, Stradbroke Is., Australia) revealed the ianthelliformisamines A–C 755–757 that are selective inhibitors of P. aeruginosa.662
The syntheses of pseudoceramines A–D (Pseudoceratina sp.)663 have been achieved,664 as have the syntheses of psammaplin C and tokaradine A,665,666 both from Psammaplysilla purpurea.667 Aplysamine-2 (Aplysina sp.),668 aplyzanzine A (Aplysina sp.),669 anomoian A (Anomoianthella popeae),670 purpurealidin E (Psammaplysilla purpurea)671 and suberedamines A and B (Suberea sp.)672 have all been synthesised for the first time, with the structure of anomoian A confirmed as 758.673 A Pseudoceratina sp. (Port Campbell, Victoria, Australia) contained aplysamine 7 759, purealins B–D 760–762, (−)-purealidin R 763, (−)-aerophobin 2 764 and the racemic purealin 765. Compounds 763 and 764 are the enantiomers of metabolites found from Psammaplysilla purpurea and Verongia aerophoba, respectively.674–676
Psammaplysins I 766 and J 767 were reported from a “Twilight Zone” (50–1000 m depth) Suberea sponge collected in Guam,677 while four individuals of Suberea ianthelliformis collected at various locations in the Solomon Islands yielded the five araplysillin congeners 768–772. Araplysillin N-20 formamide 768 and araplysillin N-20-hydroxyformamide 769 are moderate antimalarials.678
Twenty one psammaplysin variants 773–793 were isolated from Aplysinella strongylata (Tulamben Bay, Bali, Indonesia), although only 19-hydroxypsammaplysin E 773 showed any activity against P. falciparum.679
The synthesis of dioxepine bastadin-3 (Ianthella reticulata)680 has been achieved.681 A new cyclonucleoside 794 has been reported from Axinella polypoides (Calvi, Corsica, France).611 Siphonodictyal sulfate 795 and akadisulfates A 796 and B 797 are antioxidant merosesquiterpenoids from Aka coralliphaga (Quintana Roo, Mexico),682 while dysideavarones A–D 798–801 are cytotoxic merosesquiterpenes from Dysidea avara (Xisha Is., South China Sea). The absolute configurations of dysideavarones A–D were determined by comparison of DFT-calculated and experimental ECD measurements.683
A Smenospongia sponge (PP Is., Thailand) was the source of the iodine-containing 6′-iodoauerol 802 and the dimeric 6′-aueroxyaureol 803.620 The total synthesis of akaol A (Aka sp.)684 confirmed the absolute configuration as originally drawn.685
An Ircinia sponge (Weno Is., Chuuk State, Federated States of Micronesia) was the source of three linear polyprenyl hydroquinones 804–806.686 The asymmetric synthesis of (+)-akaterpin 807 (Callyspongia sp.)687 established the absolute stereostructure as shown.688
The merotriterpenoids halicloic acid A 808 and B 809 were isolated from a Haliclona sponge (Culasian Point, Leyte, Philippines). Both compounds inhibited indoleamine 2,3-dioxygenase, which is required for tumour immune-response escape, but were unstable in deuterated DMSO.689
Rhaphoxya sp. (Blue Hole, Guam) provided the sesquiterpene theonellin isocyanate 810.677 The synthesis of 10-isothiocyanato-4-cadinene 811 (Acanthella cavernosa)690 established the absolute configuration, although the spectroscopic data differed from those reported for a metabolite with the same assigned structure isolated from the nudibranch Phyllidiella pustulosa.691 This study also synthesised the structure proposed for 10-epi-10-isothiocyanato-4-cadinene (Stylissa sp.).692 However, discrepancies in the NMR data between the synthesised and reported compounds suggest that the natural product structure requires revision.693
Halichonadins K 812 and L 813 are sesquiterpene dimers from a Halichondria sponge (Unten Port, Okinawa). The absolute configurations of the compounds were determined by X-ray crystallography and chemical interconversion.694 Racemic syntheses of luffarin X (Luffariella geometrica)695 and cacospongionolide C (Fasciospongia cavernosa)696 have been achieved.697
Cavernenes A–D 814–817, kalihinenes E 818 and F 819 and kalihipyran C 820 are formamide diterpenoids from Acanthella cavernosa (Xisha Is., South China Sea).698 Also obtained from A. cavernosa (Xisha Is., South China Sea) were the antifouling compounds kalihinols M–T 821–828.699
The absolute configurations of kalihinol A,700 10-epi-kalihinol I,701 and kalihinol Y,702 all from species of Acanthella, have been confirmed by synthesis.703,704 The stereoselective synthesis of 7-isocyano-11(20),14-epiamphilectadiene (Adocia sp.),705 a potent antimalarial, has been achieved,706 while the isolation of two new formamido-amphilectane diterpenes 829 and 830 from a Thai Stylissa massa (Koh-Tao, Surat-Thani Province) have been reported.707 Jaspiferin A 831 was isolated from Jaspis stellifera (Guangdong, South China Sea) along with an oxidative degradation product jaspiferin B 832.708
Chromodorolide D 833, a heavily rearranged spongian diterpene, was reported along with 834 from an unidentified sponge (Cape Manza, Okinawa).709 Chromodorolide D was also reported as chromodorolide E from an Australian Dysidea sp., along with a second structure 835 that was also called chromodorolide D.710 Given the order in which the reports were accepted and published, 835 should be renamed chromodorolide E to reconcile the two separate papers.
A linear C21 furanoterpene 836 was reported from Coscinoderma matthewsi (Gneerings Reef, Mooloolaba, Australia),710 while Clathria compressa (Panama City Beach, Florida) was the source of three bicyclic C21 terpenoids 837–839.711
The cytotoxic diterpene alkaloids 8′-oxo-agelasine D 840 and ageloxime B 841 were isolated from Agelas mauritiana (Yongxing Is., South China Sea) along with a taurinated diterpenoid 842.628
Agelasidines E 843 and F 844 are weakly antifungal compounds from Agelas citrina (Bahamas) that were isolated along with agelasine N 845,712 while agelasines O–U 846–852 are broadly active diterpene alkaloids isolated from an Okinawan Agelas sp.713
Sarcotin P 853 is a linear furano-norsesterterpenoid isolated from a Sarcotragus sp. (Cheju Is., S. Korea),714 while diacarperoxide S 854 is a cytotoxic norsesterterpene peroxide from Diacarnus megaspinorhabdosa (Pula Baranglompo Is., Indonesia).715 The total syntheses of (−)-alotaketal A (Hamigera sp.)716 by two independent groups have confirmed the absolute configuration.717,718
Four sesterterpenoids, phorone A 855, isophorbasone A 856, ansellone B 857 and phorbasone A acetate 858, were isolated from a S. Korean Phorbas sp. Compounds 857 and 858, described in the paper's supporting information, were low μM inhibitors of NO production in LPS-stimulated RAW 264.7 cells, while 855 and 856 are the first examples of two new carbon skeletons.719
Flabelliferins A 859 and B 860 came from Carteriospongia flabellifera (Tutuba Is., Vanuatu). Both showed modest growth inhibition against human colon cancer cell lines,720 while a Hippospongia sponge (Taitung, Taiwan) was the source of hippospongides A 861 and B 862.721
The archetypical anti-inflammatory scalarane sesterterpenoid scalaradial (Cacospongia mollior)722 acted as a non-covalent binder in the active site of phospholipase A2 and chelates Ca2+ but did not covalently bind to the enzyme through dial reactivity.723 The total synthesis of solomonsterol B (Theonella swinhoei)724 has allowed for SAR studies to be performed.725
Chalinulasterol 863 came from Chalinula molitba (Little San Salvador, Caribbean) and is the first chlorinated/sulfated sterol known.726 A species of Dysidea (Ishigaki Is., Okinawa) was the source of dysideasterols F–H 864–866, all of which inhibited human epidermoid carcinoma cells.727
Theonella swinhoei from various locations in the Solomon Islands (Malaita and Vangunu Is.) have yielded conicasterols E 867,728 F 868,729 and G–K 869–873,730 along with theonellasterol I 874,729 and J 875.730 All are dual ligands of the pregnane X (PXR) and farnesol X receptors (FXR) with potential in modulating bile acid homeostasis in the liver and hence, metabolic disorders.728–730
T. swinhoei (Pingtung, Taiwan) was the source of theonellasterol K 876, acetyltheonellasterol 877 and acetyldehydroconicasterol 878, with theonellasterol K being moderately active against a panel of cancer cell lines.731
Acanthifoliosides G–J 879–882 were isolated from Pandaros acanthifolium (Marathon, Florida Keys) with acanthifolioside G activating the antioxidant response element in a dose-dependent manner without a significant increase in the activity of the detrimental xenobiotic response element.732
The sponge Lissodendryx fibrosa (North Sulawesi, Indonesia) was the source of manadosterols A 883 and B 884, dimeric sterols that are potent inhibitors of the ubiquitin Ubc13-Uev1a complex and therefore have potential as anticancer agents.733
Geoditin A (Geodia japonica)734 had antimelanogenic activity suggesting potential as a skin whitening agent,735 while sarasinosides N–R 885–889 are nortriterpenoid glycosides from Lipastrotethya sp. (Chuuk State, Micronesia).736
The isomalabaricane triterpenoids globostelletin J–R 890–898 were isolated from Rhabdastrella globostellata (Hainan Is., South China Sea), although in the paper there is no mention of the congener globostelletin S referred to in the title.737
Plakortis lita (Manado, Indonesia) was the source of the hopanoid glycoside plakohopanoid 899, although the terpenoid and carbohydrate components are reminiscent of bacterial secondary metabolites and it is likely that 899 is of symbiotic origin.738
8 Cnidarians
Although decreased from 2011, the number of new metabolites reported from cnidarians (213) is about the average number per year over the past decade. Sinularioside 900, a bis-α-D-arabinopyranosyl myristyl glycolipid,739 sinulasulfoxide 901 and sinulasulfone 902740 were isolated from Sinularia sp. (Manado, North Sulawesi, Indonesia), with sinularioside and sinulasulfoxide acting as moderate inhibitors of NO release from LPS-stimulated macrophages.Brazilian collections (Paracuru Beach, near Fortaleza) of the zoanthids Palythoa caribaeorum and Protopalythoa variabilis yielded the sulfonylated ceramides palyosulfonoceramide A 903 and B 904.741 The structure elucidation of palyosulfonoceramides A and B was aided by comparison with two known co-metabolites; none of the four ceramides exhibited cytotoxicity.
A new method of detection and quantification of palytoxin has been reported based on detection of the interaction of fluorescein-labeled Na,K-ATPase with palytoxin using fluorescence polarisation.742 The method has a limit of detection of 2 nM. Paralemnolide A 905 is an unusual bisnorsesquiterpene from Paralemnalia thyrsoides (Taitong County, Taiwan)743 and specimens of Sinularia sp. (Hainan Is., South China Sea) were the source of the cyclopentenones and butenolides sinularone A–I 906–914.744 Absolute configuration was assigned to several of the metabolites via comparison with calculated ECD, optical rotation and chemical shifts. Sinularones A, B, G, H and I exhibited antifouling activity in vitro towards Balanus amphitrite (B. amphitrite).
In addition to known congeners, fifteen new terpenes anthogorgienes A–O 915–929, based on a guaiazulene sesquiterpene scaffold, were isolated from Anthogorgia sp. (Weizhou Is., China).745 Absolute configurations were assigned to enantiomers 928 and 929 by CD analysis. Anthogorgiene G and known analogues exhibited antifouling activity (B. amphitrite) and antimicrobial properties.
Menelloide E 930, a germacrane-type sesquiterpene, and seco-germacrane anhydride 931, previously reported from fruit of a Turkish plant,746 were isolated from a deep-sea trawl collection of Menella sp. (southern coast of Taiwan).747,748 Two carbamates obtucarbamate C 932 and D 933 and two sesquiterpene-taurine conjugates 934 and 935 were reported from Melitodes squamata (Sanya, Hainan Province, South China Sea).749
Of the two epimeric sesquiterpene hydroperoxides scabralin A 936 and B 937 (Sinularia scabra, Southern Taiwan), the former was found to exhibit mild antitumour activity and to reduce levels of iNOS protein in LPS-stimulated macrophages.750 Sesquiterpenes lochmolin A–G 938–944 were reported from Sinularia lochmodes (northern coast of Taiwan); only lochmolin A was found to have an ability to reduce levels of COX-2 protein in LPS-stimulated macrophages.751
Prenylated purines 945, 946 and formamide 947 (malonganenones I–K), and a number of previously reported congeners were isolated from Euplexaura robusta (Weizhou Is., South China Sea).752 While 945–947 exhibited mild cytotoxicity, related co-metabolites malonganeones A, D and E753,754 were more cytotoxic.
Two cembranes yalongene A 948 and B 949 were identified in extracts from Sarcophyton trocheliophorum (Yalong Bay, Hainan, South China Sea), with the former exhibiting cytoprotective effects towards cells injured with H2O2.755 In addition to a number of congeners, epoxy-containing cembranes knightine 950 and related analogues 951 and 952 were isolated from Eunicea knighti (Santa Marta Bay, Colombian Caribbean Sea).756 While only 951 and 952 and a related known co-metabolite inhibited quorum sensing in Chromobacterium violaceum, many of the cembranes inhibited biofilm formation for a number of different microorganisms.
Epoxy-diol cembranes sicrassarine A 953 and B 954 (Sinularia crassa, Taitung County, Taiwan) were non-cytotoxic757 while the C-4/C-14 ether linked cembrane lobocrassin F 955 (Lobophytum crassum, Northeast Taiwan) was a moderate inhibitor of elastase release from human neutrophils.747 In contrast, cembranes 956 (a sarcophine analogue), 957 and 958 (ehrenbergol A and B) isolated from Sarcophyton ehrenbergi (Taitung County, Taiwan) exhibited cytotoxicity (P388 cells) and antiviral activity (human cytomegalovirus (CMG)).758
Of two cembranes sinumerolide A 959 and B 960 reported from Sinularia numerosa (Hainan Is., South China Sea) the latter contains the more usual combination of 5,8-epoxy linkage and C-4 norcembrane skeleton, while the former is unusual in possessing a complete C20 diterpene backbone.759 One other example of a 5,8-epoxy-C-4-norcembrane, 5-episinuleptolide acetate 961 was reported as a moderately cytotoxic component of Sinularia sp. (Taitung County, Taiwan).760
A collection of Sinularia sp. (Dongluo Is., Hainan, South China Sea) afforded sinuflexibilins A–E 962–966, with the authors speculating about the potential artefactual nature of α-methoxyfuranocembranoid 966.761 Co-metabolite flexibilide762 was the only compound in the study found to inhibit NF-κB activation.
Cembranoid diterpenes flexibilisolide C–G 967–971, flexibilisin C 972 and the ring opened 11,12-secoflexibillin 973 were reported from Sinularia flexibilis (Dongsha Atoll, South China Sea).763 Flexibilisolide C, as well as several related co-metabolites, exhibited cytotoxicity and reduced the accumulation of iNOS and COX-2 pro-inflammatory proteins.
From a suite of new diterpenes sinumaximol A–I 974–982 and known congeners (Sinularia maxima, Nha trang Bay, Vietnam), sinumaximols B and C were found to inhibit IL-12, IL-6 and TNF-α production in LPS-stimulated bone marrow dendritic cells.764
The structures of sarcophine hydroperoxide analogues 983 and 984 (Sarcophyton glaucum, Hurghada, Egyptian Red Sea) were secured by X-ray and CD analysis.765 The NMR data of the co-metabolite 8-epi-sarcophinone 985 were different to those previously reported for the isomeric iso-sarcophinone766 but could not be directly compared with earlier reported semi-synthetic isomers due to a lack of reported NMR data.767 Metabolites 984 and 985 inhibited CYP450 1A and induced glutathione-S-transferase and quinone reductase activity.
As would be expected, the α-exo-methylene-γ-lactone containing examples of michaolide L–Q 986–991 (Lobophytum michaelae, Ping-Tong County, Taiwan) exhibited more potent cytotoxicity than the seco analogue 989.768 Absolute configuration was assigned to michaolide L, though it should be noted that there is disagreement between configuration descriptors for C-4 and C-10 in the text versus the structure shown in the paper.
All three hydroperoxides sarcocrassocolide M–O 992–994 (Sarcophyton crassocaule, Dongsha Atoll, Taiwan) exhibited some degree of cytotoxicity towards tumour cell lines, with 992 and 994 and to a lesser extent 993 also inhibiting the induction of iNOS protein.769
Dihydrofuranocembranoids sarcophytonin F 995 and G 996 (Sarcophyton sp., Dongsha Atoll, Taiwan)770 are, respectively, the hydroperoxide and acetate derivatives of the co-metabolites sarcophytoxide and sarcophytonin C.771 Sarcophytonin G can also be classified as ent-crassumol C.772
A structurally diverse set of diterpenes, the pavidolides A–E 997–1001, were isolated from Sinularia pavida (Sanya Bay, Hainan Is., South China Sea). While pavidolides B and C were mildly cytotoxic, pavidolides C and D inhibited settlement of B. amphitrite.773
Cespitularia taeniata (Green Is., Taiwan) was the source of norverticillane cespitulin E 1002, secoverticillane cespitulin F 1003 and cespitulin G 1004.774 Cespitulin G was a moderate inhibitor of elastase release and superoxide production by stimulated human neutrophils. The serrulatane-skeletoned diterpene anthogorgiene P 1005 and triterpenoid anthogorgiene Q 1006 were isolated from Anthogorgia sp. (Weizhou Is., South China Sea) and the absolute configuration of the tricyclic core of anthogorgiene P was assigned.775
Dimethylamino-naphthalene 1007 and lobane 1008 (Sinularia sp., Bowden reef, Great Barrier Reef, Australia) exhibited mild to moderate cytotoxicity towards three HTCLs.776 Eunicidiol 1009 and known related diterpenes eunicol777 and fuscol777 were identified as potent topical anti-inflammatory metabolites of Eunicea fusca (Hillsboro Ledge, Florida).778
Extracts of Lobophytum pauciflorum (Taketomijima Is., Okinawa)779 provided cyclolobatriene 1010 and the related metabolites lobatriene,780 eunicol777 and fuscol.777 A low temperature (7 °C) NMR spectrum of 1010 identified three conformational isomers, while heating to 70 °C induced a thermal Cope rearrangement to lobatriene (identical by NMR and sign of [α]). A similar rearrangement was induced for eunicol, converting it to fuscol. All four natural products showed moderate cytotoxicity towards a tumour cell line.
Two metabolites calyculone H 1011 and I 1012 (Eunicea sp., Old Providence Is., Colombia)781 appear to be respectively the C-4,5-bis-epimers of previously reported calyculones C and A,782 while triangulene C 1013 (Sinularia triangula, Taitung County, Taiwan) is another (possibly C-1) stereoisomer of calyculone C.783 Calyculones A, B, C and H exhibited mild antimalarial activity, while calyculone A displayed strong cytotoxicity in testing at the NCI. In contrast, the related cubitane crassalone A 1014 (Sinularia crassa, Taitung County, Taiwan) exhibited no cytotoxicity towards a panel of tumour cell lines.784
Two separate studies of Cespitularia sp. (Zamami Is., Okinawa) afforded the mildly cytotoxic alcyonolide-type diterpenes 1015–1023.785,786
Twelve related diterpenes astrogorgin B–M 1024–1035 were isolated from Astrogorgia sp. (Beibuwan Bay, South China Sea).787 Astrogorgin L was observed to undergo dehydration of the allylic alcohol functional group in CDCl3 NMR solvent – the diene product was named astrogorgin N. Astrogorgins A–C exhibited moderate potency in a B. amphitrite antifouling bioassay.
Duplicate structures were evident in the case of metabolites simplexin P–S 1036–1039 reported from Klyxum simplex (Dongsha Atoll, Taiwan).788 Simplexin Q is identical to klysimplexin C,789 while simplexin S is identical to cladieunicellin G concurrently reported from Cladiella sp. (Indonesia).790 In addition, this latter study noted the presence of 6-epi-cladieunicellin F 1040 in the organism. The same specimen of Cladiella sp. also yielded the hemiketal congener cladieunicellin H 1041.791
Seco-briarellinone 1042 and briarellin S 1043 are C-12 keto eunicellin diterpenes (Briareum asbestinum, Bocas del Toro, Panamanian Caribbean) that mildly inhibited the production of NO by LPS-stimulated macrophages.792 Cristaxenicin A 1044 from Acanthoprimnoa cristata (dredging, Yakushima-Shinsone, Kagoshima, Japan) exhibited sub-micromolar activity towards the human protozoal targets Leishmania amazonesis (modest selectivity) and Trypanosoma congolense, but was less active towards P. falciparum.793 Absolute configuration was assigned by comparison of calculated and experimental ECD spectra.
In three separate accounts, new halimane diterpenes 1045 (echinohalimane A) and 1046 (echinoclerodane A) and labdane 1047 (echinolabdane A) were reported from a single collection of Echinomuricea sp. (Taiwan)794–796 with echinohalimane A inhibiting the release of elastase from stimulated human neutrophils.
Diepoxybriaranes briaroxalide A–G 1048–1054 (Briareum sp., Ishigaki Is., Okinawa) all share the same absolute configuration; the configuration of briaroxalide A was determined (derivative, X-ray) and related to the others by peracetylation.797
A southern Taiwanese collection of Briareum sp. afforded briarenolides E–I 1055–1059.798–800 Hydroperoxide briarenolide F was a strong inhibitor of superoxide generation by stimulated neutrophils.
The 8,17-epoxybriaranes briacavatolide A–F 1060–1065 were all reported from Briareum excavatum (Orchid Is., Taiwan).801,802 While none of the diterpenes exhibited cytotoxicity, briacavatolides C and F were modest inhibitors of CMG.
Of six 11,20-epoxybriaranes (gemmacolide T–Y 1066–1071) isolated from Dichotella gemmacea (Beihai, China), gemmacolides V and Y exhibited the most pronounced cytotoxicity towards two tumour cell lines.803
Investigation of Junceella juncea (Taitung County, Taiwan) secured three briaranes, juncenolide M–O 1072–1074;804 unfortunately the structure of juncenolide O is identical to that previously reported for juncin Z (Junceella juncea).805
Briareolate esters J 1075 and K 1076 (Briareum asbestinum, Boca Raton, Florida)806 are hexanoate esters of briareolate esters G and D,807 respectively. Briareolate ester K was a weak growth inhibitor of human embryonic stem cells.
Sinularia crassa (Taitung County, Taiwan) yielded crassarosterol A 1077 and glycosides crassarosteroside A–D 1078–1081; crassarosteroside A inhibited the expression of iNOS protein in stimulated macrophages.808
Of the four tetraols anthogorgsteroid A–D 1082–1085 isolated from Anthogorgia sp. (Beihai, South China Sea), anthogorgsteroid A appears to be identical to the previously reported sterol menellsteroid C809 (Menella sp.).810 Mildly cytotoxic pentaols 1086 and 1087 were isolated from Subergorgia suberosa (Naozhou Is., South China Sea).811
Two studies reported sterols with antagonistic activity towards FXR. In the first study, two new sterols 1088 and 1089 were isolated from Dendronephthya gigantea (Geo-je Is., S. Korea);812 although three sterols were claimed to be new, one had in fact been reported the previous year from a Chinese specimen of Astrogorgia sp. (astrogorgol N).813 Sterol 1088 was the most active of those isolated at inhibiting FXR transactivation induced by chenodeoxycholic acid.
In addition to a number of known sterols, new examples 1090–1093 (new natural products) and 1094 were reported from Sinularia sp. (Bunaken Marine Park, Manado, Indonesia).814 The most potent antagonist of FXR identified in this second study was gorgosterol.
Extraction of Echinomuricea sp. (Taiwan) that had afforded a new labdane diterpene 1047 (see earlier) also yielded 6-epi-yonarasterol B 1095 which was notable for the ability to inhibit the generation of superoxide and the release of elastase from stimulated human neutrophils.796 Three examples of C-19 oxygenated sterols, nebrosteroid N–P 1096–1098 (Nephthea cabrolii, Taitung County, Taiwan), were moderately cytotoxic towards a range of tumour cell lines.815
In addition to two cembranes 959 and 960 (see earlier), specimens of Sinularia numerosa (Hainan Is., South China Sea) also yielded the 7β-hydroxy analogue of gorgosterol 1099,759 while triol 1100 and tetraols 1101 and 1102 were characterised from extracts of Sinularia sp. (Weizhou Is., South China Sea).816
The 9,11-secosterol 1103 (Sinularia granosa, Pingtung, Taiwan) is an H-8, 5,6-epoxy diastereomer of a previously reported co-metabolite (Sinularia lochmodes);817 both compounds exhibited cytotoxicity and inhibited expression of iNOS and COX-2 proteins in stimulated macrophages.818 The first examples of 9,11-secosterol glycosides, sinularoside A 1104 and B 1105 (Sinularia humilis, South China Sea) exhibited growth inhibition properties towards two fungi, a microalga and a Gram-positive bacterium, but not a Gram-negative bacterium.819
Of a diverse range of pregnanes, including new examples sclerosteroid A–I 1106–1114 (Scleronephthya gracillimum, Green Is., Taiwan), only sclerosteroids A, B and E inhibited expression of the pro-inflammatory proteins iNOS and COX-2 in stimulated macrophages.820
Mass-guided fractionation of the hydroid Thuiaria breitfussi (Bear Is., Arctic) identified the halogenated alkaloids breitfussin A 1115 and B 1116. These structures were established using atomic force microscopy in combination with structure elucidation software and chemical shift calculations.821
The structure of a second γ-lactone ‘trocheliophorolide B’, originally reported from Sarcophyton trocheliophorum,822 has been shown to be incorrect.823 The trivial name trocheliophorol, ascribed to a cembranoid metabolite isolated from cultured specimens of S. trocheliophorum,824 has been used before.825 As a consequence the authors have changed the name to trocheliol.826
Syntheses of the modestly cytotoxic seco-caryophyllane rumphellaone A827 and clovane rumphellclovane A828 (Rumphella antipathies) have been reported;829–831 the latter study used the filamentous fungi Pestalotiopsis palustris to biotransform synthetically prepared (1S,2S,5S,8R,9R)-2-methoxyclovan-9-ol into the soft coral metabolite, simultaneously establishing the absolute configuration.
Several studies have been reported on further biological investigation of purified cembranoids. Sinularin (Sinularia flexibilis)832 inhibits the up-regulation of pro-inflammatory proteins iNOS and COX-2 as well as TGF-β in stimulated macrophages.833 Such in vitro results also translated to in vivo studies, where sub-cutaneous application led to observable analgesic effects. While one study of 11-dehydrosinulariolide834 noted an ability to induce apoptosis and act as an antiproliferative and antimigration agent towards human melanoma cells,835 a second study noted the same cembranoid significantly reduced 6-hydrodopamine-induced cytotoxicity and apoptosis of a human neuroblastoma cell line, mediated by caspase-3/7 and PI3K, suggesting a potential role as a neuroprotective candidate.836 The related cembrane sinulariolide837 exhibited antiproliferative, antimigratory and apoptosis-inducing activities, the latter via mitochondrial and p38MAP kinase pathways against human urinary bladder carcinoma cells.838
Investigation of the antiproliferative activity of an unnamed γ-lactone cembranoid (Sinularia mayi)839 revealed that it functions by activation of apoptosis via reactive oxygen species (ROS) generation, which has further downstream consequences of inhibiting signal transduction.840 A semi-synthetic analogue of cembranoid sarcophine, sarcophine diol, inhibited mouse melanoma cell proliferation by arresting cell division at G0 and by also activating apoptosis.841
A configurational reassignment of 11-gorgiacerol842 (= 11-pseudopteranol?)843 and 11-epi-gorgiacerol (aka 11-epi-pseudopteranol)844 to those shown in 1117 and 1118 has been reported; the syntheses made use of a stereospecific photochemical ring contraction reaction.845
The absolute configurations of bis-cembranoid ximaolide A8461119 and cembrane (+)-sinulaparvalide A8471120 were established by comparison of calculated ECD spectra (derived from X-ray structure conformation) with spectra observed for micro-crystalline solids.62 Comparison with solution ECD spectra also allowed assignment of absolute configurations to ximaolide B and E, methyl tortuosoate (= methyl tetrahydrosarcoate)848 and (+)-sinulaparvalide B.
By stereospecific synthesis of a stereoisomer, the absolute configuration of elisabethatriene,849 a diterpene representing the first committed step in pseudopterosin biosynthesis, has been corrected to that shown here, 1121.850
Purified pterosins and seco-pterosins (Pseudopterogorgia elisabethae, Providencia Is., Colombia) demonstrated selective ability to inhibit the growth and biofilm of Gram-positive bacteria, suggesting that the natural products play a role in regulation of the gorgonian surface bacterial communities.851 Further investigation of the antimycobacterial activity demonstrated by pseudopteroxazole852 and homopseudopteroxazole853 has identified a semi-synthetic histidine analogue with similar levels of potency and selectivity.854 Theoretical studies suggested that the photochemical [2 + 2] cycloaddition reaction proposed for the biogenesis of the cyclobutane-containing diterpene plumisclerin A855 could also be realised by a thermal step-wise proton-promoted process.856
The absolute configuration of clavulactone 1122 (Sinularia sp.)857 has been established by enantioselective synthesis.858 As indicated in the previous review in this series,1 the structure of gemmacolide H859 has been confirmed860 as identical to the previously reported briarane 12-epi-fragilide G.861
The structure of pregnane krempene B (Cladiella krempfi)862 has been confirmed by synthesis from 3β-acetoxy-5-pregnen-20-one,863 while two cholestane-dienones previously reported from Minabea sp.864 and Anthomastus bathyproctus865 have been prepared from (25S)-Δ4-dafachronic acid.866
A systematic nomenclature for sea anemone toxins has been proposed, combining descriptors of biological activity, generic family name, genus and species of original source and relationship to known isoforms.867 Further proof of the need of a consistent nomenclature system was demonstrated in a report on digital marine bioprospecting, whereby deep sequencing of transcriptomes of cold-water anemones Bolocera tuediae and Hormathia digitata and subsequent homology searching identified four highly similar and 15 additional new neurotoxin peptide candidates.868 A concise synthesis of the guanidine hydantoin alkaloid parazoanthine A (Parazoanthus axinellae)869 and the methyl ether analogue has been reported.870
9 Bryozoans
Only two investigations of bryozoan chemistry have been reported in the last year. A collection of Amathia tortuosa (northern New South Wales, Australia) provided the tribrominated indole alkaloid kororamide A 1123 which was marginally active against chloroquine-sensitive and resistant strains of P. falciparum.871Total synthesis of the published structure of amathamide D, a metabolite of Amathia wilsoni,872 indicated that the structure should be revised to that indicated by other researchers.873 This revised structure was also synthesised.874 Convolutamine H, a metabolite of Amathia convoluta,875 was synthesised via a Grob fragmentation-aromatisation strategy.874
10 Molluscs
There was a pronounced increase in the number of new metabolites reported from molluscs when compared with recent years. The antioxidant activity detected in Japanese samples of the Pacific Oyster (Crassostrea gigas) was attributed to 3,5-dihydroxy-4-methoxybenzyl alcohol,876 previously reported from the brown alga Leathesia nana.877The new C-terminus pyroglutamate gonadotropin-releasing hormone related peptides Cg-GnRH-a 1124 and Cg-GnRH-G 1125 were isolated from French (Normandy) specimens of the Pacific Oyster.878
Optimised purification using extracts of Mytilus edulis (Bruckless, Donegal, Ireland) afforded sufficient quantities of the toxin azaspiracid-6 1126, previously characterised by MS,879 to enable the characterisation by NMR spectroscopy.880 While 1126 was stable in aqueous acetonitrile solution, storage in methanol formed a C-21 methyl ketal.
A diverse array of fatty acid esters of pinnatoxin G were identified in extracts of M. edulis (Atlantic coast, Canada).881 Specimens of the sea snails Gibbula umbilicalis (flat top shell) and Monodonta lineata (thick top shell) collected on the Portuguese coast were found to contain low levels of tetrodotoxin and/or analogues, highlighting these species as new toxin vectors.882
A number of new conotoxins continue to be isolated from snail venom. Conus regius (Plantation Key, Florida) yielded an α4/7-conotoxin, RegIIA 1127, comprised of 16 residues with two disulfide bonds.883 The structure was confirmed by synthesis and the 3D-conformation established by NMR methods. The peptide was a low nM blocker of α3β4 nAChRs. In contrast, the amidated 11-residue peptide pc16a 1128 (Conus pictus, Port Elizabeth, South Africa), which contained a rare cysteine framework XVI, was found to be inactive in a range of bioassays.884
A novel framework XXIII was present in two toxins im23a (42 residues) and im23b (43 residues) isolated from Conus imperialis (South China Sea).885 The conformation of recombinant im23a was determined by NMR spectroscopy; the sequence and cysteine framework suggests that this peptide is the first member of the K-superfamily. Intracranial injections of either peptide caused excitatory symptoms. μ- and μO-Conotoxins are well known as blockers of voltage-gated sodium channels (VGSC) and nAChRs, making them of interest as potential analgesics.
A new μ-conotoxin congener, CnIIIC 1129 (Conus consors, Chesterfield Is., New Caledonia) was isolated, synthesised and tested against a range of skeletal muscle and VGSC targets.886 Potent blocking of skeletal muscle (NaV 1.4) and neuronal (NaV 1.2) VGSCs was observed.
A hydrophobic 32-residue peptide, μO-conotoxin MfVIA (Conus magnificus, unspecified location) preferentially inhibited NaV 1.8 and NaV 1.4 sodium channel isoforms.887 Synthesis of the peptide made use of an interesting sequence of selective oxidative deprotections of cysteine residues that allowed for synthesis without requiring chromatographic purification of intermediates.
A combined proteomic/transcriptomic analysis of the venom duct of Conus consors (Chesterfield Is., New Caledonia) identified 105 components (of over 400 detected) covering A-, M- and O1-superfamily toxins, as well as unusual disulfide-free peptides and actinoprin- and hyaluronidase-like proteins.888 However, the ability to automate such analysis is confounded somewhat by the complexity of post-translational modifications in Conus venoms. The interplay of three Conus imperialis venom duct proteins, protein-disulfide isomerase, peptidyl-prolyl cis–trans isomerase and immunoglobulin-binding protein in directing the oxidative folding of cysteine-rich peptides has been studied.889
Investigation of the chemistry of Siphonaria oculus (Eastern Cape, South Africa) afforded new polypropionates 1130–1132.890 The potential artefactual nature of cyclic products 1130, 1131 and a fourth known polypropionate was suggested when analysis of the crude mucous released from individual molluscs revealed 1H NMR signals attributable to only 1132 in the extract.
The more structurally complex labdane diterpene 1133 was reported from specimens of the limpet Trimusculus peruvianus (Bahía de Pichidangui, Chile).891 Enantioselective synthesis of ent-caloundrin B (Siphonaria zelandica)892 confirmed the proposed relative and absolute configuration and in the presence of imidazole was found to isomerise to ent-siphonarin B.893
The absolute configurations of the bis-γ-pyrone polyproprionate onchidione (Onchidium sp.)894 and two methanolysis products have been assigned by analysis of X-ray diffraction data and TDDFT calculated ECD spectra.895Dolabrifera dolabrifera (El Escambron, Puerto Rico) afforded the known non-contiguous polyproprionate ester dolabriferol896 and two new congeners dolabriferol B 1134 and C 1135.897 The artefactual nature of all three metabolites was suggested.
Although not the first synthesis, a potential biomimetic synthesis of (−)-dolabriferol has been reported, utilising a retro-Claisen rearrangement of a 1,3-diketone to yield the non-contiguous polypropionate skeleton.898 Dactylomelatriol 1136 (Aplysia dactylomela, La Gomera, Canary Is.) contains a rare sesquiterpene skeleton previously seen in a metabolite isolated899 from a liverwort.900
Use of either silica gel or AgNO3-impregnated silica gel chromatography yielded diterpenes thuridillin D–F 1137–1139 from the sacoglossan mollusc Thuridilla splendens (Mooloolaba, Queensland).901 Alkenes 1138 and 1139 may be purification artefacts of tertiary alcohol 1137.
Five new aplyronine congeners D–H 1140–1144 were reported as cytotoxic constituents of Aplysia kurodai (Mie Prefecture, Japan).902 While aplyronines D–G were equipotent or more cytotoxic (HeLa S3 cells) than aplyronine A,903 aplyronine H was less cytotoxic highlighting that translocation of the di (or tri) methylserine residue to C-9 is detrimental to cell-based activity. Photoaffinity probes of aplyronine A have been used to identify two actin-related proteins Arp2 and Arp3 as target binding proteins.904 As neither Arp2 nor Arp3 were covalently bound directly by the probes, it was suggested these proteins bind to the aplyronine A-actin complex or to oligomeric actin. Further evidence for the disconnect between actin-depolymerising activity and whole cell cytotoxicity of aplyronine A was observed for a synthetic hybrid combining the macrolactone of aplyronine A and the sidechain of the actin-depolymerising sponge metabolite mycalolide B.905 While the hybrid was as potent as aplyronine A as an actin depolymeriser it was ca. 1000-fold less cytotoxic towards HeLa S3 cells.906
Biosynthetic incorporation studies have established that the C3 units present in the opistobranch mollusc Ercolania funerea metabolite 7-methyl-cyercene907 result from intact incorporation of propionic acid.908 What is intriguing about this story is that the same metabolite is biosynthesised by the terrestrial fungus Leptosphaeria maculans/Phoma lingam via the acetate/SAM pathway.909 The mollusc incorporation study made use of analysis of cross-peak volumes in HSQC and HMBC data to determine sites of isotopic enrichment.
SAR studies on the cytotoxic cyclic depsipeptide kulokekahilide-2 (Philinopsis speciosa)910,911 have identified the importance of the cyclic structure (but not the ring size), and the configuration at 21-L-Ala and 24-D-MePhe as important determinants of potency.912,913para-Chloro-24-MePhe analogues were found to be particularly potent cytotoxins. The structures of α-pyrone polyketides aplysiopsene A–D (Aplysiopsis formosa)914 have been confirmed by synthesis, with the absolute configuration of aplysiopsene D 1145 defined by synthesis of the enantiomer.915
Also confirmed by synthesis are the structures of the 1,2,4-oxadiazole ring-containing alkaloids phidianidine A and B (Phidiana militaris).916–918 In addition to a number of known PUFAs, heneicosa-5,8,11,14-tetraenoic acid (21;4 n-7) was reported as a new natural product from the opistobranch mollusc Scaphander lignarius (Arctic).919 Mild, non-specific cytotoxicity was observed.
The biosynthesis of the aromatic polyketides previously reported from Mediterranean specimens of S. lignarius920,921 has been investigated identifying the functional expression of a phenylalanine ammonia lyase (PAL), representing the first report of PAL in animal cells, and that biosynthesis is located specifically to the mantle border Blochmann's glands cells.922
The γ-pyrone tridachiahydropyrone (Tridachia crispata)923,924 and two putative polyene precursors insert themselves into two phospholipid vesicle models of membranes, indicative perhaps of their location in molluscan cells.925 Mass spectrometry imaging, using a perfluorinated siloxane-pretreated porous silicon support, of the hypobranchial gland of the mollusc Dicathais orbita, has established the spatial distribution of brominated precursors of Tyrian purple dye.926
New indigoids, of undefined structure, were identified by LC-MS/MS (Orbitrap) analysis of dye from Hexaplex trunculus (French Mediterranean coast).927 In addition to three previously reported palmadorin diterpene glyceride esters,928 sixteen new congeners 1146–1161 were isolated from specimens of the nudibranch Austrodoris kerguelenensis (Anvers Is., Palmer Station, Antarctic).929 Six of the diterpenes inhibited (μM) the growth of human erythroleukaemia cells, with the most potent analogue 1155 also inhibiting Jak2-STAT5 and Erk1/2 activation pathways.
Investigation of the organelle distribution of metabolites in a single specimen of Chromodoris albopunctata (Inner Gneerings Reef, Mooloolaba, Queensland) yielded the sponge-derived diterpene 1162 and known analogues930,931 from the internal organs, while 1163–1165 were isolated from the mantle extract.932
New phorboxazole congeners 1166 and 1167 were isolated from the dorid nudibranch Aldisa andersoni (Muttom Coast, India).933 Both metabolites were feeding deterrents in a shrimp bioassay and mildly growth inhibitory to a panel of HTCLs, while 1167 was determined to exert cytostatic effects towards two of the tumour cell lines.
Tambjamine alkaloids C, E, F and BE-18591,934–936 isolated from nudibranchs, ascidians or a bacterium, acted as efficient transmembrane anion transporters, releasing chloride from phospholipid vesicles.937 Syntheses of the Hexabranchus sanguineus cyclic peptides938 sanguinamide A 1168939 and B940 have been reported. The cis,cis proline configuration originally proposed for sanguinamide A was corrected to Pro 4 cis, Pro 6 trans as shown – the resultant structure is stabilised by transannular hydrogen bonds making the natural product orally bioavailable in rats. In the case of sanguinamide B, final step macrocyclisation yielded the cis,cis-conformer as the dominant (kinetic control) product, while heating afforded a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the trans,trans natural product and trans,cis-conformer. The mixture of the latter two conformers inhibited P. aeruginosa fimbriae twitching motility.
1 mixture of the trans,trans natural product and trans,cis-conformer. The mixture of the latter two conformers inhibited P. aeruginosa fimbriae twitching motility.
Analogues of (−)-jorumycin (Jorunna funebris),941 with variation at the pendant methylene C-22, exhibited varied levels of potency towards a panel of HTCLs. A hippuric acid ester derivative exhibited broad spectrum nM potency.942
11 Tunicates (ascidians)
The 51 new tunicate-derived natural products presented in this review is one of the highest number reported per annum over the last decade. New alkyl sulfates 1169 and 1170 (Aplidium elegans) and 1171 (Ciona edwardsii) were reported from ascidians collected in the Bay of Naples.943 While 1169 and 1170 exhibited cytotoxicity towards murine macrophage cells, 1171 was inactive, highlighting the importance of sulfate groups for biological activity.Two unusual dimethylamino lipidsulfoxides aplisulfamine A 1172 and B 1173 were isolated from Aplidium sp. (Pozzuoli, Naples).944 A comprehensive combination of nOe analysis, J-based configurational analysis, and calculated 13C chemical shift and ECD data secured the absolute configurations of the alkaloids.
Three separate studies reported new congeners of the rubrolide and cadiolide families of furanones. In the first, South African specimens of Synoicum globosum (Algoa Bay) yielded known rubrolides E and F945 in addition to new more highly brominated analogues 1174–1177.946 Variable levels of antibacterial activity were observed for all six metabolites.
As well as rubrolide P 1178 and 1179 and 1177 (3′-bromorubrolide F, which the authors of this second study named rubrolide Q), a collection of Pseudodistoma antinboja (Tong-Yeong City, South Sea, S. Korea) also afforded four new cadiolide analogues C–F 1180–1184.947 Some of the latter examples, in addition to the known co-metabolite cadiolide B,948 exhibited potent antibacterial activity towards MSSA and MRSA strains. As well as cadiolide E, related furanones cadiolide G 1185, H 1186,1187 and I 1188 and diesters synoilide A 1189,1190 and B 1191,1192 (Synoicum sp., Chuja-do, S. Korea) were evaluated for antibacterial activity as well as activity in sortase A, isocitrate lyase (ICL) and Na+/K+-ATPase assays.949 Cadiolides E, G–I exhibited antibacterial activity towards Gram-positive and Gram-negative bacteria, while the diesters were inactive, helping define an important SAR parameter. All metabolites exhibited activity towards the ATPase target, and all except cadiolide H were active against ICL but only cadiolide E inhibited sortase A (weak). Using calculated ECD data, the absolute configuration of 1185 was also assigned.
A small library of semi-synthetic analogues of eudistomin Y2 and Y3 was prepared and evaluated against the same set of biological targets used for the cadiolides; only one analogue exhibited increased cytotoxic potency, while all other analogues were less active than the natural products in the respective assays.950 New efficient syntheses of rubrolides C and E have been reported.951
Potently cytotoxic macrolides mandelalide A–D 1193–1196 were isolated from Lissoclinum sp. (Algoa Bay, South Africa); relative configurations were assigned by extensive J-based and rOe analysis and absolute configuration was assigned to 1193 by a combination of sugar analysis (GC-MS) and rOe data.952,953 Note that the structure diagram in the original paper952 implying mandelalide C to be a hydroperoxide at C-24, is a typographical error, and that the macrolide is indeed a C-24 carbinol.954
New rossinone congeners 1197–1199 (Aplidium fuegiense, dredge Western Weddell Sea, Antarctica) were localised in extracts of the ascidian internal organs.955,956 Both rossinone B957 and a mixture of meridianin alkaloids exhibited feeding deterrence towards the sea star Odontaser validus and amphipod Cheirimedon femoratus.958
In contrast to the monomeric, presumed precursor 1,8-dihydroxy-9,10-anthraquinone, the anthrone-anthraquinone dimer albopunctatone 1200 (Didemnum albopunctatum, Swain Reefs, Great Barrier Reef) was equipotent towards chloroquine-sensitive and chloroquine-resistant strains of P. falciparum.959 While a previous study of an Australian collection of Leptoclinides durus afforded, amongst other metabolites, (+)-(S)-leptoclinidamine B,960 Indonesian (Lembeh Strait, North Sulawesi) specimens of L. dubius have yielded leptoclinidamide 1201 and (−)-(R)-leptoclinidamine B 1202.961
Further investigation of an extract of Herdmania momus (Jeju Is., S. Korea), from which anti-inflammatory amino acid derivatives had been previously identified,962 afforded new congeners herdmanine E–L 1203–1210, in addition to (−)-(R)-leptoclinidamine B.963,964 The latter compound and herdmanine I and K all transactivated PPAR-γ in a cell-based luciferase reporter assay.
Mollamides E 1211 and F 1212 and molleurea A 1213 were obtained from Didemnum molle (New Britain, Papua New Guinea) with NMR chemical shift and nOe data defining the proline conformations as cis in 1211 and cis and trans in 1212.965 These same two peptides have previously been identified as predicted products from the genome sequence of Prochloron spp., and detected by LC-MS in an ascidian966 or in E. coli heterologously expressing the pathway.967
Australian collections of Didemnum sp. (Wasp Is., New South Wales, and Northern Rottnest Shelf, Western Australia) afforded an extensive number of lamellarin alkaloids including the new members lamellarin A1–A6 1214–1219.968 Evaluation of the library identified examples exhibiting cytotoxicity towards a human carcinoma cell line and a P-gp-overexpressing variant, while addition to doxorubicin (P-gp substrate) in the same assays identified a permethylated semi-synthetic analogue as being the best inhibitor of P-gp activity.
Further investigation of the Northern Rottnest Shelf specimens of Didemnum sp. also yielded ningalins E–G 1220–1222.969 Ningalins C, D and G were potent inhibitors of CK1δ, CDK5 and GSK3β kinases.
Biological evaluation of a mixture of two new staurosporine analogues 1223 and 1224 (Eudistoma vannamei, Ceara state, Brazil) identified the mixture as more potent than the parent compound.970 Synthesis of the structure originally proposed for palmerolide C (Synoicum adareanum)971 has required correction of configurations at C-9 and C-10 to those shown in 1225.972 Synthesis of the structure originally proposed for haouamine B (Aplidium haouarianum)973 combined with re-examination/re-acquisition of NMR data for haouamine B peracetate requires the structure to be corrected to the ortho-diphenol (catechol) shown in 1226.974
A stereoselective and convergent synthesis of lepadiformines A–C (Clavelina lepadiformis and C. moluccensis)975,976 has been reported, confirming the absolute configuration of lepadiformine B.977 A thorough investigation of different methods used to determine the absolute configuration of synoxazolidinone natural products (Synoicum pulmonaria)978,979 has highlighted the value and reliability of VCD.980 The structures and absolute configurations of the brominated 1,2,3,4-tetrahydro-β-carboline alkaloids eudistomidin G–I (Eudistoma glaucus)981,982 have been confirmed by synthesis983 as have those of an iodinated nucleoside984 originally reported from Diplosoma sp.985,986
Biomimetic syntheses of the apoptosis-inducing prenylated thiazinoquinones thiaplidiaquinone A and B (Aplidium conicum)987 have been reported.988,989 Two different routes for the synthesis of the antimalarial pyrroloquinoline aplidiopsamine A (Aplidiopsis confluata)990 have been reported,991 with one992 of the studies also identifying the natural product as a moderate inhibitor of rat and human PDE4. Total synthesis of the structure proposed for didemnaketal A (Didemnum sp.)993 gave a product that exhibited different NMR spectra compared to those reported for the natural product, calling into question the original stereochemical assignments.994
12 Echinoderms
The 37 new metabolites reported from echinoderms in 2012 is a modest increase from the number reported in 2011. Known sulfated alkanes 2,6-dimethylheptyl sulfate, octyl sulfate and decyl sulfate as well as new sulfated alkenes (5Z)-dec-5-en-1-yl sulfate and (3E)-dec-3-en-1-yl sulfate were isolated from the sea cucumber Apostichopus japonicus (commercially supplied).995 All of the metabolites exhibited moderate to potent cytotoxicity and antibiotic activity towards E. coli. Cerebrosides 1227 and 1228 were obtained as pure entities for the first time (Cucumaria frondosa, undefined location),996 with 1227 having previously been reported as a component of a mixture from Stichopus japonicus.997Crude preparations of cerebrosides from the sea cucumber Acaudina molpadioides (Zhejiang Province, China) and the starfish Asterias amurensis (S. Hokkaido, Japan) exhibited pronounced in vitro and in vivo antitumour activity and appear to function by induction of apoptosis via a mitochondrial-mediated pathway.998 A series of complex ganglioside molecular species PNG-1 (disaccharide), PNG-2A and PNG-2B (tetraosides) were reported from the starfish Protoreaster nodosus (Katsuren, Okinawa).999 The sterol sulfate mithrotriol 1229 was isolated as a non-cytotoxic component of the starfish Mithrodia clavigera (Maldives Is.).1000
An unusual peroxy-ester lucunterperacetate 1230 and the more classical peroxy-bridged sterol peroxylucunterine 1231 were reported from the urchin Echinometra lucunter (Dakar, Senegal).1001
An amidotaurine β-D-xyloside, fisherioside A 1232, was isolated from the starfish Leptasterias fisheri (Sakhalin Is., Sea of Okhotsk),1002 while pentaosides lethasteriosides A 1233 and B 1234 were reported from L. fusca (Posyet Bay, Sea of Japan).1003 Lethasterioside A, while being weakly cytotoxic, had pronounced ability to inhibit colony formation of tumour cells in a soft agar clonogenic assay.
In five separate accounts, a series of tri-, tetra- and pentaosides were reported from the same collection of the sea cucumber Eupentacta fraudatrix (Peter the Great Gulf, Sea of Japan). Of the two side-chain isomeric triosides, cucumarioside B11235 and B21236, only the latter demonstrated cytotoxicity and haemolytic activity (mild).1004 Minor metabolite tetraosides cucumarioside A1–A151237–1251 all incorporated the same tetrasaccharide unit.1005–1007 Cucumariosides A1, A5, A10 and A11 are desulfated derivatives of previously reported cucumariosides G1, G3, G2 and G4, respectively1008–1011 and cucumarioside A15 appears to be identical to a previously reported starfish metabolite.1012 Cucumariosides A1, A2, A6, A8 and A13 were the most active of the series in a range of biological studies assessing cytotoxicity, antifungal and haemolytic activities. Out of the three pentaosides cucumarioside H2–H41252–1254, there was more pronounced cytotoxicity and haemolytic activity for the likely artefactual ethyl ether, cucumarioside H4.1013
The sea cucumber Apostichopus japonicus is used as a food and a tonic in China – investigation of constituents of specimens (Dalian coast, Bohai Sea of China) identified penta- and hexaosides 1255, holotoxin D–G 1256–12591014 and holotoxin D11260 and 24,25-dihydroxyholotoxin A11261.1015 Broad spectrum antifungal activity was observed for holotoxins D–G and holotoxin D1, with indications that the presence of a lactone and a double bond in the sidechain at C-25 increases activity.
The antifungal properties of the closely related hexaoside, bivittoside D (Bohadschia vitiensis),1016 have also been investigated1017 while tetraosides echinosides A and B (Actinopyga echinites and Holothuria polii)1018 demonstrated schistomicidal activity in vitro.1019 The antitumour effects of the echinosides A and B have also been investigated, establishing that each echinoside can induce apoptosis in human HepG2 cells via the mitochondrial pathway, induce cell cycle arrest in G0/G1 phase and reduce tumour growth in vivo (ip dosing).1020 Interestingly, desulfated echinoside B suppressed NF-κB expression, while echinoside A did not. The related tetraoside neothyonidioside1021 (Australostichopus mollis) demonstrated antifungal activity by binding to ergosterol, leading to altered membrane curvature and fusion capability.1022
13 Mangroves
Three known dimeric alkyl aromatics integracin A 1262, B 1263 and dehydroxy-B 1264 were isolated from Sonneratia hainanensis (Hainan Province, China), and absolute configurations assigned to 1262 (degradation and Mosher), 1263 (degradation and optical rotation) and 1264 (loosely on biogenetic grounds).1023Investigation of the chemistry of the bark of Aegiceras corniculatum (Nizampatnam coast, India) yielded corniculatolide macrolides 1265–1268.1024
Four polyphenols excoecariphenol A–D 1269–1272, including two unusual thioglycosides, were reported from Excoecaria agallocha (China).1025 Excoecariphenol D inhibited NS3-4A protease and exhibited anti-hepatitis C virus (HCV) activity in two assays.
Extracts of seeds of Xylocarpus moluccensis collected at two sites (Godavari and Krishna estuaries, Andhra Pradesh, India) yielded new limonoids 1273–1280 in addition to several known congeners1026 including 1281.1027 Absolute configuration was assigned to both 1277 and 1281. The known 2-hydroxyfissinolide and 1273, 1274 and 1281 exhibited antifeedant activity towards third-instar larvae of Brontispa longissima, with 2-hydroxyfissinolide being the most active.
The structurally rare or unique limonoids andhraxylocarpin A–E 1282–1286 were isolated from seeds of X. moluccensis and X. granatum (Godavari and Krishna estuaries, Andhra Pradesh, India) with relative configurations of 1282 and 1284 established by X-ray analysis and absolute configurations assigned to all metabolites via quantum chemical calculations of CD or optical rotation properties.1028 A bioinspired [4 + 2] dimerisation of a 4-hydroxybutenolide precursor has been used to confirm the structure of paracaseolide A,1029 recently reported from stem bark extract of Sonneratia paracaseolaris.1030
14 Miscellaneous
The cyanobacterial neurotoxin β-N-methylamino-L-alanine has been detected in fins of seven species of sharks in South Florida, raising the possibility of human exposure through consumption.1031 Using a previously reported cuttlefish (Sepia officinalis) neuropeptide1032 as a biologically inactive starting point, substitution of aza-β3-amino acids led to the discovery of analogues with increased potency.1033 The data suggested that a peptide helical structure was not necessary for biological activity and that structural flexibility was important.15 Conclusion
MNP research can be conveniently divided into two areas; the discovery of new compounds from macro and micro marine sources, and all other aspects including those involved with syntheses of newly discovered compounds, corrections of structure and/or stereochemistry, assignment of stereochemistry, reviews, bioactivity and biosynthetic studies along with ecological and general surveys of marine species. All but the ecological and survey aspects are normally covered each year in this annual review. Just how many corresponding authors are involved worldwide in this effort? This information is shown in Table 1 below. A survey of the literature from 2007 to 2012 was undertaken to identify those corresponding authors (hereinafter referred to as ‘authors’) involved in these two broad areas as well as the distribution of effort by country. Interrogation of the marine literature database MarinLit1034 identified all papers reporting new compounds published over that period, the year of publication and the author responsible (Compounds) as well establishing a comparable set of results for papers reporting syntheses, corrections of structure, stereochemistry, reviews, ecological studies, surveys, bioactivities etc (Other Aspects). After compilation by author the results were assessed and placed into one of three categories, A, B or C. The placement was based on the numbers of papers published by each author over the six-year period and the frequency at which they were published. For the Compounds group the numbers of papers published by an individual author ranged down from 65 to just one and the frequency from every year to one year out of the six. Typically for an A placement in Compounds the authors needed to have published ≥10 papers and published in at least three of the years between 2007–2012. As the numbers and/or frequency diminished the rankings decreased. A comparable system based on ≥7 papers was used for Other Aspects where the range of papers from one author was from 19 downwards. While varying the ranking system might change the relative populations of the A, B and C categories, the totals remain the same. A comparable system based on ≥7 papers was used for Other Aspects where the range of papers from one author was from 19 downwards. While varying the ranking system might change the relative populations of the A, B and C categories, the totals remain the same. That is, 668 authors contributed papers on isolation only, while 286 of these authors, along with a further 1842 authors, published in the Other Aspects area. The crossover was usually in the direction of specialist isolation chemists also undertaking the likes of synthetic/stereochemical work. The countries that were dominant (>15 authors) in the Compounds area are (in alphabetical order): Australia, China, Egypt, France, Germany, India, Italy, Japan, Russia, South Korea, Spain, Taiwan and the USA. For Other Aspects it is a comparable list with the addition of Brazil, Canada, New Zealand, Norway, and the UK.| Compounds | Other Aspects | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |||
| a There were 40 countries that each had fewer than 4 corresponding authors in the Compounds section. | ||||||||
| Australia | 5 | 5 | 5 | 15 | 2 | 8 | 50 | 60 |
| Brazil | 2 | 5 | 7 | 4 | 8 | 73 | 85 | |
| Canada | 2 | 1 | 6 | 9 | 2 | 46 | 48 | |
| China | 23 | 23 | 134 | 180 | 5 | 17 | 190 | 212 |
| Colombia | 1 | 3 | 4 | 1 | 1 | 3 | 5 | |
| Egypt | 1 | 2 | 14 | 17 | 1 | 13 | 14 | |
| France | 3 | 2 | 20 | 25 | 3 | 9 | 109 | 121 |
| Germany | 4 | 5 | 22 | 31 | 5 | 14 | 101 | 120 |
| India | 17 | 17 | 6 | 11 | 105 | 122 | ||
| Italy | 6 | 4 | 11 | 21 | 1 | 7 | 50 | 58 |
| Japan | 9 | 20 | 68 | 97 | 12 | 35 | 188 | 235 |
| Malaysia | 1 | 4 | 5 | 2 | 9 | 11 | ||
| New Zealand | 1 | 1 | 6 | 8 | 4 | 2 | 15 | 21 |
| Norway | 1 | 3 | 4 | 1 | 1 | 13 | 15 | |
| Russia | 8 | 4 | 4 | 16 | 1 | 5 | 46 | 52 |
| South Korea | 6 | 5 | 17 | 28 | 2 | 7 | 67 | 76 |
| Saudi Arabia | 1 | 3 | 4 | 5 | 5 | |||
| Spain | 2 | 2 | 12 | 16 | 4 | 6 | 94 | 104 |
| Taiwan | 4 | 5 | 7 | 16 | 3 | 25 | 28 | |
| Thailand | 2 | 3 | 5 | 10 | 12 | 12 | ||
| UK | 1 | 1 | 7 | 9 | 4 | 10 | 75 | 89 |
| USA | 15 | 13 | 67 | 95 | 29 | 51 | 341 | 421 |
| Other countriesa | 3 | 7 | 24 | 34 | 3 | 16 | 195 | 214 |
| 96 | 108 | 464 | 668 | 90 | 213 | 1825 | 2128 | |
The 96 A-ranked authors in Compounds reported 4033 compounds in 1234 papers, those in B (108) were responsible for 1264 compounds in 435 papers and for C (464) a total of 1282 compounds in 549 papers. In Other Aspects the A-ranked authors (90) published 712 papers, those in B (213) 772 papers while the 1825 authors in C published 2142 papers. Of the overall 2510 corresponding authors across the 2007–2012 period, 499 authors published more than two papers. Possible trends across this publication period were evaluated based on the numbers of publications and the years in which they were published: Constant if publications for 2007–2009 = 2010–2012; Upward if numbers for 2007–2009 < 2010–2012; Downward if numbers for 2007–2009 > 2010–2012. These data were included as it was felt that the method of assessment used could have disadvantaged younger, emerging scientists who only started to publish in the 2010–2012 period. It was found that the Upward/Downward trend for the two broad areas of research across all scientists was relatively constant, but at the country level there was an Upward trend in Compounds for China and Taiwan. The crossover between the two areas is interesting. Of the 93 A-ranked authors in Compounds, 12 were also A in Other Aspects and 24 were ranked B. If the rankings for Compounds and Other Aspects are combined the top 50 ranked authors are almost all well-known isolation chemists confirming the breadth of interests of the marine natural product community.
The marine natural product database MarinLit1034 has been an essential tool for the authors in assembling all aspects of this review. The ownership of MarinLit has now been transferred from the University of Canterbury, New Zealand to the Royal Society of Chemistry, London.1035 The database was maintained by the University of Canterbury until the end of 2013. In 2014 a web-based version of MarinLit will become available.
16 References
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2013, 30, 237–323 RSC.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2012, 29, 144–222 RSC.
- R. A. Hill, Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2012, 108, 131–146 RSC.
- Handbook of Marine Natural Products, ed. E. Fattorusso, W. H. Gerwick and O. Taglialatela-Scafati, Springer, Dordrecht, 2012 Search PubMed.
- http://marinepharmacology.midwestern.edu/ Accessed 24 May 2013.
- A. M. S. Mayer, Toxicon, 2012, 60, 104 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2012, 75, 311–335 CrossRef CAS PubMed.
- C. I. Schroeder and D. J. Craik, Future Med. Chem., 2012, 4, 1243–1255 CrossRef CAS PubMed.
- R. J. Lewis, S. Dutertre, I. Vetter and M. J. Christie, Pharmacol. Rev., 2012, 64, 259–298 CrossRef CAS PubMed.
- J. Lee, J. N. Currano, P. J. Carroll and M. M. Joullié, Nat. Prod. Rep., 2012, 29, 404–424 RSC.
- J. Y. Hong and H. Luesch, Nat. Prod. Rep., 2012, 29, 449–456 RSC.
- P. A. Harnedy and R. J. FitzGerald, J. Funct. Foods, 2012, 4, 6–24 CrossRef CAS PubMed.
- G.-M. Suarez-Jimenez, A. Burgos-Hernandez and J.-M. Ezquerra-Brauer, Mar. Drugs, 2012, 10, 963–986 CrossRef CAS PubMed.
- F. Lazcano-Perez, S. A. Roman-Gonzalez, N. Sanchez-Puig and R. Arreguin-Espinosa, Protein Pept. Lett., 2012, 19, 700–707 CrossRef CAS.
- S. N. Sunassee and M. T. Davies-Coleman, Nat. Prod. Rep., 2012, 29, 513–535 RSC.
- V. Sele, J. J. Sloth, A. K. Lundebye, E. H. Larsen, M. H. G. Berntssen and H. Amlund, Food Chem., 2012, 133, 618–630 CrossRef CAS PubMed.
- B. F. Ruan and H. L. Zhu, Curr. Med. Chem., 2012, 19, 2652–2664 CrossRef CAS.
- C.-S. Jiang, W. E. G. Muller, H. C. Schröder and Y.-W. Guo, Chem. Rev., 2012, 112, 2179–2207 CrossRef CAS PubMed.
- S. B. Bharate, S. Manda, N. Mupparapu, N. Battini and R. A. Vishwakarma, Mini-Rev. Med. Chem., 2012, 12, 650–664 CrossRef CAS.
- M. Sugumaran and W. E. Robinson, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2012, 163, 1–25 CrossRef CAS PubMed.
- V. I. Kalinin, N. V. Ivanchina, V. B. Krasokhin, T. N. Makarieva and V. A. Stonik, Mar. Drugs, 2012, 10, 1671–1710 CrossRef CAS PubMed.
- B. Frazão, V. Vasconcelos and A. Antunes, Mar. Drugs, 2012, 10, 1812–1851 CrossRef PubMed.
- R. A. Mosey and P. E. Floreancig, Nat. Prod. Rep., 2012, 29, 980–995 RSC.
- Z. J. Witczak, R. M. Rampulla and A. Bommareddy, Mini-Rev. Med. Chem., 2012, 12, 1520–1532 CrossRef CAS.
- Q.-F. Lin, J.-G. Cui, C.-F. Gan, L. Liu, Q.-C. Yao and Y.-M. Huang, Chin. J. Org. Chem., 2012, 32, 2214–2222 CrossRef CAS.
- B. M. Fraga, Nat. Prod. Rep., 2012, 29, 1334–1366 RSC.
- B. Yang, X.-F. Zhou, X.-P. Lin, J. Liu, Y. Peng, X.-W. Yang and Y. Liu, Curr. Org. Chem., 2012, 16, 1512–1539 CrossRef CAS.
- A. D. Borthwick, Chem. Rev., 2012, 112, 3641–3716 CrossRef CAS PubMed.
- M. Nagarajan, V. Maruthanayagam and M. Sundararaman, J. Appl. Toxicol., 2012, 32, 153–185 CrossRef CAS PubMed.
- M. Costa, J. Costa-Rodrigues, M. H. Fernandes, P. Barros, V. Vasconcelos and R. Martins, Mar. Drugs, 2012, 10, 2181–2207 CrossRef CAS PubMed.
- J. Gallardo-Rodríguez, A. Sánchez-Mirón, F. García-Camacho, L. López-Rosales, Y. Chisti and E. Molina-Grima, Biotechnol. Adv., 2012, 30, 1673–1684 CrossRef PubMed.
- R. Pistocchi, F. Guerrini, L. Pezzolesi, M. Riccardi, S. Vanucci, P. Ciminiello, C. Dell'Aversano, M. Forino, E. Fattorusso, L. Tartaglione, A. Milandri, M. Pompei, M. Cangini, S. Pigozzi and E. Riccardi, Mar. Drugs, 2012, 10, 140–162 CrossRef CAS PubMed.
- S. B. Zotchev, J. Biotechnol., 2012, 158, 168–175 CrossRef CAS PubMed.
- L.-H. Wang, J.-H. Sheu, S.-Y. Kao, J.-H. Su, Y.-H. Chen, Y.-H. Chen, Y.-D. Su, Y.-C. Chang, L.-S. Fang, W.-H. Wang, Y.-C. Wu and P.-J. Sung, Mar. Drugs, 2012, 10, 2415–2434 CrossRef PubMed.
- E. W. Schmidt, M. S. Donia, J. A. McIntosh, W. F. Fricke and J. Ravel, J. Nat. Prod., 2012, 75, 295–304 CrossRef CAS PubMed.
- E. L. Cooper and D. Yao, Drug Discovery Today, 2012, 17, 636–648 CrossRef CAS PubMed.
- P. L. Kiew and M. M. Don, Int. J. Food Sci. Nutr., 2012, 63, 616–636 CrossRef CAS PubMed.
- M. L. C. da Frota, R. B. da Silva, B. Mothes, A. T. Henriques and J. C. F. Moreira, Curr. Pharm. Biotechnol., 2012, 13, 235–244 Search PubMed.
- J. C. Noro, J. A. Kalaitzis and B. A. Neilan, Chem. Biodiversity, 2012, 9, 2077–2095 CAS.
- M. Roué, E. Quévrain, I. Domart-Coulon and M. L. Bourguet-Kondracki, Nat. Prod. Rep., 2012, 29, 739–751 RSC.
- R. C. Pereira and L. V. Costa-Lotufo, Rev. Bras. Farmacogn., 2012, 22, 894–905 CrossRef CAS PubMed.
- A. L. L. de Oliveira, R. de Felicio and H. M. Debonsi, Rev. Bras. Farmacogn., 2012, 22, 906–920 CrossRef PubMed.
- K. D. Feussner, K. Ragini, R. Kumar, K. M. Soapi, W. G. Aalbersberg, M. K. Harper, B. Carte and C. M. Ireland, Nat. Prod. Rep., 2012, 29, 1424–1462 RSC.
- O. Sacristán–Soriano, B. Banaigs and M. A. Becerro, Mar. Drugs, 2012, 10, 677–693 CrossRef PubMed.
- S. Rohde, D. J. Gochfeld, S. Ankisetty, B. Avula, P. J. Schupp and M. Slattery, J. Chem. Ecol., 2012, 38, 463–475 CrossRef CAS PubMed.
- F. Amir, Y. C. Koay and W. S. Yam, Trop. J. Pharm. Res., 2012, 11, 485–498 CAS.
- F. Amir, Y. C. Koay and W. S. Yam, Trop. J. Pharm. Res., 2012, 11, 499–517 CAS.
- C. Nastrucci, A. Cesario and P. Russo, Recent Pat. Anti-Cancer Drug Discovery, 2012, 7, 218–232 CrossRef CAS.
- E. Delfourne, Mini-Rev. Med. Chem., 2012, 12, 988–996 CrossRef CAS.
- J. Liu, Y. Hu, D. L. Waller, J. Wang and Q. Liu, Nat. Prod. Rep., 2012, 29, 392–403 RSC.
- C.-S. Jiang, L.-F. Liang and Y.-W. Guo, Acta Pharmacol. Sin., 2012, 33, 1217–1245 CrossRef CAS PubMed.
- I. Abraham, K. El Sayed, Z.-S. Chen and H. Guo, Mar. Drugs, 2012, 10, 2312–2321 CrossRef CAS PubMed.
- S. Fiorucci, E. Distrutti, G. Bifulco, M. V. D'Auria and A. Zampella, Trends Pharmacol. Sci., 2012, 33, 591–601 CrossRef CAS PubMed.
- Y. Mizushina, I. Kuriyama and H. Yoshida, Curr. Org. Chem., 2012, 16, 2961–2969 CrossRef CAS.
- N. D'Orazio, M. A. Gammone, E. Gemello, M. De Girolamo, S. Cusenza and G. Riccioni, Mar. Drugs, 2012, 10, 812–833 CrossRef CAS PubMed.
- P. N. Leão, N. Engene, A. Antunes, W. H. Gerwick and V. Vasconcelos, Nat. Prod. Rep., 2012, 29, 372–391 RSC.
- D. J. Gochfeld, H. N. Kamel, J. B. Olson and R. W. Thacker, J. Chem. Ecol., 2012, 38, 451–462 CrossRef CAS PubMed.
- M. C. Leal, C. Madeira, C. A. Brandão, J. Puga and R. Calado, Molecules, 2012, 17, 9842–9854 CrossRef CAS PubMed.
- M. C. Leal, J. Puga, J. Serôdio, N. C. M. Gomes and R. Calado, PLoS One, 2012, 7, e30580 CAS.
- J. C. Morris, Nat. Prod. Rep., 2013, 30, 783–805 RSC.
- T. F. Molinski and B. I. Morinaka, Tetrahedron, 2012, 68, 9307–9343 CrossRef CAS PubMed.
- T. Kurtán, R. Jia, Y. Li, G. Pescitelli and Y.-W. Guo, Eur. J. Org. Chem., 2012, 34, 6722–6728 CrossRef.
- M. Slattery, S. Ankisetty, J. Corrales, K. E. Marsh-Hunkin, D. J. Gochfeld, K. L. Willett and J. M. Rimoldi, J. Nat. Prod., 2012, 75, 1833–1877 CrossRef CAS PubMed.
- D. Camp, R. A. Davis, E. A. Evans-Illidge and R. J. Quinn, Future Med. Chem., 2012, 4, 1067–1084 CrossRef CAS PubMed.
- S. Goulitquer, P. Potin and T. Tonon, Mar. Drugs, 2012, 10, 849–880 CrossRef CAS PubMed.
- K. Duarte, T. A. P. Rocha–Santos, A. C. Freitas and A. C. Duarte, TrAC, Trends Anal. Chem., 2012, 34, 97–110 CrossRef CAS PubMed.
- W. H. Gerwick and B. S. Moore, Chem. Biol., 2012, 19, 85–98 CrossRef CAS PubMed.
- S. Sato, F. Iwata, S. Yamada and M. Katayama, J. Nat. Prod., 2012, 75, 1974–1982 CrossRef CAS PubMed.
- F. S. Tareq, J. H. Kim, M. A. Lee, H.-S. Lee, Y.-J. Lee, J. S. Lee and H. J. Shin, Org. Lett., 2012, 14, 1464–1467 CrossRef CAS PubMed.
- Z. Ma, N. Wang, J. Hu and S. Wang, J. Antibiot., 2012, 65, 317–322 CrossRef CAS PubMed.
- M. Azumi, K. Ogawa, T. Fujita, M. Takeshita, R. Yoshida, T. Furumai and Y. Igarashi, Tetrahedron, 2008, 64, 6420–6425 CrossRef CAS PubMed.
- J. Itoh, T. Shomura, S. Omoto, S. Miyado, Y. Yuda, U. Shibata and S. Inouye, Agric. Biol. Chem., 1982, 46, 1255–1259 CrossRef CAS.
- Y. Li, Y. Xu, L. Liu, Z. Han, P. Y. Lai, X. Guo, X. Zhang, W. Lin and P.-Y. Qian, Mar. Drugs, 2012, 10, 319–328 CrossRef CAS PubMed.
- A. P. Terent'ev, A. N. Grinev and A. B. Terent'ev, Zhurnal Obshchei Khimii, 1954, 24, 1433–1435 CAS.
- N. Moriya, N. Ikeda and Y. Tada, Jpn. Kokai Tokkyo Koho, 2011, JP 2011088873 A 20110506.
- A. S. Leutou, K. Yun, H. D. Choi, J. S. Kang and B. W. Son, J. Microbiol. Biotechnol., 2012, 22, 80–83 CrossRef CAS.
- Y. Hu, A. G. Legako, A. P. D. M. Espindola and J. B. MacMillan, J. Org. Chem., 2012, 77, 3401–3407 CrossRef CAS PubMed.
- S. H. Kim, Y. K. Shin, Y. C. Sohn and H. C. Kwon, Molecules, 2012, 17, 12357–12364 CrossRef CAS PubMed.
- X. Zhou, H. Huang, Y. Chen, J. Tan, Y. Song, J. Zou, X. Tian, Y. Hua and J. Ju, J. Nat. Prod., 2012, 75, 2251–2255 CrossRef CAS PubMed.
- H. A. Kirst, D. E. Dorman, J. L. Occolowitz, N. D. Jones, J. W. Paschal, R. L. Hamill and E. F. Szymanski, J. Antibiot., 1985, 38, 575–586 CrossRef CAS.
- Q. Zhu, J. Li, J. Ma, M. Luo, B. Wang, H. Huang, X. Tian, W. Li, S. Zhang, C. Zhang and J. Ju, Antimicrob. Agents Chemother., 2012, 56, 110–114 CrossRef CAS PubMed.
- S. Baur, J. Niehaus, A. D. Karagouni, E. A. Katsifas, K. Chalkou, C. Meintanis, A. L. Jones, M. Goodfellow, A. C. Ward, W. Beil, K. Schneider, R. D. Süssmuth and H. P. Fiedler, J. Antibiot., 2006, 59, 293–297 CrossRef CAS PubMed.
- Z. Feng, J. H. Kim and S. F. Brady, J. Am. Chem. Soc., 2010, 132, 11902–11903 CrossRef CAS PubMed.
- G. Fendrich, W. Zimmermann, J. Gruner and J. A. L. Auden, Eur. Pat. Appl., 1989, EP 304400 A2 19890222.
- W. Zhang, Z. Liu, S. Li, Y. Lu, Y. Chen, H. Zhang, G. Zhang, Y. Zhu, J. Li and C. Zhang, J. Nat. Prod., 2012, 75, 1937–1943 CrossRef CAS PubMed.
- T. D. S. Sousa, P. C. Jimenez, E. G. Ferreira, E. R. Silveira, R. Braz-Filho, O. D. L. Pessoa and L. V. Costa-Lotufo, J. Nat. Prod., 2012, 75, 489–493 CrossRef CAS PubMed.
- T. P. Wyche, Y. Hou, E. Vazquez-Rivera, D. Braun and T. S. Bugni, J. Nat. Prod., 2012, 75, 735–740 CrossRef CAS PubMed.
- J. Y. Cho, P. G. Williams, H. C. Kwon, P. R. Jensen and W. Fenical, J. Nat. Prod., 2007, 70, 1321–1328 CrossRef CAS PubMed.
- J. W. Cha, J.-S. Park, T. Sim, S.-J. Nam, H. C. Kwon, J. R. Del Valle and W. Fenical, J. Nat. Prod., 2012, 75, 1648–1651 CrossRef CAS PubMed.
- S. Ranatunga, C.-H. A. Tang, C.-C. A. Hu and J. R. Del Valle, J. Org. Chem., 2012, 77, 9859–9864 CrossRef CAS PubMed.
- K. B. Selim, B. K. Lee and T. Sim, Tetrahedron Lett., 2012, 53, 5895–5898 CrossRef CAS PubMed.
- B. Elazari-Volcani, Arch. Microbiol., 1939, 10, 343–58 CAS.
- W. N. Cude, J. Mooney, A. A. Tavanaei, M. K. Hadden, A. M. Frank, C. A. Gulvik, A. L. May and A. Buchan, Appl. Environ. Microbiol., 2012, 78, 4771–4780 CrossRef CAS PubMed.
- Y.-H. Chen, M.-C. Lu, Y.-C. Chang, T.-L. Hwang, W.-H. Wang, C.-F. Weng, J. Kuo and P.-J. Sung, Tetrahedron Lett., 2012, 53, 1675–1677 CrossRef CAS PubMed.
- Y.-H. Chen, J. Kuo, J.-H. Su, T.-L. Hwang, Y.-H. Chen, C.-H. Lee, C.-F. Weng and P.-J. Sung, Mar. Drugs, 2012, 10, 1566–1571 CrossRef CAS PubMed.
- D. Kim, J. S. Lee, Y. K. Park, J. F. Kim, H. Jeong, T.-K. Oh, B. S. Kim and C. Lee, J. Appl. Microbiol., 2007, 102, 937–944 CAS.
- D. Feher, R. S. Barlow, P. S. Lorenzo and T. K. Hemscheidt, J. Nat. Prod., 2008, 71, 1970–1972 CrossRef CAS PubMed.
- Y. Wang, A. Nakajima, K. Hosokawa, A. B. Soliev, I. Osaka, R. Arakawa and K. Enomoto, Biosci. Biotechnol. Biochem., 2012, 76, 1229–1232 CAS.
- H. He, W. D. Ding, V. S. Bernan, A. D. Richardson, C. M. Ireland, M. Greenstein, G. A. Ellestad and G. T. Carter, J. Am. Chem. Soc., 2001, 123, 5362–5363 CrossRef CAS.
- C. M. Woo, N. E. Beizer, J. E. Janso and S. B. Herzon, J. Am. Chem. Soc., 2012, 134, 15285–15288 CrossRef CAS PubMed.
- Y. Hu and J. B. MacMillan, Nat. Prod. Commun., 2012, 7, 211–214 CAS.
- L.-L. Liu, Y. Xu, Z. Han, Y.-X. Li, L. Lu, P.-Y. Lai, J.-L. Zhong, X.-R. Guo, X.-X. Zhang and P.-Y. Qian, Mar. Drugs, 2012, 10, 2571–2583 CrossRef CAS PubMed.
- M. Igarashi, R. Utsumi and T. Watanabe, Jpn. Kokai Tokkyo Koho, 2011, JP 2011201843, A 20111013.
- W. Xin, X. Ye, S. Yu, X.-Y. Lian and Z. Zhang, Mar. Drugs, 2012, 10, 2388–2402 CrossRef CAS PubMed.
- P. Fu, Y. Zhuang, Y. Wang, P. Liu, X. Qi, K. Gu, D. Zhang and W. Zhu, Org. Lett., 2012, 14, 6194–6197 CrossRef CAS PubMed.
- T. Yamada, K. Minoura and A. Numata, Tetrahedron Lett., 2002, 43, 1721–1724 CrossRef CAS.
- T. Yamada, K. Minoura and A. Numata, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 2001, 43, 455–460 Search PubMed.
- T. Yamada, T. Kikuchi, R. Tanaka and A. Numata, Tetrahedron Lett., 2012, 53, 2842–2846 CrossRef CAS PubMed.
- H. Huang, T. Yang, X. Ren, J. Liu, Y. Song, A. Sun, J. Ma, B. Wang, Y. Zhang, C. Huang, C. Zhang and J. Ju, J. Nat. Prod., 2012, 75, 202–208 CrossRef CAS PubMed.
- Y. Hu, E. D. Martinez and J. B. MacMillan, J. Nat. Prod., 2012, 75, 1759–1764 CrossRef CAS PubMed.
- N. A. Mahyudin, J. W. Blunt, A. L. J. Cole and M. H. G. Munro, J. Biomed. Biotechnol., 2012, 894708, DOI:10.1155/2012/894708.
- T. Hosoya, T. Hirokawa, M. Takagi and K. Shin-ya, J. Nat. Prod., 2012, 75, 285–289 CrossRef CAS PubMed.
- S. Imai, K. Furihata, Y. Hayakawa, T. Noguchi and H. Seto, J. Antibiot., 1989, 42, 1196–1198 CrossRef CAS.
- T. P. Kondratyuk, E.-J. Park, R. Yu, R. B. van Breemen, R. N. Asolkar, B. T. Murphy, W. Fenical and J. M. Pezzuto, Mar. Drugs, 2012, 10, 451–464 CrossRef CAS PubMed.
- U. R. Abdelmohsen, G. Zhang, A. Philippe, W. Schmitz, S. M. Pimentel-Elardo, B. Hertlein- Amslinger, U. Hentschel and G. Bringmann, Tetrahedron Lett., 2012, 53, 23–29 CrossRef CAS PubMed.
- R. Raju, A. M. Piggott, Z. Khalil, P. V. Bernhardt and R. J. Capon, Tetrahedron Lett., 2012, 53, 1063–1065 CrossRef CAS PubMed.
- Z. Lin, M. Flores, I. Forteza, N. M. Henriksen, G. P. Concepcion, G. Rosenberg, M. G. Haygood, B. M. Olivera, A. R. Light, T. E. Cheatham III and E. W. Schmidt, J. Nat. Prod., 2012, 75, 644–649 CrossRef CAS PubMed.
- D.-G. Kim, K. Moon, S.-H. Kim, S.-H. Park, S. Park, S. K. Lee, K.-B. Oh, J. Shin and D.-C. Oh, J. Nat. Prod., 2012, 75, 959–967 CrossRef CAS PubMed.
- W. Zhang, Z. Liu, S. Li, T. Yang, Q. Zhang, L. Ma, X. Tian, H. Zhang, C. Huang, S. Zhang, J. Ju, Y. Shen and C. Zhang, Org. Lett., 2012, 14, 3364–3367 CrossRef CAS PubMed.
- B. Ohlendorf, D. Schulz, A. Erhard, K. Nagel and J. F. Imhoff, J. Nat. Prod., 2012, 75, 1400–1404 CrossRef CAS PubMed.
- Z. Xie, B. Liu, H. Wang, S. Yang, H. Zhang, Y. Wang, N. Ji, S. Qin and H. Laatsch, Mar. Drugs, 2012, 10, 551–558 CrossRef CAS PubMed.
- G. Sakoulas, S.-J. Nam, S. Loesgen, W. Fenical, P. R. Jensen, V. Nizet and M. Hensler, PLoS One, 2012, 7, e29439 CAS.
- L. Kaysser, P. Bernhardt, S.-J. Nam, S. Loesgen, J. G. Ruby, P. Skewes-Cox, P. R. Jensen, W. Fenical and B. S. Moore, J. Am. Chem. Soc., 2012, 134, 11988–11991 CrossRef CAS PubMed.
- L. Ding, J. Münch, H. Goerls, A. Maier, H.-H. Fiebig, W.-H. Lin and C. Hertweck, Bioorg. Med. Chem. Lett., 2010, 20, 6685–6687 CrossRef CAS PubMed.
- Q. Zhang, A. Mándi, S. Li, Y. Chen, W. Zhang, X. Tian, H. Zhang, H. Li, W. Zhang, S. Zhang, J. Ju, T. Kurtán and C. Zhang, Eur. J. Org. Chem., 2012, 27, 5256–5262 CrossRef.
- H. Li, Q. Zhang, S. Li, Y. Zhu, G. Zhang, H. Zhang, X. Tian, S. Zhang, J. Ju and C. Zhang, J. Am. Chem. Soc., 2012, 134, 8996–9005 CrossRef CAS PubMed.
- T. Amagata, J. Xiao, Y.-P. Chen, N. Holsopple, A. G. Oliver, T. Gokey, A. B. Guliaev and K. Minoura, J. Nat. Prod., 2012, 75, 2193–2199 CrossRef CAS PubMed.
- J. Rohr and A. Zeeck, J. Antibiot., 1987, 40, 459–467 CrossRef CAS.
- M. Sezaki, S. Kondo, K. Maeda, H. Umezawa and M. Ohno, Tetrahedron, 1970, 26, 5171–5190 CrossRef CAS.
- K. Supong, C. Thawai, K. Suwanborirux, W. Choowong, S. Supothina and P. Pittayakhajonwut, Phytochem. Lett., 2012, 5, 651–656 CrossRef CAS PubMed.
- E. Pan, M. Jamison, M. Yousufuddin and J. B. MacMillan, Org. Lett., 2012, 14, 2390–2393 CrossRef CAS PubMed.
- Y. Igarashi, S. Miura, T. Fujita and T. Furumai, J. Antibiot., 2006, 59, 193–195 CrossRef CAS PubMed.
- Y. Igarashi, D. Asano, K. Furihata, N. Oku, S. Miyanaga, H. Sakurai and I. Saiki, Tetrahedron Lett., 2012, 53, 654–656 CrossRef CAS PubMed.
- M.-J. Xu, X.-J. Liu, Y.-L. Zhao, D. Liu, Z.-H. Xu, X.-M. Lang, P. Ao, W.-H. Lin, S.-L. Yang, Z.-G. Zhang and J. Xu, Mar. Drugs, 2012, 10, 639–654 CrossRef CAS PubMed.
- N. Kawamura, E. Tsuji, Y. Watanabe, K. Tsuchihashi and T. Takako, Jpn. Kokai Tokkyo Koho, 2000, JP 2000072766 A 20000307.
- E. H. Andrianasolo, L. Haramaty, R. Rosario-Passapera, C. Vetriani, P. Falkowski, E. White and R. Lutz, Mar. Drugs, 2012, 10, 2300–2311 CrossRef CAS PubMed.
- L. Gram, J. Melchiorsen and J. B. Bruhn, Mar. Biotechnol., 2010, 12, 439–451 CrossRef CAS PubMed.
- A. Nielsen, M. Mansson, M. Wietz, A. N. Varming, R. K. Phipps, T. O. Larsen, L. Gram and H. Ingmer, Mar. Drugs, 2012, 10, 2584–2595 CrossRef CAS PubMed.
- J. M. Gauglitz, H. Zhou and A. Butler, J. Inorg. Biochem., 2012, 107, 90–95 CrossRef CAS PubMed.
- G. G. Harrigan, B. L. Harrigan and B. S. Davidson, Tetrahedron, 1997, 53, 1577–1582 CrossRef CAS.
- Y. Kawabata, K. Mochida, M. Nishishima and M. Sugi, Jpn. Kokai Tokkyo Koho, 2000, JP 2000245497 A 20000912.
- R. Raju, K. Kawabata, M. Nishijima and W. G. L. Aalbersberg, Tetrahedron Lett., 2012, 53, 6905–6907 CrossRef CAS PubMed.
- Z. Han, Y. Xu, O. McConnell, L. Liu, Y. Li, S. Qi, X. Huang and P. Qian, Mar. Drugs, 2012, 10, 668–676 CrossRef CAS PubMed.
- P. Fu, C. Yang, Y. Wang, P. Liu, Y. Ma, L. Xu, M. Su, K. Hong and W. Zhu, Org. Lett., 2012, 14, 2422–2425 CrossRef CAS PubMed.
- L. Ding, A. Maier, H.-H. Fiebig, W.-H. Lin, G. Peschel and C. Hertweck, J. Nat. Prod., 2012, 75, 2223–2227 CrossRef CAS PubMed.
- Z. Chen, Y. Song, Y. Chen, H. Huang, W. Zhang and J. Ju, J. Nat. Prod., 2012, 75, 1215–1219 CrossRef CAS PubMed.
- E. Julianti, H. Oh, H.-S. Lee, D.-C. Oh, K.-B. Oh and J. Shin, Tetrahedron Lett., 2012, 53, 2885–2886 CrossRef CAS PubMed.
- K. Banert, Tetrahedron Lett., 2012, 53, 6443–6445 CrossRef CAS PubMed.
- F.-Z. Wang, Z. Huang, X.-F. Shi, Y.-C. Chen, W.-M. Zhang, X.-P. Tian, J. Li and S. Zhang, Bioorg. Med. Chem. Lett., 2012, 22, 7265–7267 CrossRef CAS PubMed.
- X. Xu, S. Zhao, J. Wei, N. Fang, L. Yin and J. Sun, Chem. Nat. Compd., 2012, 47, 893–895 CrossRef CAS PubMed.
- C.-J. Zheng, C.-L. Shao, Z.-Y. Guo, J.-F. Chen, D.-S. Deng, K.-L. Yang, Y.-Y. Chen, X.-M. Fu, Z.-G. She, Y.-C. Lin and C.-Y. Wang, J. Nat. Prod., 2012, 75, 189–197 CrossRef CAS PubMed.
- J. J. Irwin and B. K. Shoichet, J. Chem. Inf. Model., 2005, 45, 177–182 CrossRef CAS PubMed.
- C. Hopmann, M. A. Knauf, K. Weithmann and J. Wink, PCT Int. Appl., 2001, WO 2001044264 A2 20010621.
- G. Bringmann, G. Lang, S. Steffens, E. Günther and K. Schaumann, Phytochemistry, 2003, 63, 437–443 CrossRef CAS.
- O. I. Zhuravleva, S. S. Afiyatullov, V. A. Denisenko, S. P. Ermakova, N. N. Slinkina, P. S. Dmitrenok and N. Y. Kim, Phytochemistry, 2012, 80, 123–131 CrossRef CAS PubMed.
- D. Zhang, M. Satake, S. Fukuzawa, K. Sugahara, A. Niitsu, T. Shirai and K. Tachibana, J. Nat. Med., 2012, 66, 222–226 CrossRef CAS PubMed.
- S. S. Afiyatullov, O. I. Zhuravleva, E. L. Chaikina and M. M. Anisimov, Chem. Nat. Compd., 2012, 48, 95–98 CrossRef CAS.
- S. S. Afiyatullov, O. I. Zhuravleva, A. S. Antonov, A. I. Kalinovsky, M. V. Pivkin, E. S. Menchinskaya and D. L. Aminin, Nat. Prod. Commun., 2012, 7, 497–500 CAS.
- Y. Wang, Z.-L. Li, J. Bai, L.-M. Zhang, X. Wu, L. Zhang, Y.-H. Pei, Y.-K. Jing and H.-M. Hua, Chem. Biodiversity, 2012, 9, 385–393 CAS.
- F. He, Y.-L. Sun, K.-S. Liu, X.-Y. Zhang, P.-Y. Qian, Y.-F. Wang and S.-H. Qi, J. Antibiot., 2012, 65, 109–111 CrossRef CAS PubMed.
- X.-J. Li, Q. Zhang, A.-L. Zhang and J.-M. Gao, J. Agric. Food Chem., 2012, 60, 3424–3431 CrossRef CAS PubMed.
- R. P. Ubillas, Ph.D. thesis, University of Missouri-Columbia, 1990, pp. 61–74.
- M. Kitano, T. Yamada, T. Amagata, K. Minoura, R. Tanaka and A. Numata, Tetrahedron Lett., 2012, 53, 4192–4194 CrossRef CAS PubMed.
- Q. X. Wu, X. J. Jin, M. Draskovic, M. S. Crews, K. Tenney, F. A. Valeriote, X. J. Yao and P. Crews, Phytochem. Lett., 2012, 5, 114–117 CrossRef CAS PubMed.
- D. Li, Y. Xu, C.-L. Shao, R.-Y. Yang, C.-J. Zheng, Y.-Y. Chen, X.-M. Fu, P.-Y. Qian, Z.-G. She, N. J. de Voogd and C.-Y. Wang, Mar. Drugs, 2012, 10, 234–241 CrossRef CAS PubMed.
- M. W. Sumarah, J. R. Kesting, D. Soerensen and J. D. Miller, Phytochemistry, 2011, 72, 1833–1837 CrossRef CAS PubMed.
- J. D. Miller, G. W. Adams and M. Sumarah, Can. Pat. Appl., 2012, CA 2766412 A1 20120728.
- L.-L. Sun, C.-L. Shao, J.-F. Chen, Z.-Y. Guo, X.-M. Fu, M. Chen, Y.-Y. Chen, R. Li, N. J. de Voogd, Z.-G. She, Y.-C. Lin and C.-Y. Wang, Bioorg. Med. Chem. Lett., 2012, 22, 1326–1329 CrossRef CAS PubMed.
- S. A. Neff, S. U. Lee, Y. Asami, J. S. Ahn, H. Oh, J. Baltrusaitis, J. B. Gloer and D. T. Wicklow, J. Nat. Prod., 2012, 75, 464–472 CrossRef CAS PubMed.
- H. Huang, F. Wang, M. Luo, Y. Chen, Y. Song, W. Zhang, S. Zhang and J. Ju, J. Nat. Prod., 2012, 75, 1346–1352 CrossRef CAS PubMed.
- S. Liu, C. Lu, J. Huang and Y. Shen, Rec. Nat. Prod., 2012, 6, 334–338 CAS.
- S.-S. Liu, B.-B. Zhao, C.-H. Lu, J.-J. Huang and Y.-M. Shen, Nat. Prod. Commun., 2012, 7, 1057–1062 CAS.
- R. Wang, T.-M. Liu, M.-H. Shen, M.-Q. Yang, Q.-Y. Feng, X.-M. Tang and X.-M. Li, Molecules, 2012, 17, 13175–13182 CrossRef CAS PubMed.
- D. Zhang, S. Fukuzawa, M. Satake, X. Li, T. Kuranaga, A. Niitsu, K. Yoshizawa and K. Tachibana, Nat. Prod. Commun., 2012, 7, 1411–1414 CAS.
- T. Yang, Z. Lu, L. Meng, S. Wei, K. Hong, W. Zhu and C. Huang, Bioorg. Med. Chem. Lett., 2012, 22, 579–585 CrossRef CAS PubMed.
- O. I. Zhuravleva, S. S. Afiyatullov, O. S. Vishchuk, V. A. Denisenko, N. N. Slinkina and O. F. Smetanina, Arch. Pharmacal Res., 2012, 35, 1757–1762 CrossRef CAS PubMed.
- H. Guo, T. Feng, Z.-H. Li and J.-K. Liu, Nat. Prod. Bioprospect., 2012, 2, 170–173 CrossRef CAS PubMed.
- H. N. Abramson and H. C. Wormser, J. Heterocycl. Chem., 1981, 18, 363–366 CrossRef CAS.
- F. M. Dean, A. Robertson, J. C. Roberts and K. B. Raper, Nature, 1953, 172, 344 CrossRef CAS.
- W. E. Doering, R. J. Dubos, D. S. Noyce and R. Dreyfus, J. Am. Chem. Soc., 1946, 68, 725–726 CrossRef CAS.
- N. Kawahara, K. Nozawa, S. Nakajima, K. Kawai and M. Yamazaki, J. Chem. Soc., Perkin Trans. 1, 1988, 2611–2614 RSC.
- S. Sureram, S. Wiyakrutta, N. Ngamrojanavanich, C. Mahidol, S. Ruchirawat and P. Kittakoop, Planta Med., 2012, 78, 582–588 CrossRef CAS PubMed.
- G.-Y. Li, T. Yang, Y.-G. Luo, X.-Z. Chen, D.-M. Fang and G.-L. Zhang, Org. Lett., 2009, 11, 3714–3717 CrossRef CAS PubMed.
- K. Ito, A. Yamane, T. Hamasaki and Y. Hatsuda, Agric. Biol. Chem., 1976, 40, 2099–2100 CrossRef CAS.
- P. S. Steyn, R. Vleggaar, P. L. Wessels, R. J. Cole and D. B. Scott, J. Chem. Soc., Perkin Trans. 1, 1979, 451–459 RSC.
- F.-P. Miao, X.-D. Li, X.-H. Liu, R. H. Cichewicz and N.-Y. Ji, Mar. Drugs, 2012, 10, 131–139 CrossRef CAS PubMed.
- X.-H. Liu, F.-P. Miao, X.-D. Li, X.-L. Yin and N.-Y. Ji, Nat. Prod. Commun., 2012, 7, 819–820 CAS.
- Y. Zhang, X.-M. Li and B.-G. Wang, Biosci., Biotechnol., Biochem., 2012, 76, 1774–1776 CrossRef CAS.
- C. Shao, Z. She, Z. Guo, H. Peng, X. Cai, S. Zhou, Y. Gu and Y. Lin, Magn. Reson. Chem., 2007, 45, 434–438 CrossRef CAS PubMed.
- D. G. I. Kingston, P. N. Chen and J. R. Vercellotti, Phytochemistry, 1976, 15, 1037–1039 CrossRef CAS.
- F. Song, X. Liu, H. Guo, B. Ren, C. Chen, A. M. Piggott, K. Yu, H. Gao, Q. Wang, M. Liu, X. Liu, H. Dai, L. Zhang and R. J. Capon, Org. Lett., 2012, 14, 4770–4773 CrossRef CAS PubMed.
- U. W. Hawas, A. A. El-Beih and A. M. El-Halawany, Arch. Pharmacal Res., 2012, 35, 1749–1756 CrossRef CAS PubMed.
- H.-F. Sun, X.-M. Li, L. Meng, C.-M. Cui, S.-S. Gao, C.-S. Li, C.-G. Huang and B.-G. Wang, J. Nat. Prod., 2012, 75, 148–152 CrossRef CAS PubMed.
- J. W. Dorner, R. J. Cole, J. P. Springer, R. H. Cox, H. Cutler and D. T. Wicklow, Phytochemistry, 1980, 19, 1157–1161 CrossRef CAS.
- G. A. Ellestad, R. H. Evans Jr. and M. P. Kunstmann, Tetrahedron Lett., 1971, 497–500 CrossRef CAS.
- H. M. T. B. Herath, W. H. M. W. Herath, P. Carvalho, S. I. Khan, B. L. Tekwani, S. O. Duke, M. Tomaso-Peterson and N. P. D. Nanayakkara, J. Nat. Prod., 2009, 72, 2091–2097 CrossRef PubMed.
- T. A. M. Gulder, H. Hong, J. Correa, E. Egereva, J. Wiese, J. F. Imhoff and H. Gross, Mar. Drugs, 2012, 10, 2912–2935 CrossRef.
- H. Yamazaki, H. Rotinsulu, T. Kaneko, K. Murakami, H. Fujiwara, K. Ukai and M. Namikoshi, Mar. Drugs, 2012, 10, 2691–2697 CrossRef CAS.
- M. P. Kuntsmann and L. A. Mitscher, J. Org. Chem., 1966, 31, 2920–2925 CrossRef CAS.
- E. M. K. Wijeratne, T. J. Turbyville, A. Fritz, L. Whitesell and A. A. L. Gunatilaka, Bioorg. Med. Chem., 2006, 14, 7917–7923 CrossRef CAS PubMed.
- M.-Y. Kim, J. H. Sohn, J.-H. Jang, J. S. Ahn and H. Oh, J. Antibiot., 2012, 65, 161–164 CrossRef CAS PubMed.
- J. L. Reino, R. M. Duran-Patron, M. Daoubi, I. G. Collado and R. Hernandez-Galan, J. Org. Chem., 2006, 71, 562–565 CrossRef CAS PubMed.
- J. Moraga, C. Pinedo, R. Durán-Patrón, I. G. Collado and R. Hernández-Galán, Tetrahedron, 2011, 67, 417–420 CrossRef CAS PubMed.
- J. Moraga, C. Pinedo, R. Durán-Patrón, I. G. Collado and R. Hernández-Galán, Tetrahedron, 2011, 67, 8583 CrossRef CAS PubMed.
- M. Yasuhide, T. Yamada, A. Numata and R. Tanaka, J. Antibiot., 2008, 61, 615–622 CrossRef CAS PubMed.
- T. Yamada, M. Yasuhide, H. Shigeta, A. Numata and R. Tanaka, J. Antibiot., 2009, 62, 353–357 CrossRef CAS PubMed.
- Y. Muroga, T. Yamada, A. Numata and R. Tanaka, Tetrahedron, 2009, 65, 7580–7586 CrossRef CAS PubMed.
- T. Yamada, Y. Muroga, M. Jinno, T. Kajimoto, Y. Usami, A. Numata and R. Tanaka, Bioorg. Med. Chem., 2011, 19, 4106–4113 CrossRef CAS PubMed.
- T. Yamada, M. Jinno, T. Kikuchi, T. Kajimoto, A. Numata and R. Tanaka, J. Antibiot., 2012, 65, 413–417 CrossRef CAS PubMed.
- W. Lan, H. Li and Y. Xie, Faming Zhuanli Shenqing, 2011, CN 102267884 A 20111207.
- H.-J. Li, Y.-L. Xie, Z.-L. Xie, Y. Chen, C.-K. Lam and W.-J. Lan, Mar. Drugs, 2012, 10, 627–638 CrossRef CAS PubMed.
- M. F. Elsebai, L. Natesan, S. Kehraus, I. E. Mohamed, G. Schnakenburg, F. Sasse, S. Shaaban, M. Gütschow and G. M. König, J. Nat. Prod., 2011, 74, 2282–2285 CrossRef CAS PubMed.
- M. F. Elsebai, M. Nazir, S. Kehraus, E. Egereva, K. N. Ioset, L. Marcourt, D. Jeannerat, M. Gütschow, J.-L. Wolfender and G. M. König, Eur. J. Org. Chem., 2012, 31, 6197–6203 CrossRef.
- K. Tarman, G. J. Palm, A. Porzel, K. Merzweiler, N. Arnold, L. A. Wessjohann, M. Unterseher and U. Lindequist, Phytochem. Lett., 2012, 5, 83–86 CrossRef CAS PubMed.
- G. K. Poch and J. B. Gloer, J. Nat. Prod., 1989, 52, 257–260 CrossRef CAS.
- P. R. Krishna, V. S. Mallula and P. V. A. Kumar, Tetrahedron Lett., 2012, 53, 4997–4999 CrossRef PubMed.
- A. Pinheiro, T. Dethoup, J. Bessa, A. M. S. Silva and A. Kijjoa, Phytochem. Lett., 2012, 5, 68–70 CrossRef CAS PubMed.
- J. Kimura, M. Furui, M. Kanda and M. Sugiyama, Jpn. Kokai Tokkyo Koho, 2002, JP 2002047281 20020212.
- J. Peng, J. Jiao, J. Li, W. Wang, Q. Gu, T. Zhu and D. Li, Bioorg. Med. Chem. Lett., 2012, 22, 3188–3190 CrossRef CAS PubMed.
- H. Fujimoto, T. Fujimaki, E. Okuyama and M. Yamazaki, Chem. Pharm. Bull., 1999, 47, 1426–1432 CrossRef CAS.
- H. Nagasawa, A. Isogai, A. Suzuki and S. Tamura, Tetrahedron Lett., 1976, 1601–1604 CrossRef CAS.
- F.-Y. Du, X.-M. Li, C.-S. Li, Z. Shang and B.-G. Wang, Bioorg. Med. Chem. Lett., 2012, 22, 4650–4653 CrossRef CAS PubMed.
- A. P. Almeida, T. Dethoup, N. Singburaudom, R. Lima, M. H. Vasconcelos, M. Pinto and A. Kijjoa, J. Nat. Pharm., 2010, 1, 25–29 CrossRef PubMed.
- N. M. Gomes, T. Dethoup, N. Singburaudom, L. Gales, A. M. S. Silva and A. Kijjoa, Phytochem. Lett., 2012, 5, 717–720 CrossRef CAS PubMed.
- L. Sun, D. Li, M. Tao, Y. Chen, F. Dan and W. Zhang, Mar. Drugs, 2012, 10, 539–550 CrossRef CAS PubMed.
- X. Xia, J. Zhang, Y. Zhang, F. Wei, X. Liu, A. Jia, C. Liu, W. Li, Z. She and Y. Lin, Bioorg. Med. Chem. Lett., 2012, 22, 3017–3019 CrossRef CAS PubMed.
- L. Sun, D.-L. Li, M.-H. Tao, F.-J. Dan and W.-M. Zhang, Helv. Chim. Acta, 2012, 95, 157–162 CrossRef CAS.
- S. Takahashi, Y. Itoh, M. Takeuchi, K. Furuya, K. Kodama, A. Naito, T. Haneishi, S. Sato and C. Tamura, J. Antibiot., 1983, 36, 1418–1420 CrossRef CAS.
- O. F. Smetanina, A. N. Yurchenko, S. S. Afiyatullov, A. I. Kalinovsky, M. A. Pushilin, Y. V. Khudyakova, N. N. Slinkina, S. P. Ermakova and E. A. Yurchenko, Phytochem. Lett., 2012, 5, 165–169 CrossRef CAS PubMed.
- K.-L. Yang, M.-Y. Wei, C.-L. Shao, X.-M. Fu, Z.-Y. Guo, R.-F. Xu, C.-J. Zheng, Z.-G. She, Y.-C. Lin and C.-Y. Wang, J. Nat. Prod., 2012, 75, 935–941 CrossRef CAS PubMed.
- F. Song, B. Ren, K. Yu, C. Chen, H. Guo, N. Yang, H. Gao, X. Liu, M. Liu, Y. Tong, H. Dai, H. Bai, J. Wang and L. Zhang, Mar. Drugs, 2012, 10, 1297–1306 CrossRef CAS PubMed.
- T. Kawahara, M. Takagi and K. Shin-ya, J. Antibiot., 2012, 65, 45–47 CrossRef CAS PubMed.
- N. Khamthong, V. Rukachaisirikul, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Tetrahedron, 2012, 68, 8245–8250 CrossRef CAS PubMed.
- L. Chen, K. Huang, P. Zhong, X. Hu, Z.-X. Fang, J. Wu and Q.-Q. Zhang, Heterocycles, 2012, 85, 413–419 CrossRef CAS.
- S.-S. Gao, Z. Shang, X.-M. Li, C.-S. Li, C.-M. Cui and B.-G. Wang, Biosci., Biotechnol., Biochem., 2012, 76, 358–360 CrossRef CAS.
- J. Wang, P. Liu, Y. Wang, H. Wang, J. Li, Y. Zhuang and W. Zhu, Chin. J. Chem., 2012, 30, 1236–1242 CrossRef CAS.
- J. Wang, P. Liu, Y. Wang, H. Wang, J. Li, Y. Zhuang and W. Zhu, Chin. J. Chem., 2012, 30, 2880 CrossRef CAS.
- G. Wu, H. Ma, T. Zhu, J. Li, Q. Gu and D. Li, Tetrahedron, 2012, 68, 9745–9749 CrossRef CAS PubMed.
- T. Sassa, H. Kachi and M. Nukina, J. Antibiot., 1985, 38, 439–441 CrossRef CAS.
- Y.-L. Sun, F. He, K.-S. Liu, X.-Y. Zhang, J. Bao, Y.-F. Wang, X.-H. Nong, X.-Y. Xu and S.-H. Qi, Planta Med., 2012, 78, 1957–1961 CrossRef CAS PubMed.
- S.-W. Yang, T.-M. Chan, J. Terracciano, R. Patel, M. Patel, V. Gullo and M. Chu, J. Antibiot., 2006, 59, 720–723 CrossRef CAS PubMed.
- Y. Myobatake, T. Takeuchi, K. Kuramochi, I. Kuriyama, T. Ishido, K. Hirano, F. Sugawara, H. Yoshida and Y. Mizushina, J. Nat. Prod., 2012, 75, 135–141 CrossRef CAS PubMed.
- A. A. Stierle, D. B. Stierle and T. Girtsman, J. Nat. Prod., 2012, 75, 344–350 CrossRef CAS PubMed.
- T. Takeuchi, Y. Mizushina, S. Takaichi, N. Inoue, K. Kuramochi, S. Shimura, Y. Myobatake, Y. Katayama, K. Takemoto, S. Endo, S. Kamisuki and F. Sugawara, Org. Lett., 2012, 14, 4303–4305 CrossRef CAS PubMed.
- S.-M. Fang, C.-B. Cui, C.-W. Li, C.-J. Wu, Z.-J. Zhang, L. Li, X.-J. Huang and W.-C. Ye, Mar. Drugs, 2012, 10, 1266–1287 CrossRef CAS PubMed.
- X. Lin, X. Zhou, F. Wang, K. Liu, B. Yang, X. Yang, Y. Peng, J. Liu, Z. Ren and Y. Liu, Mar. Drugs, 2012, 10, 106–115 CrossRef CAS PubMed.
- L.-Y. Ma, W.-Z. Liu, L. Shen, Y.-L. Huang, X.-G. Rong, Y.-Y. Xu and X.-D. Gao, Tetrahedron, 2012, 68, 2276–2282 CrossRef CAS PubMed.
- L.-Y. Ma, W.-Z. Liu, L. Shen, Y.-L. Huang, X.-G. Rong, Y.-Y. Xu and X.-D. Gao, Tetrahedron, 2013, 69, 8316 CrossRef CAS PubMed.
- J. He, U. Lion, I. Sattler, F. A. Gollmick, S. Grabley, J. Cai, M. Meiners, H. Schünke, K. Schaumann, U. Dechert and M. Krohn, J. Nat. Prod., 2005, 68, 1397–1399 CrossRef CAS PubMed.
- H. Hayashi, T. Nakatani, Y. Inoue, S. Teraguchi, M. Nakayama and H. Nozaki, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1996, 38, 271–276 Search PubMed.
- T. Liu, L. Zhang, Z. Li, Y. Wang, L. Tian, Y. Pei and H. Hua, Chem. Nat. Compd., 2012, 48, 771–773 CrossRef CAS.
- F. A. Macias, R. M. Varela, A. M. Simonet, H. G. Cutler, S. J. Cutler, S. A. Ross, D. C. Dunbar, F. M. Dugan and R. A. Hill, Tetrahedron Lett., 2000, 41, 2683–2686 CrossRef CAS.
- Y. Li, D. Ye, Z. Shao, C. Cui and Y. Che, Mar. Drugs, 2012, 10, 497–508 CrossRef CAS PubMed.
- G. W. Kirby, D. J. Robins, M. A. Sefton and R. R. Talekar, J. Chem. Soc., Perkin Trans. 1, 1980, 119–121 RSC.
- G. Lowe, A. Taylor and L. C. Vining, J. Chem. Soc. C, 1966, 1799–1803 RSC.
- J. A. Mills, Aust. J. Exp. Biol. Med., 1946, 24, 136–138 CAS.
- G. W. Kirby, G. V. Rao and D. J. Robins, J. Chem. Soc., Perkin Trans. 1, 1988, 301–304 RSC.
- Y. Sun, K. Takada, Y. Takemoto, M. Yoshida, Y. Nogi, S. Okada and S. Matsunaga, J. Nat. Prod., 2012, 75, 111–114 CrossRef CAS PubMed.
- P. Zhuang, X.-X. Tang, Z.-W. Yi, Y.-K. Qiu and Z. Wu, J. Asian Nat. Prod. Res., 2012, 14, 197–203 CrossRef CAS PubMed.
- CAS 913690-73-0, SciFinder Database, accessed 26 September 2013.
- T. Kawahara, M. Takagi and K. Shin-ya, J. Antibiot., 2012, 65, 147–150 CrossRef CAS PubMed.
- T. Haishi, K. Furuya, M. Nakajima, T. Kinoshita, T. Kagazaki and Y. Sakaida, Jpn. Kokai Tokkyo Koho, 1989, JP 01290667 A 19891122.
- Z. Chen, Z. Zheng, H. Huang, Y. Song, X. Zhang, J. Ma, B. Wang, C. Zhang and J. Ju, Bioorg. Med. Chem. Lett., 2012, 22, 3332–3335 CrossRef CAS PubMed.
- F. He, Z. Liu, J. Yang, P. Fu, J. Peng, W.-M. Zhu and S.-H. Qi, Tetrahedron Lett., 2012, 53, 2280–2283 CrossRef CAS PubMed.
- H. Gao, L. Zhang, T. Zhu, Q. Gu and D. Li, Chem. Pharm. Bull., 2012, 60, 1458–1460 CrossRef CAS PubMed.
- L. Chen, T. Zhu, Y. Ding, I. A. Khan, Q. Gu and D. Li, Tetrahedron Lett., 2012, 53, 325–328 CrossRef CAS PubMed.
- K. Krohn, Zia-Ullah, H. Hussain, U. Flörke, B. Schulz, S. Draeger, G. Pescitelli, P. Salvadori, S. Antus and T. Kurtán, Chirality, 2007, 19, 464–470 CrossRef CAS PubMed.
- H. Oh, D. C. Swenson, J. B. Gloer and C. A. Shearer, J. Nat. Prod., 2003, 66, 73–79 CrossRef CAS PubMed.
- Y. Lin, X. Wu, Z. Deng, J. Wang, S. Zhou, L. L. P. Vrijmoed and E. B. G. Jones, Phytochemistry, 2002, 59, 469–471 CrossRef CAS.
- G. F. Zhang, W. B. Han, J. T. Cui, S. W. Ng, Z. K. Guo, R. X. Tan and H. M. Ge, Planta Med., 2012, 78, 76–78 CrossRef CAS PubMed.
- E. L. Kim, J. L. Li, B. Xiao, J. Hong, E. S. Yoo, W. D. Yoon and J. H. Jung, Chem. Pharm. Bull., 2012, 60, 1590–1593 CAS.
- E. L. Kim, J. L. Li, H. T. Dang, J. Hong, C.-O. Lee, D.-K. Kim, W. D. Yoon, E. Kim, Y. Liu and J. H. Jung, Bioorg. Med. Chem. Lett., 2012, 22, 3126–3129 CrossRef CAS PubMed.
- E. L. Kim, J. L. Li, H. T. Dang, J. Hong, C.-O. Lee, D.-K. Kim, W. D. Yoon, E. Kim, Y. Liu and J. H. Jung, Bioorg. Med. Chem. Lett., 2012, 22, 5752 CrossRef CAS PubMed.
- C. Almeida, Y. Hemberger, S. M. Schmitt, S. Bouhired, L. Natesan, S. Kehraus, K. Dimas, M. Gütschow, G. Bringmann and G. M. König, Chem.–Eur. J., 2012, 18, 8827–8834 CrossRef CAS PubMed.
- N. Narasimhachari and K. S. Gopalkrishnan, J. Antibiot., 1974, 27, 283–287 CrossRef CAS.
- W.-L. Geng, X.-Y. Wang, T. Kurtán, A. Mándi, H. Tang, B. Schulz, P. Sun and W. Zhang, J. Nat. Prod., 2012, 75, 1828–1832 CrossRef CAS PubMed.
- N. Khamthong, V. Rukachaisirikul, K. Tadpetch, M. Kaewpet, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Arch. Pharmacal Res., 2012, 35, 461–468 CrossRef CAS PubMed.
- W.-J. Lan, Y. Zhao, Z.-L. Xie, L.-Z. Liang, W.-Y. Shao, L.-P. Zhu, D.-P. Yang, X.-F. Zhu and H.-J. Li, Nat. Prod. Commun., 2012, 7, 1337–1340 CAS.
- M. Ishino, K. Kinoshita, K. Takahashi, T. Sugita, M. Shiro, K. Hasegawa and K. Koyama, Tetrahedron, 2012, 68, 8572–8576 CrossRef CAS PubMed.
- V. Rukachaisirikul, A. Rodglin, Y. Sukpondma, S. Phongpaichit, J. Buatong and J. Sakayaroj, J. Nat. Prod., 2012, 75, 853–858 CrossRef CAS PubMed.
- H. Gao, W. Liu, T. Zhu, X. Mo, A. Mándi, T. Kurtán, J. Li, J. Ai, Q. Gu and D. Li, Org. Biomol. Chem., 2012, 10, 9501–9506 CAS.
- H. Wang, Z. Lu, H.-J. Qu, P. Liu, C. Miao, T. Zhu, J. Li, K. Hong and W. Zhu, Arch. Pharmacal Res., 2012, 35, 1387–1392 CrossRef CAS PubMed.
- S. Li, M. Wei, G. Chen and Y. Lin, Chem. Nat. Compd., 2012, 48, 371–373 CrossRef CAS PubMed.
- S. Cai, X. Kong, W. Wang, H. Zhou, T. Zhu, D. Li and Q. Gu, Tetrahedron Lett., 2012, 53, 2615–2617 CrossRef CAS PubMed.
- Y. Shen, J. Zou, D. Xie, H. Ge, X. Cao and J. Dai, Chem. Pharm. Bull., 2012, 60, 1437–1441 CrossRef CAS PubMed.
- B. K. Joshi, J. B. Gloer and D. T. Wicklow, J. Nat. Prod., 1999, 62, 730–733 CrossRef CAS PubMed.
- M. Isaka, P. Berkaew, K. Intereya, S. Komwijit and T. Sathitkunanon, Tetrahedron, 2007, 63, 6855–6860 CrossRef CAS PubMed.
- W. Ebrahim, J. Kjer, M. El Amrani, V. Wray, W. Lin, R. Ebel, D. Lai and P. Proksch, Mar. Drugs, 2012, 10, 1081–1091 CrossRef CAS PubMed.
- P. Chomcheon, S. Wiyakrutta, N. Sriubolmas, N. Ngamrojanavanich, S. Kengtong, C. Mahidol, S. Ruchirawat and P. Kittakoop, Phytochemistry, 2009, 70, 407–413 CrossRef CAS PubMed.
- H. Chimura, T. Sawa, Y. Kumada, F. Nakamura, M. Matsuzaki, T. Takita, T. Takeuchi and H. Umezawa, J. Antibiot., 1973, 26, 618–620 CrossRef CAS.
- W. Ebrahim, A. H. Aly, A. Mándi, F. Totzke, M. H. G. Kubbutat, V. Wray, W.-H. Lin, H. Dai, P. Proksch, T. Kurtán and A. Debbab, Eur. J. Org. Chem., 2012, 18, 3476–3484 CrossRef.
- L. Y. Zang, W. Wei, Y. Guo, T. Wang, R. H. Jiao, S. W. Ng, R. X. Tan and H. M. Ge, J. Nat. Prod., 2012, 75, 1744–1749 CrossRef CAS PubMed.
- K. Shishido, T. Omodani and M. Shibuya, J. Chem. Soc., Perkin Trans. 1, 1991, 2285–2287 RSC.
- A. J. Aasen, C. H. G. Vogt and C. R. Enzell, Acta Chem. Scand., Ser. B, 1975, 29, 51–55 CrossRef PubMed.
- M. N. Koltsa, G. N. Mironov, A. N. Aryku and P. F. Vlad, Khim. Prir. Soedin., 1991, 43–50 CAS.
- H.-J. Yan, X.-M. Li, C.-S. Li and B.-G. Wang, Helv. Chim. Acta, 2012, 95, 163–168 CrossRef CAS.
- W.-L. Wang, Z.-Y. Lu, H.-W. Tao, T.-J. Zhu, Y.-C. Fang, Q.-Q. Gu and W.-M. Zhu, J. Nat. Prod., 2007, 70, 1558–1564 CrossRef CAS PubMed.
- S. Inoue, J. Murata, N. Takamatsu, H. Nagano and Y. Kishi, Yakugaku Zasshi, 1977, 97, 576–581 CAS.
- L. Ding, H.-M. Dahse and C. Hertweck, J. Nat. Prod., 2012, 75, 617–621 CrossRef CAS PubMed.
- Z. J. Vejdelek, V. Treka and M. Protiva, J. Med. Pharm. Chem., 1961, 3, 427–40 CrossRef CAS.
- J. D. Edwards, J. E. Page and M. Pianka, J. Chem. Soc., 1964, 5200–5206 RSC.
- G. A. Zou, S. Mansur, S. C. Hu, H. A. Aisa and K. M. Shakhidoyatov, Chem. Nat. Compd., 2012, 48, 635–637 CrossRef CAS.
- Z. Huang, J. Yang, Z. She and Y. Lin, Nat. Prod. Res., 2012, 26, 11–15 CrossRef CAS PubMed.
- W.-L. Mei, B. Zheng, Y.-X. Zhao, H.-M. Zhong, X. L. W. Chen, Y.-B. Zeng, W.-H. Dong, J.-L. Huang, P. Proksch and H.-F. Dai, Mar. Drugs, 2012, 10, 1993–2001 CrossRef CAS PubMed.
- E. Molinar, N. Rios, C. Spadafora, E. A. Arnold, P. D. Coley, T. A. Kursar, W. H. Gerwick and L. Cubilla-Rios, Tetrahedron Lett., 2012, 53, 919–922 CrossRef CAS PubMed.
- W. H. Yuan, M. Liu, N. Jiang, Z. K. Guo, J. Ma, J. Zhang, Y. C. Song and X. R. Tan, Eur. J. Org. Chem., 2010, 33, 6348–6353 CrossRef.
- S. F. Brady, M. M. Wagenaar, M. P. Singh, J. E. Janso and J. Clardy, Org. Lett., 2000, 2, 4043–4046 CrossRef CAS PubMed.
- J. Beau, N. Mahid, W. N. Burda, L. Harrington, L. N. Shaw, T. Mutka, D. E. Kyle, B. Barisic, A. van Olphen and B. J. Baker, Mar. Drugs, 2012, 10, 762–774 CrossRef CAS PubMed.
- D. Broadbent, R. P. Mabelis and H. Spencer, Phytochemistry, 1975, 14, 2082–2083 CrossRef CAS.
- Z. Shang, X.-M. Li, C.-S. Li and B.-G. Wang, Chem. Biodiversity, 2012, 9, 1338–1348 CAS.
- C. G. Naik, P. Devi and E. Rodrigues, U.S. Pat. Appl. Publ., 2005, US 20050143392, A1 20050630.
- P. Devi, C. Rodrigues, C. G. Naik and L. D'Souza, Indian J. Microbiol., 2012, 52, 617–623 CrossRef CAS PubMed.
- J. F. Wang, Z. Y. Lu, P. P. Liu, Y. Wang, J. Li, K. Hong and W. M. Zhu, Planta Med., 2012, 78, 1861–1866 CrossRef CAS PubMed.
- Y. Zhang, X.-M. Li, Z. Shang, C.-S. Li, N.-Y. Ji and B.-G. Wang, J. Nat. Prod., 2012, 75, 1888–1895 CrossRef CAS PubMed.
- T. Sassa, G. Niwa, H. Unno, M. Ikeda and Y. Miura, Tetrahedron Lett., 1974, 3941–3942 CrossRef CAS.
- K. Nonaka, T. Abe, M. Iwatsuki, M. Mori, T. Yamamoto, K. Shiomi, S. Omura and R. Masuma, J. Antibiot., 2011, 64, 769–774 CrossRef CAS PubMed.
- K. Suzuki, K. Nozawa, S. Udagawa, S. Nakajima and K. Kawai, Phytochemistry, 1991, 30, 2096–2098 CrossRef CAS.
- H. Kawamura, T. Kaneko, H. Koshino, Y. Esumi, J. Uzawa and F. Sugawara, Nat. Prod. Lett., 2000, 14, 477–484 CrossRef CAS.
- V. Rukachaisirikul, A. Rodglin, S. Phongpaichit, J. Buatong and J. Sakayaroj, Phytochem. Lett., 2012, 5, 13–17 CrossRef CAS PubMed.
- S. Klaiklay, V. Rukachaisirikul, K. Tadpetch, Y. Sukpondma, S. Phongpaichit, J. Buatong and J. Sakayaroj, Tetrahedron, 2012, 68, 2299–2305 CrossRef CAS PubMed.
- S. Klaiklay, V. Rukachaisirikul, S. Phongpaichit, C. Pakawatchai, S. Saithong, J. Buatong, S. Preedanon and J. Sakayaroj, Phytochem. Lett., 2012, 5, 738–742 CrossRef CAS PubMed.
- A. A. Freer, D. Gardner, D. Greatbanks, J. P. Poyser and G. A. Sim, J. Chem. Soc., Chem. Commun., 1982, 1160–1162 RSC.
- M. Izumikawa, T. Hosoya, M. Takagi and K. Shin-ya, J. Antibiot., 2012, 65, 41–43 CrossRef CAS PubMed.
- X. Lu, L. Tian, G. Chen, Y. Xu, H.-F. Wang, Z.-Q. Li and Y.-H. Pei, J. Asian Nat. Prod. Res., 2012, 14, 647–651 CrossRef CAS PubMed.
- S. Klaiklay, V. Rukachaisirikul, Y. Sukpondma, S. Phongpaichit, J. Buatong and B. Bussaban, Arch. Pharmacal Res., 2012, 35, 1127–1131 CrossRef CAS PubMed.
- W. C. Tayone, S. Kanamaru, M. Honma, K. Tanaka, T. Nehira and M. Hashimoto, Biosci., Biotechnol., Biochem., 2011, 75, 2390–2393 CrossRef CAS.
- Y.-X. Song, J. Wang, S.-W. Li, B. Cheng, L. Li, B. Chen, L. Liu, Y.-C. Lin and Y.-C. Gu, Planta Med., 2012, 78, 172–176 CrossRef CAS PubMed.
- Y. Song, J. Wang, H. Huang, L. Ma, J. Wang, Y. Gu, L. Liu and Y. Lin, Mar. Drugs, 2012, 10, 340–348 CrossRef CAS PubMed.
- L. A. McDonald, L. R. Barbieri, V. S. Bernan, J. Janso, P. Lassota and G. T. Carter, J. Nat. Prod., 2004, 67, 1565–1567 CrossRef CAS PubMed.
- Y.-B. Zeng, H. Wang, W.-J. Zuo, B. Zheng, T. Yang, H.-F. Dai and W.-L. Mei, Mar. Drugs, 2012, 10, 598–603 CrossRef CAS PubMed.
- M. El Amrani, A. Debbab, A. H. Aly, V. Wray, S. Dobretsov, W. E. G. Müller, W. Lin, D. Lai and P. Proksch, Tetrahedron Lett., 2012, 53, 6721–6724 CrossRef CAS PubMed.
- S. P. Putri, H. Kinoshita, F. Ihara, Y. Igarashi and T. Nihira, J. Nat. Prod., 2009, 72, 544–1546 CrossRef PubMed.
- H. Choi, S. J. Mascuch, F. A. Villa, T. Byrum, M. E. Teasdale, J. E. Smith, L. B. Preskitt, D. C. Rowley, L. Gerwick and W. H. Gerwick, Chem. Biol., 2012, 19, 589–598 CrossRef CAS PubMed.
- M. Morita, O. Ohno and K. Suenaga, Chem. Lett., 2012, 41, 165–167 CrossRef CAS.
- T. Teruya, H. Sasaki, K. Kitamura, T. Nakayama and K. Suenaga, Org. Lett., 2009, 11, 2421–2424 CrossRef CAS PubMed.
- M. Morita, O. Ohno, T. Teruya, T. Yamori, T. Inuzuka and K. Suenaga, Tetrahedron, 2012, 68, 5984–5990 CrossRef CAS PubMed.
- J. H. Cardellina II, D. Dalietos, F. J. Marner, J. S. Mynderse and R. E. Moore, Phytochemistry, 1978, 17, 2091–2095 CrossRef.
- R. Montaser, V. J. Paul and H. Luesch, ChemBioChem, 2012, 13, 2676–2681 CrossRef CAS PubMed.
- H. Choi, E. Mevers, T. Byrum, F. A. Valeriote and W. H. Gerwick, Eur. J. Org. Chem., 2012, 27, 5141–5150 CrossRef.
- J. Orjala and W. H. Gerwick, J. Nat. Prod., 1996, 59, 427–30 CrossRef CAS PubMed.
- E. J. Kim, J. H. Lee, H. Choi, A. R. Pereira, Y. H. Ban, Y. J. Yoo, E. Kim, J. W. Park, D. H. Sherman, W. H. Gerwick and Y. J. Yoon, Org. Lett., 2012, 14, 5824–5827 CrossRef CAS PubMed.
- M. J. Balunas, M. F. Grosso, F. A. Villa, N. Engene, K. L. McPhail, K. Tidgewell, L. M. Pineda, L. Gerwick, C. Spadafora, D. E. Kyle and W. H. Gerwick, Org. Lett., 2012, 14, 3878–3881 CrossRef CAS PubMed.
- P. D. Boudreau, T. Byrum, W.-T. Liu, P. C. Dorrestein and W. H. Gerwick, J. Nat. Prod., 2012, 75, 1560–1570 CrossRef CAS PubMed.
- K. L. Malloy, H. Choi, C. Fiorilla, F. A. Valeriote, T. Matainaho and W. H. Gerwick, Bioorg. Med. Chem. Lett., 2012, 22, 683–688 CrossRef CAS PubMed.
- T. F. Molinski, K. A. Reynolds and B. I. Morinaka, J. Nat. Prod., 2012, 75, 425–431 CrossRef CAS PubMed.
- A. R. Pereira, A. J. Kale, A. T. Fenley, T. Byrum, H. M. Debonsi, M. K. Gilson, F. A. Valeriote, B. S. Moore and W. H. Gerwick, ChemBioChem, 2012, 13, 810–817 CrossRef CAS PubMed.
- J. K. Nunnery, N. Engene, T. Byrum, Z. Cao, S. V. Jabba, A. R. Pereira, T. Matainaho, T. F. Murray and W. H. Gerwick, J. Org. Chem., 2012, 77, 4198–4208 CrossRef CAS PubMed.
- T. Inuzuka, Y. Yamamoto, K. Yamada and D. Uemura, Tetrahedron Lett., 2012, 53, 239–242 CrossRef CAS PubMed.
- P. T. Holland, F. Shi, M. Satake, Y. Hamamoto, E. Ito, V. Beuzenberg, P. McNabb, R. Munday, L. Briggs, P. Truman, R. Gooneratne, P. Edwards and S. M. Pascal, Harmful Algae, 2012, 13, 47–57 CrossRef CAS PubMed.
- Y. Hamamoto, K. Tachibana, P. T. Holland, F. Shi, V. Beuzenberg, Y. Itoh and M. Satake, J. Am. Chem. Soc., 2012, 134, 4963–4968 CrossRef CAS PubMed.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, L. Tartaglione, C. Grillo and N. Melchiorre, J. Am. Soc. Mass Spectrom., 2008, 19, 111–120 CrossRef CAS PubMed.
- P. Ciminiello, C. Dell'Aversano, E. Dello Iacovo, E. Fattorusso, M. Forino, L. Grauso and L. Tartaglione, Chem.–Eur. J., 2012, 18, 16836–16843 CrossRef CAS PubMed.
- P. Ciminiello, C. Dell'Aversano, E. Dello Iacovo, E. Fattorusso, M. Forino, L. Grauso, L. Tartaglione, F. Guerrini, L. Pezzolesi, R. Pistocchi and S. Vanucci, J. Am. Chem. Soc., 2012, 134, 1869–1875 CrossRef CAS PubMed.
- P. Ciminiello, C. Dell'Aversano, E. Dello Iacovo, E. Fattorusso, M. Forino, L. Tartaglione, C. Battochi, R. Crinelli, E. Carloni, M. Magnani and A. Penna, Chem. Res. Toxicol., 2012, 25, 1243–1252 CrossRef CAS PubMed.
- G. Guella, E. Callone, I. Mancini, F. Dini and G. Di Giuseppe, Eur. J. Org. Chem., 2012, 27, 5208–5216 CrossRef.
- M. I. Mitova, G. Lang, J. Wiese and J. F. Imhoff, J. Nat. Prod., 2008, 71, 824–827 CrossRef CAS PubMed.
- Z. Yang, X. Jin, M. Guaciaro and B. F. Molino, J. Org. Chem., 2012, 77, 3191–3196 CrossRef CAS PubMed.
- Z. Yang, X. Jin, M. Guaciaro, B. F. Molino, U. Mocek, R. Reategui, J. Rhea and T. Morley, Org. Lett., 2011, 13, 5436–5439 CrossRef CAS PubMed.
- R. Raju, A. M. Piggott, L. X. Barrientos Diaz, Z. Khalil and R. J. Capon, Org. Lett., 2010, 12, 5158–5161 CrossRef CAS PubMed.
- J. Schmidt and C. B. W. Stark, Org. Lett., 2012, 14, 4042–4045 CrossRef CAS PubMed.
- S. Sato, F. Iwata, T. Mukai, S. Yamada, J. Takeo, A. Abe and H. Kawahara, J. Org. Chem., 2009, 74, 5502–5509 CrossRef CAS PubMed.
- O. F. Jeker and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3474–3477 CrossRef CAS PubMed.
- X.-L. Li, M.-J. Xu, Y.-L. Zhao and J. Xu, Molecules, 2010, 15, 9298–9307 CrossRef CAS PubMed.
- C. Tian, X. Jiao, X. Liu, R. Li, L. Dong, X. Liu, Z. Zhang, J. Xu, M. Xu and P. Xie, Tetrahedron Lett., 2012, 53, 4892–4895 CrossRef CAS PubMed.
- C. Klemke, S. Kehraus, A. D. Wright and G. M. König, J. Nat. Prod., 2004, 67, 1058–1063 CrossRef CAS PubMed.
- M. Gärtner, D. Kossler, D. Pflästerer and G. Helmchen, J. Org. Chem., 2012, 77, 4491–4495 CrossRef PubMed.
- Y. Usami, J. Yamaguchi and A. Numata, Heterocycles, 2004, 63, 1123–1129 CrossRef CAS.
- N. Boyer and M. Movassaghi, Chem. Sci., 2012, 3, 1798–1803 RSC.
- X. Li, Y. Yao, Y. Zheng, I. Sattler and W. Lin, Arch. Pharmacal Res., 2007, 30, 812–815 CrossRef CAS.
- S. F. Tlais and G. B. Dudley, Org. Lett., 2010, 12, 4698–4701 CrossRef CAS PubMed.
- S. F. Tlais and G. B. Dudley, Beilstein J. Org. Chem., 2012, 8, 1287–1292 CrossRef CAS PubMed.
- Y. Lin, X. Wu, S. Feng, G. Jiang, S. Zhou, L. L. P. Vrijmoed and E. B. G. Jones, Tetrahedron Lett., 2001, 42, 449–451 CrossRef CAS.
- S.-Y. Wang, Z.-L. Xu, H. Wang, C.-R. Li, L. W. Fu, J.-Y. Pang, J. Li, Z.-G. She and Y.-C. Lin, Helv. Chim. Acta, 2012, 95, 973–982 CrossRef CAS.
- A. R. Pereira, Z. Cao, N. Engene, I. E. Soria-Mercado, T. F. Murray and W. H. Gerwick, Org. Lett., 2010, 12, 4490–4493 CrossRef CAS PubMed.
- R. Tello-Aburto, E. M. Johnson, C. K. Valdez and W. A. Maio, Org. Lett., 2012, 14, 2150–2153 CrossRef CAS PubMed.
- R. Tello-Aburto, T. D. Newar and W. A. Maio, J. Org. Chem., 2012, 77, 6271–6289 CrossRef CAS PubMed.
- L. T. Tan, B. L. Marquez and W. H. Gerwick, J. Nat. Prod., 2002, 65, 925–928 CrossRef CAS PubMed.
- A. ElMarrouni, R. Lebeuf, J. Gebauer, M. Heras, S. Arseniyadis and J. Cossy, Org. Lett., 2012, 14, 314–317 CrossRef CAS PubMed.
- D. Webb, A. van den Heuvel, M. Kogl and S. V. Ley, Synlett, 2009, 2320–2324 CAS.
- H. Luesch, W. Y. Yoshida, G. G. Harrigan, J. P. Doom, R. E. Moore and V. J. Paul, J. Nat. Prod., 2002, 65, 1945–1948 CrossRef CAS PubMed.
- L. A. Salvador, J. S. Biggs, V. J. Paul and H. Luesch, J. Nat. Prod., 2011, 74, 917–927 CrossRef CAS PubMed.
- E. Mevers, W.-T. Liu, N. Engene, H. Mohimani, T. Byrum, P. A. Pevzner, P. C. Dorrestein, C. Spadafora and W. H. Gerwick, J. Nat. Prod., 2011, 74, 928–936 CrossRef CAS PubMed.
- D. Wang, X. Jia and A. Zhang, Org. Biomol. Chem., 2012, 10, 7027–7030 CAS.
- K. N. Maloney, J. B. MacMillan, C. A. Kauffman, P. R. Jensen, A. G. Di Pasquale, A. L. Rheingold and W. Fenical, Org. Lett., 2009, 11, 5422–5424 CrossRef CAS PubMed.
- T. Burckhardt, K. Harms and U. Koert, Org. Lett., 2012, 14, 4674–4677 CrossRef CAS PubMed.
- A. Kanjana-opas, S. Panphon, H. K. Fun and S. Chantrapromma, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, 2728–2730 Search PubMed.
- Y. Sangnoi, O. Sakulkeo, S. Yuenyongsawad, A. Kanjana-opas, K. Ingkaninan, A. Plubrukarn and K. Suwanborirux, Mar. Drugs, 2008, 6, 578–586 CrossRef CAS.
- L. Ni, Z. Li, F. Wu, J. Xu, X. Wu, L. Kong and H. Yao, Tetrahedron Lett., 2012, 53, 1271–1274 CrossRef CAS PubMed.
- J. C. Carlson, S. Li, D. A. Burr and D. H. Sherman, J. Nat. Prod., 2009, 72, 2076–2079 CrossRef CAS PubMed.
- M. Chen and W. R. Roush, Org. Lett., 2012, 14, 426–428 CrossRef CAS PubMed.
- W. Balk-Bindseil, E. Helmke, H. Weyland and H. Laatsch, Liebigs Ann., 1995, 1291–1294 CrossRef CAS.
- Y. Liu, L. Zhang and Y. Jia, Tetrahedron Lett., 2012, 53, 684–687 CrossRef CAS PubMed.
- D.-C. Oh, E. A. Gontang, C. A. Kauffman, P. R. Jensen and W. Fenical, J. Nat. Prod., 2008, 71, 570–575 CrossRef CAS PubMed.
- P. Ramesh and H. M. Meshram, Tetrahedron, 2012, 68, 9289–9292 CrossRef CAS PubMed.
- T. Yamada, K. Minoura, R. Tanaka and A. Numata, J. Antibiot., 2007, 60, 370–375 CrossRef CAS PubMed.
- G. V. M. Sharma and P. S. Reddy, Eur. J. Org. Chem., 2012, 12, 2414–2421 CrossRef.
- D. P. Curran, M. K. Sinha, K. Zhang, J. J. Sabatini and D.-H. Cho, Nat. Chem., 2012, 4, 124–129 CrossRef CAS PubMed.
- C. Takahashi, A. Numata, T. Yamada, K. Minoura, S. Enomoto, K. Konishi, M. Nakai, C. Matsuda and K. Nomoto, Tetrahedron Lett., 1996, 37, 655–658 CrossRef CAS.
- K. Fujioka, H. Yokoe, M. Yoshida and K. Shishido, Org. Lett., 2012, 14, 244–247 CrossRef CAS PubMed.
- Y. C. Park, S. P. Gunasekera, J. V. Lopez, P. J. McCarthy and A. E. Wright, J. Nat. Prod., 2006, 69, 580–584 CrossRef CAS PubMed.
- Y. Kothapalli, R. K. Puthukanoori and S. R. Alapati, Tetrahedron Lett., 2012, 53, 1891–1893 CrossRef CAS PubMed.
- K. C. Nicolaou, M. Lu, S. Totokotsopoulos, P. Heretsch, D. Giguère, Y.-P. Sun, D. Sarlah, T. H. Nguyen, I. C. Wolf, D. F. Smee, C. W. Day, S. Bopp and E. A. Winzeler, J. Am. Chem. Soc., 2012, 134, 17320–17332 CrossRef CAS PubMed.
- M. A. M. Shushni, R. Singh, R. Mentel and U. Lindequist, Mar. Drugs, 2011, 9, 844–851 CrossRef CAS PubMed.
- P. R. Krishna, S. Prabhakar and D. V. Ramana, Tetrahedron Lett., 2012, 53, 6843–6845 CrossRef PubMed.
- Y.-Y. Li, M.-Z. Wang, Y.-J. Huang and Y.-M. Shen, Mycology, 2010, 1, 254–261 CrossRef CAS.
- P. Ramesh, B. C. Reddy and H. M. Meshram, Tetrahedron Lett., 2012, 53, 3735–3738 CrossRef CAS PubMed.
- M. Isaka, A. Yangchum, S. Intamas, K. Kocharin, E. B. G. Jones, P. Kongsaeree and S. Prabpai, Tetrahedron, 2009, 65, 4396–4403 CrossRef CAS PubMed.
- T.-Y. Yuen and M. A. Brimble, Org. Lett., 2012, 14, 5154–5157 CrossRef CAS PubMed.
- M. Gutierrez, T. L. Suyama, N. Engene, J. S. Wingerd, T. Matainaho and W. H. Gerwick, J. Nat. Prod., 2008, 71, 1099–1103 CrossRef CAS PubMed.
- B. D. Robertson, S. E. Wengryniuk and D. M. Coltart, Org. Lett., 2012, 14, 5192–5195 CrossRef CAS PubMed.
- J. C. Kwan, E. A. Eksioglu, C. Liu, V. J. Paul and H. Luesch, J. Med. Chem., 2009, 52, 5732–5747 CrossRef CAS PubMed.
- S. Yang, W. Zhang, N. Ding, J. Lo, Y. Liu, M. J. Clare-Salzler, H. Luesch and Y. Li, Bioorg. Med. Chem., 2012, 20, 4774–4780 CrossRef CAS PubMed.
- H. Nagai, M. Murata, K. Torigoe, M. Satake and T. Yasumoto, J. Org. Chem., 1992, 57, 5448–5453 CrossRef CAS.
- H. Fuwa, K. Ishigai, K. Hashizume and M. Sasaki, J. Am. Chem. Soc., 2012, 134, 11984–11987 CrossRef CAS PubMed.
- H. Fuwa, K. Ishigai, T. Goto, A. Suzuki and M. Sasaki, J. Org. Chem., 2009, 74, 4024–4040 CrossRef CAS PubMed.
- J. Kobayashi, M. Tsuda, M. Ishibashi, H. Shigemori, T. Yamasu, H. Hirota and T. Sasaki, J. Antibiot., 1991, 44, 1259–1261 CrossRef CAS.
- S. Mahapatra and R. G. Carter, Angew. Chem., Int. Ed., 2012, 51, 7948–7951 CrossRef CAS PubMed.
- R. D. Charan, G. Schlingmann, J. Janso, V. Bernan, X. Feng and G. T. Carter, J. Nat. Prod., 2004, 67, 1431–1433 CrossRef CAS PubMed.
- U. R. Abdelmohsen, M. Szesny, E. M. Othman, T. Schirmeister, S. Grond, H. Stopper and U. Hentschel, Mar. Drugs, 2012, 10, 2208–2221 CrossRef CAS PubMed.
- H. H. Wasserman, J. E. McKeon, L. Smith and P. Forgione, J. Am. Chem. Soc., 1960, 82, 506–507 CrossRef CAS.
- I. D. B. Arthaud, F. A. R. Rodrigues, P. C. Jimenez, R. C. Montenegro, A. L. Angelim, V. M. M. Maciel, E. R. Silveira, H. P. S. Freitas, T. S. Sousa, O. D. L. Pessoa, T. M. C. Lotufo and L. V. Costa-Lotufo, Chem. Biodiversity, 2012, 9, 418–427 CAS.
- W. Qin, Q. Kong, Z. Fan, X. Su and L. Li, Zhongcaoyao, 1984, 15, 490–492 CAS.
- F. Caesar, K. Jansson and E. Mutschler, Pharmaceutica Acta Helvetiae, 1969, 44, 676–690 CAS.
- J. Y. Cho, J. Y. Kang, Y. K. Hong, H. H. Baek, H. W. Shin and M. S. Kim, Biosci., Biotechnol., Biochem., 2012, 76, 1116–1121 CAS.
- M. Bernart and W. H. Gerwick, Phytochemistry, 1990, 29, 3697–3698 CrossRef CAS.
- Y. Chen, A. Zeeck, Z. Chen and H. Zahner, J. Antibiot., 1983, 36, 913–915 CrossRef CAS.
- S. Martínez-Luis, J. F. Gómez, C. Spadafora, H. M. Guzmán and M. Gutiérrez, Molecules, 2012, 17, 11146–11155 CrossRef PubMed.
- V. Bultel-Ponce, J.-P. Berge, C. Debitus, J.-L. Nicolas and M. Guyot, Mar. Biotechnol., 1999, 1, 384–390 CrossRef CAS.
- M. Chu, R. Mierzwa, L. Xu, L. He, J. Terracciano, M. Patel, W. Zhao, T. A. Black and T.-M. Chan, J. Antibiot., 2002, 55, 215–218 CrossRef CAS.
- F. Kong, M. P. Singh and G. T. Carter, J. Nat. Prod., 2005, 68, 920–923 CrossRef CAS PubMed.
- J. Y. Cho, Biosci., Biotechnol., Biochem., 2012, 76, 1452–1458 CrossRef CAS.
- C. V. Minh, P. V. Kiem, X. N. Nguyen, X. C. Nguyen, P. T. Nguyen, H. N. Nguyen, L. T. A. Hoang, D. C. Thung, D. T. T. Thuy, H.-K. Kang, H. D. Jang and Y. H. Kim, Bioorg. Med. Chem. Lett., 2011, 21, 2155–2159 CrossRef CAS PubMed.
- J. Y. Cho and M. S. Kim, Biosci., Biotechnol., Biochem., 2012, 76, 1849–1854 CrossRef CAS.
- C. C. Hughes, A. Prieto-Davo, P. R. Jensen and W. Fenical, Org. Lett., 2008, 10, 629–631 CrossRef CAS PubMed.
- C. C. Hughes, C. A. Kauffman, P. R. Jensen and W. Fenical, J. Org. Chem., 2010, 75, 3240–3250 CrossRef CAS PubMed.
- K. Yamanaka, K. S. Ryan, T. A. M. Gulder, C. C. Hughes and B. S. Moore, J. Am. Chem. Soc., 2012, 134, 12434–12437 CrossRef CAS PubMed.
- G. Strobel, J. Y. Li, F. Sugawara, H. Koshino, J. Harper and W. M. Hess, Microbiology (Reading, U.K.), 1999, 145, 3557–3564 CrossRef CAS PubMed.
- J. J. Levenfors, R. Hedman, C. Thaning, B. Gerhardson and C. J. Welch, Soil Biol. Biochem., 2004, 36, 677–685 CrossRef CAS PubMed.
- N. Takada, H. Sato, K. Suenaga, H. Arimoto, K. Yamada, K. Ueda and D. Uemura, Tetrahedron Lett., 1999, 40, 6309–6312 CrossRef CAS.
- K. Ueda and Y. Hu, Tetrahedron Lett., 1999, 40, 6305–6308 CrossRef CAS.
- M. A. Matilla, H. Stöckmann, F. J. Leeper and G. P. C. Salmond, J. Biol. Chem., 2012, 287, 39125–39138 CrossRef CAS PubMed.
- C. E. Meyer, J. Antibiot., 1971, 24, 558–560 CrossRef CAS.
- Z. Yu, S. Vodanovic-Jankovic, N. Ledeboer, S.-X. Huang, S. R. Rajski, M. Kron and B. Shen, Org. Lett., 2011, 13, 2034–2037 CrossRef CAS PubMed.
- X. Mo, J. Ma, H. Huang, B. Wang, Y. Song, S. Zhang, C. Zhang and J. Ju, J. Am. Chem. Soc., 2012, 134, 2844–2847 CrossRef CAS PubMed.
- P. Fu, S.-X. Wang, K. Hong, X. Li, P.-P. Liu, Y. Wang and W.-M. Zhu, J. Nat. Prod., 2011, 74, 1751–1756 CrossRef CAS PubMed.
- X. Qu, B. Pang, Z. Zhang, M. Chen, Z. Wu, Q. Zhao, Q. Zhang, Y. Wang, Y. Liu and W. Liu, J. Am. Chem. Soc., 2012, 134, 9038–9041 CrossRef CAS PubMed.
- A. M. do Rosario Marinho, E. Rodrigues-Filho, M. d. L. R. Moitinho and L. S. Santos, J. Braz. Chem. Soc., 2005, 16, 280–283 CrossRef CAS PubMed.
- K. Arai, K. Kimura, T. Mushiroda and Y. Yamamoto, Chem. Pharm. Bull., 1989, 37, 2937–2939 CrossRef CAS.
- Y. Tsuda, M. Kaneda, A. Tada, K. Nitta, Y. Yamamoto and Y. Iitaka, J. Chem. Soc., Chem. Commun., 1978, 160–161 RSC.
- F. P. Coyne, H. Raistrick and R. Robinson, Philos. Trans. R. Soc. London, Ser. B, 1931, B220, 297–300 CrossRef CAS.
- Y.-J. Chai, C.-B. Cui, C.-W. Li, C.-J. Wu, C.-K. Tian and W. Hua, Mar. Drugs, 2012, 10, 559–582 CrossRef CAS PubMed.
- G. Xie, X. Zhu, Q. Li, M. Gu, Z. He, J. Wu, J. Li, Y. Lin, M. Li, Z. She and J. Yuan, Br. J. Pharmacol., 2010, 159, 689–697 CrossRef CAS PubMed.
- C. Niu, M. Cai, Y. Zhang and X. Zhou, Biotechnol. Lett., 2012, 34, 2119–2124 CrossRef CAS PubMed.
- P. Moya, A. Cantin, M.-A. Castillo, J. Primo, M. A. Miranda and E. Primo-Yufera, J. Org. Chem., 1998, 63, 8530–8535 CrossRef CAS.
- E. F. Pimenta, A. M. Vita-Marques, A. Tininis, M. H. R. Seleghim, L. D. Sette, K. Veloso, A. G. Ferreira, D. E. Williams, B. O. Patrick, D. S. Dalisay, R. J. Andersen and R. G. S. Berlinck, J. Nat. Prod., 2010, 73, 1821–1832 CrossRef CAS PubMed.
- S. Romminger, E. F. Pimenta, E. S. Nascimento, A. G. Ferreira and R. G. S. Berlinck, J. Braz. Chem. Soc., 2012, 23, 1783–1788 CrossRef CAS PubMed.
- R. Liu, W. Zhu, Y. Zhang, T. Zhu, H. Liu, Y. Fang and Q. Gu, J. Antibiot., 2006, 59, 362–365 CrossRef CAS PubMed.
- I. Kurobane, L. C. Vining and A. G. McInnes, J. Antibiot., 1979, 32, 1256–1266 CrossRef CAS.
- A. Krick, S. Kehraus, C. Gerhaüser, K. Klimo, M. Nieger, A. Maier, H.-H. Fiebig, I. Atodiresei, G. Raabe, J. Fleischhauer and G. M. König, J. Nat. Prod., 2007, 70, 353–360 CrossRef CAS PubMed.
- J. Lin, S. Liu, B. Sun, S. Niu, E. Li, X. Liu and Y. Che, J. Nat. Prod., 2010, 73, 905–910 CrossRef CAS PubMed.
- M. A. Schätzle, S. M. Husain, S. Ferlaino and M. Müller, J. Am. Chem. Soc., 2012, 134, 14742–14745 CrossRef PubMed.
- J. M. Finefield, T. J. Greshock, D. H. Sherman, S. Tsukamoto and R. M. Williams, Tetrahedron Lett., 2011, 52, 1987–1989 CrossRef CAS PubMed.
- Y. Ding, J. R. de Wet, J. Cavalcoli, S. Li, T. J. Greshock, K. A. Miller, J. M. Finefield, J. D. Sunderhaus, T. J. McAfoos, S. Tsukamoto, R. M. Williams and D. H. Sherman, J. Am. Chem. Soc., 2010, 132, 12733–12740 CrossRef CAS PubMed.
- S. Li, J. M. Finefield, J. D. Sunderhaus, T. J. McAfoos, R. M. Williams and D. H. Sherman, J. Am. Chem. Soc., 2012, 134, 788–791 CrossRef CAS PubMed.
- S. Li, J. M. Finefield, J. D. Sunderhaus, T. J. McAfoos, R. M. Williams and D. H. Sherman, J. Am. Chem. Soc., 2012, 134, 20565–20565 CrossRef CAS.
- L. Ding, G. Peschel and C. Hertweck, ChemBioChem, 2012, 13, 2661–2664 CrossRef CAS PubMed.
- M. Yamazaki, M. Izumikawa, K. Tachibana, M. Satake, Y. Itoh and M. Hashimoto, J. Org. Chem., 2012, 77, 4902–4906 CrossRef CAS PubMed.
- P. G. Cruz, A. H. Daranas, J. J. Fernández, M. L. Souto and M. Norte, Toxicon, 2006, 47, 920–924 CrossRef CAS PubMed.
- T. S. Vilches, M. Norte, A. H. Daranas and J. J. Fernández, Mar. Drugs, 2012, 10, 2234–2245 CrossRef CAS PubMed.
- M. Tsukimoto, M. Nagaoka, Y. Shishido, J. Fujimoto, F. Nishisaka, S. Matsumoto, E. Harunari, C. Imada and T. Matsuzaki, J. Nat. Prod., 2011, 74, 2329–2331 CrossRef CAS PubMed.
- Y. Xu, R. D. Kersten, S.-J. Nam, L. Lu, A. M. Al-Suwailem, H. Zheng, W. Fenical, P. C. Dorrestein, B. S. Moore and P.-Y. Qian, J. Am. Chem. Soc., 2012, 134, 8625–8632 CrossRef CAS PubMed.
- J.-L. Zhang, H.-Y. Tian, J. Li, L. Jin, C. Luo, W.-C. Ye and R.-W. Jiang, Fitoterapia, 2012, 83, 973–978 CrossRef CAS PubMed.
- D.-Q. Liu, S.-C. Mao, X.-Q. Yu, L.-H. Feng and X.-P. Lai, Heterocycles, 2012, 85, 661–666 CrossRef CAS.
- M. B. Miller, B. A. Haubrich, Q. Wang, W. J. Snell and W. D. Nes, J. Lipid Res., 2012, 53, 1636–1645 CrossRef CAS PubMed.
- N. R. P. V. Macedo, M. S. Ribeiro, R. C. Villaça, W. Ferreira, A. M. Pinto, V. L. Teixeira, C. Cirne-Santos, I. C. N. P. Paixão and V. Giongo, Rev. Bras. Farmacogn., 2012, 22, 861–867 CrossRef CAS PubMed.
- Y.-V. Tang, S.-M. Phang, W.-L. Chu, A. Ho, S. H. Teo and H.-B. Lee, J. Appl. Phycol., 2012, 24, 783–790 CrossRef CAS.
- D. Kelman, E. K. Posner, K. J. McDermid, N. K. Tabandera, P. R. Wright and A. D. Wright, Mar. Drugs, 2012, 10, 403–416 CrossRef CAS PubMed.
- S.-M. Sun, G.-H. Chung and T.-S. Shin, J. Appl. Phycol., 2012, 24, 1003–1013 CrossRef CAS.
- H. Choi, P. J. Proteau, T. Byrum and W. H. Gerwick, Phytochemistry, 2012, 73, 134–141 CrossRef CAS PubMed.
- H. Choi, P. J. Proteau, T. Byrum, A. R. Pereira and W. H. Gerwick, Phytochemistry, 2012, 78, 197–197 CrossRef CAS PubMed.
- Q. Göthel, J. Muñoz and M. Köck, Phytochem. Lett., 2012, 5, 693–695 CrossRef PubMed.
- Q. Göthel, E. Lichte and M. Köck, Tetrahedron Lett., 2012, 53, 1873–1877 CrossRef PubMed.
- C. Francisco, G. Combaut, J. Teste and M. Prost, Phytochemistry, 1978, 17, 1003–1005 CrossRef CAS.
- G. Culioli, M. Daoudi, V. Mesguiche, R. Valls and L. Piovetti, Phytochemistry, 1999, 52, 1447–1454 CrossRef CAS.
- A. Ortalo-Magne, G. Culioli, R. Valls, B. Pucci and L. Piovetti, Phytochemistry, 2005, 66, 2316–2323 CrossRef CAS PubMed.
- L. Hougaard, U. Anthoni, C. Christopherson and P. H. Nielsen, Phytochemistry, 1991, 30, 3049–3051 CrossRef CAS.
- R. Valls, L. Piovetti, B. Banaigs, A. Archavlis and M. Pellegrini, Phytochemistry, 1995, 39, 145–149 CrossRef CAS.
- R. Valls, B. Banaigs, L. Piovetti, A. Archavlis and J. Artaud, Phytochemistry, 1993, 34, 1585–1588 CrossRef CAS.
- F. Bohlmann, A. Adler, J. Jakupovic, R. M. Kings and H. Robinson, Phytochemistry, 1982, 21, 1349–1355 CrossRef CAS.
- E. Ioannou, A. Quesada, M. M. Rahman, S. Gibbons, C. Vagias and V. Roussis, Eur. J. Org. Chem., 2012, 5177–5186 CrossRef CAS.
- H. Zhang, X. Xiao, M. M. Conte, Z. Khalil and R. J. Capon, Org. Biomol. Chem., 2012, 10, 9671–9676 CAS.
- C. Kim, I.-K. Lee, G. Y. Cho, K.-H. Oh, Y. W. Lim and B.-S. Yun, J. Antibiot., 2012, 65, 87–89 CrossRef CAS PubMed.
- H. Nozaki, S. Ohira, D. Takaoka, N. Senda and M. Nakayama, Chem. Lett., 1995, 24, 331–331 CrossRef.
- Y. Kawamura-Konishi, N. Watanabe, M. Saito, N. Nakajima, T. Sakaki, T. Katayama and T. Enomoto, J. Agric. Food Chem., 2012, 60, 5565–5570 CrossRef CAS PubMed.
- R. P. Gregson and J. J. Daly, Aust. J. Chem., 1982, 35, 649–657 CrossRef CAS.
- S.-H. Lee, S.-M. Kang, S.-C. Ko, D.-H. Lee and Y.-J. Jeon, Biochem. Biophys. Res. Commun., 2012, 420, 576–581 CrossRef CAS PubMed.
- S.-M. Kang, S.-J. Heo, K.-N. Kim, S.-H. Lee and Y.-J. Jeon, J. Funct. Foods, 2012, 4, 158–166 CrossRef CAS PubMed.
- E. Ioannou, C. Vagias and V. Roussis, Tetrahedron Lett., 2011, 52, 3054–3056 CrossRef CAS PubMed.
- G. Genta-Jouve, E. Ioannou, V. Mathieu, C. Bruyère, F. Lefranc, O. P. Thomas, R. Kiss and V. Roussis, Phytochem. Lett., 2012, 5, 747–751 CrossRef CAS PubMed.
- A. G. González, J. Darias and J. D. Martín, Tetrahedron Lett., 1971, 12, 2729–2732 CrossRef.
- A. G. González, J. Darias, J. D. Martín and C. Pascual, Tetrahedron, 1973, 29, 1605–1609 CrossRef.
- W. H. Gerwick and W. Fenical, J. Org. Chem., 1981, 46, 22–27 CrossRef CAS.
- M. A. Muñoz, C. Areche, J. Rovirosa, A. San Martin, B. Gordillo-Román and P. Joseph-Nathan, Heterocycles, 2012, 85, 1961–1973 CrossRef PubMed.
- K. C. Pullaiah, R. K. Surapaneni, C. B. Rao, K. F. Albizati, B. W. Sullivan, D. J. Faulkner, H. Cun-heng and J. Clardy, J. Org. Chem., 1985, 50, 3665–3666 CrossRef CAS.
- B. Defaut, T. B. Parsons, N. Spencer, L. Male, B. M. Kariuki and R. S Grainger, Org. Biomol. Chem., 2012, 10, 4926–4932 CAS.
- D. D. Dixon, J. W. Lockner, Q. Zhou and P. S. Baran, J. Am. Chem. Soc., 2012, 134, 8432–8435 CrossRef CAS PubMed.
- T. Kajikawa, S. Okumura, T. Iwashita, D. Kosumi, H. Hashimoto and S. Katsumura, Org. Lett., 2012, 14, 808–811 CrossRef CAS PubMed.
- J. C. De-Paula, D. N. Cavalcanti, Y. Yoneshigue-Valentin and V. L. Teixeira, Rev. Bras. Farmacogn., 2012, 22, 736–740 CrossRef CAS PubMed.
- V. Amico, F. Cunsolo, M. Piatelli and G. Ruberto, Phytochemistry, 1984, 23, 2017–2020 CrossRef CAS.
- C. Jégou, G. Culioli and V. Stiger-Pouvreau, Biochem. Syst. Ecol., 2012, 44, 202–204 CrossRef PubMed.
- G. Blunden, M. D. Guiry, L. D. Druehl, K. Kogame and H. Kawai, Nat. Prod. Commun., 2012, 7, 863–865 CAS.
- K. H. Jang, B. H. Lee, B. W. Choi, H.-S. Lee and J. Shin, J. Nat. Prod., 2005, 68, 710–723 Search PubMed.
- W.-J. Yoon, S.-J. Heo, S.-C. Han, H.-J. Lee, G.-J. Kang, H.-K. Kang, J.-W. Hyun, Y.-S. Koh and E.-S. Yoo, Arch. Pharmacal Res., 2012, 35, 1421–1430 CrossRef CAS PubMed.
- W.-J. Yoon, S.-J. Heo, S.-C. Han, H.-J. Lee, G.-J. Kang, E.-J. Yang, S.-S. Park, H.-K. Kang and E.-S. Yoo, Food Chem. Toxicol., 2012, 50, 3273–3279 CrossRef CAS PubMed.
- S.-H. Lee, M.-H. Park, S.-J. Heo, S.-M. Kang, S.-C. Ko, J.-S. Han and Y.-J. Jeon, Food Chem. Toxicol., 2010, 48, 2633–2637 CrossRef CAS PubMed.
- S.-M. Kang, S.-J. Heo, K.-N. Kim, S.-H. Lee, H.-M. Yang, A.-D. Kim and Y.-J. Jeon, Bioorg. Med. Chem., 2012, 20, 311–316 CrossRef CAS PubMed.
- N. Ragubeer, J. L. Limson and D. R. Beukes, Food Chem., 2012, 131, 286–290 CrossRef CAS PubMed.
- T. Kamada and C. S. Vairappan, Molecules, 2012, 17, 2119–2125 CrossRef CAS PubMed.
- X.-D. Li, F.-P. Miao, K. Li and N.-Y. Ji, Fitoterapia, 2012, 83, 518–522 CrossRef CAS PubMed.
- Y. Liang, X.-M. Li, C.-M. Cui, C.-S. Li, H. Sun and B.-G. Wang, Mar. Drugs, 2012, 10, 2817–2825 CrossRef PubMed.
- M. A. Timmers, D. A. Dias and S. Urban, Mar. Drugs, 2012, 10, 2089–2102 CrossRef CAS PubMed.
- S. Naylor, L. V. Manes and P. Crews, J. Nat. Prod., 1985, 48, 72–75 CrossRef CAS.
- X.-D. Li, W. Ding, F.-P. Miao and N.-Y. Ji, Magn. Reson. Chem., 2012, 50, 174–177 CrossRef CAS PubMed.
- X.-D. Li, F.-P. Miao, X.-L. Yin, J.-L. Liu and N.-Y. Ji, Fitoterapia, 2012, 83, 1191–1195 CrossRef CAS PubMed.
- W. M. Alarif, S. S. Al-Lihaibi, S.-E. N. Ayyad, M. H. Abdel-Rhman and F. A. Badria, Eur. J. Med. Chem., 2012, 55, 462–466 CrossRef CAS PubMed.
- L. R. De Carvalho, M. T. Fujii, N. F. Roque and J. H. G. Largo, Phytochemistry, 2006, 67, 1331–1335 CrossRef CAS PubMed.
- M. T. Crimmins and C. O. Hughes, Org. Lett., 2012, 14, 2168–2171 CrossRef CAS PubMed.
- M. E. Teasdale, T. L. Shearer, S. Engel, T. S. Alexander, C. R. Fairchild, J. Prudhomme, M. Torres, K. Le Roch, W. Aalbersberg, M. E. Hay and J. Kubanek, J. Org. Chem., 2012, 77, 8000–8006 CrossRef CAS PubMed.
- W. M. Alarif, S.-E. N. Ayyad, S. M. El-Assouli and S. S. Al-Lihaibi, Nat. Prod. Res., 2012, 26, 785–791 CrossRef CAS PubMed.
- F. Cen-Pacheco, F. Mollinedo, J. A. Villa-Pulgarín, M. Norte, J. J. Fernández and A. H. Daranas, Tetrahedron, 2012, 68, 7275–7279 CrossRef CAS PubMed.
- A. R. B. Ola, A.-M. Babey, C. Moti and B. F. Bowden, Aust. J. Chem., 2010, 63, 907–914 CrossRef CAS.
- K. Li, X.-M. Li, J. B. Gloer and B.-G. Wang, Food Chem., 2012, 135, 868–872 CrossRef CAS PubMed.
- X. Xu, L. Yin, N. Fang, X. Fan and F. Song, Chem. Nat. Compd., 2012, 48, 622–624 CrossRef CAS.
- X. Xu, A. M. Piggott, L. Yin, R. J. Capon and F. Song, Tetrahedron Lett., 2012, 53, 2103–2106 CrossRef CAS PubMed.
- L. M. de Souza, G. L. Sassaki, M. T. V. Romanos and E. Barreto-Bergter, Mar. Drugs, 2012, 10, 918–931 CrossRef CAS PubMed.
- A. Campos, C. B. Souza, C. Lhullier, M. Falkenberg, E. P. Schenkel, R. M. Ribeiro-do-Valle and J. M. Siqueira, J. Pharm. Pharmacol., 2012, 64, 1146–1154 CrossRef CAS PubMed.
- L. H. A. Cavalcante-Silva, C. B. B. da Matta, M. V. D. de Araújo, J. M. Barbosa-Filho, D. P. de Lira, B. V. de Oiliveira Santos, G. E. C. de Miranda and M. S. Alexandre-Moreira, Mar. Drugs, 2012, 10, 1977–1992 CrossRef PubMed.
- S.-H. Park, J.-H. Song, T. Kim, W.-S. Shin, G. M. Park, S. Lee, Y.-J. Kim, P. Choi, H. Kim, H.-S. Kim, D.-H. Kwon, H.-J. Choi and J. Ham, Mar. Drugs, 2012, 10, 2222–2233 CrossRef CAS PubMed.
- A. Abbas, M. Josefson, G. M. Nylund, H. Pavia and K. Abrahamsson, Anal. Chim. Acta, 2012, 737, 37–44 CrossRef CAS PubMed.
- I. Kuroda, M. Musman, I. I. Ohtani, T. Ichiba, J. Tanaka, D. Garcia Gravalos and T. Higa, J. Nat. Prod., 2002, 65, 1505–1506 CrossRef CAS PubMed.
- H. Yoo, Y. S. Lee, S. Lee, S. Kim and T.-Y. Kim, Phytother. Res., 2012, 26, 1927–1933 CrossRef CAS PubMed.
- L. G. Meimetis, D. E. Williams, N. R. Mawji, C. A. Banuelos, A. A. Lal, J. J. Park, A. H. Tien, J. G. Fernandez, N. J. de Voogd, M. D. Sadar and R. J. Andersen, J. Med. Chem., 2012, 55, 503–514 CrossRef CAS PubMed.
- S. Tsukamoto, T. Takeuchi, H. Rotinsulu, R. E. P. Mangindaan, R. W. M. van Soest, K. Ukai, H. Kobayashi, M. Namikoshi, T. Ohta and H. Yokosawa, Bioorg. Med. Chem. Lett., 2008, 24, 6319–6320 CrossRef PubMed.
- G. Chianese, E. Fattorusso, M. Y. Putra, B. Calcinai, G. Bavestrello, A. S. Moriello, L. De Petrocellis, V. Di Marzo and O. Taglialatela-Scafati, Mar. Drugs, 2012, 10, 2435–2447 CrossRef CAS PubMed.
- K. H. Jang, Y. Lee, C. J. Sim, K.-B. Oh and J. Shin, Bioorg. Med. Chem. Lett., 2012, 22, 1078–1081 CrossRef CAS PubMed.
- F. Farokhi, G. Wielgosz-Collin, A. Robic, C. Debitus, M. Malleter, C. Roussakis, J.-M. Kornprobst and G. Barnathan, Eur. J. Med. Chem., 2012, 49, 406–410 CrossRef CAS PubMed.
- S. Ankisetty and M. Slattery, Mar. Drugs, 2012, 10, 1037–1043 CrossRef CAS PubMed.
- G. Nuzzo, M. L. Ciavatta, G. Villani, E. Manzo, A. Zanfardino, M. Varcamonti and M. Gavagnin, Tetrahedron, 2012, 68, 754–760 CrossRef CAS PubMed.
- J. Kobayashi, S. Takeuchi, M. Ishibashi, H. Shigemori and T. Sasaki, Tetrahedron Lett., 1992, 33, 2579–2580 CrossRef CAS.
- A. Qureshi, C. S. Stevenson, C. L. Albert, R. S. Jacobs and D. J. Faulkner, J. Nat. Prod., 1999, 62, 1205–1207 CrossRef CAS PubMed.
- E. Bourcet, L. Kaufmann, S. Arzt, A. Bihlmeier, W. Klopper, U. Schepers and S. Bräse, Chem.–Eur. J., 2012, 18, 15004–15020 CrossRef CAS PubMed.
- A. Bihlmeier, E. Bourcet, S. Arzt, T. Muller, S. Bräse and W. Klopper, J. Am. Chem. Soc., 2012, 134, 2154–2160 CrossRef CAS PubMed.
- M. Varoglu, B. M. Peters and P. Crews, J. Nat. Prod., 1995, 58, 27–36 CrossRef CAS.
- A. Rudi, R. Afanii, L. Garcia Gravalos, M. Aknin, E. Gaydou, J. Vacelet and Y. Kashman, J. Nat. Prod., 2003, 66, 682–685 CrossRef CAS PubMed.
- A. Qureshi, J. Salvá, M.-K. Harper and D. J. Faulkner, J. Nat. Prod., 1998, 61, 1539–1542 CrossRef CAS PubMed.
- K. W. L. Yong, B. Barnych, J. J. De Voss, J.-M. Vatèle and M. J. Garson, J. Nat. Prod., 2012, 75, 1792–1797 CrossRef CAS PubMed.
- B. Barnych and J.-M. Vatèle, Org. Lett., 2012, 14, 564–567 CrossRef CAS PubMed.
- K. W. L. Yong, L. K. Lambert, P. Y. Hayes, J. J. De Voss and M. J. Garson, J. Nat. Prod., 2012, 75, 351–360 CrossRef CAS PubMed.
- G. Chianese, E. Fattorusso, F. Scala, R. Teta, B. Calcinai, G. Bavestrello, H. A. Dien, M. Kaiser, D. Tasdemir and O. Taglialatela-Scafati, Org. Biomol. Chem., 2012, 10, 7197–7207 CAS.
- V. Venturi, C. Davies, A. J. Singh, J. H. Matthews, D. S. Bellows, P. T. Northcote, R. A. Keyzers and P. H. Teesdale-Spittle, J. Biochem. Mol. Toxicol., 2012, 26, 94–100 CrossRef CAS PubMed.
- H.-B. Yu, X.-F. Liu, Y. Xu, J.-H. Gan, W.-H. Jiao, Y. Shen and H.-W. Lin, Mar. Drugs, 2012, 10, 1027–1036 CrossRef CAS PubMed.
- X.-F. Liu, Y. Shen, F. Yang, M. T. Hamann, W.-H. Jiao, H.-J. Zhang, W.-S. Chen and H.-W. Lin, Tetrahedron, 2012, 68, 4635–4640 CrossRef CAS PubMed.
- C. Festa, G. Lauro, S. De Marino, M. V. D'Auria, M. C. Monti, A. Casapullo, C. D'Amore, B. Renga, A. Mencarelli, S. Petek, G. Bifulco, S. Fiorucci and A. Zampella, J. Med. Chem., 2012, 55, 8303–8317 CrossRef CAS PubMed.
- C. Festa, S. De Marino, M. V. D'Auria, E. Deharo, G. Gonzalez, C. Deyssard, S. Petek, G. Bifulco and A. Zampella, Tetrahedron, 2012, 68, 10157–10163 CrossRef CAS PubMed.
- V. Costantino, E. Fattorusso, A. Mangoni, C. Perinu, R. Teta, E. Panza and A. Ianaro, J. Org. Chem., 2012, 77, 6377–6383 CrossRef CAS PubMed.
- J. Dai, Y. Liu, Y.-D. Zhou and D. G. Nagle, J. Nat. Prod., 2007, 70, 1824–1826 CrossRef CAS PubMed.
- E. E. Podlesny and M. C. Kozlowski, J. Nat. Prod., 2012, 75, 1125–1129 CrossRef CAS PubMed.
- L. Du, F. Mahdi, S. Datta, M. B. Jekabsons, Y.-D. Zhou and D. G. Nagle, J. Nat. Prod., 2012, 75, 1553–1559 CrossRef CAS PubMed.
- Y.-J. Lee, C.-K. Kim, S.-K. Park, J. S. Kang, J. S. Lee, H. J. Shin and H.-S. Lee, Heterocycles, 2012, 85, 895–901 CrossRef CAS PubMed.
- R. Kumar, R. Subramani, K.-D. Feussner and W. Aalbersberg, Mar. Drugs, 2012, 10, 200–208 CrossRef CAS PubMed.
- Y. Feng, R. A. Davis, M. L. Sykes, V. M. Avery and R. J. Quinn, Bioorg. Med. Chem. Lett., 2012, 22, 4873–4876 CrossRef CAS PubMed.
- G. R. Pettit, J.-P. Xu, J.-C. Chapuis, R. K. Pettit, L. P. Tackett, D. L. Doubek, J. N. A. Hooper and J. M. Schmidt, J. Med. Chem., 2004, 47, 1149–1152 CrossRef CAS PubMed.
- C. H. An, A. T. Hoye and A. B. Smith III, Org. Lett., 2012, 14, 4350–4353 CrossRef CAS PubMed.
- W. D. Clark, T. Corbett, F. Valeriote and P. Crews, J. Am. Chem. Soc., 1997, 119, 9285–9286 CrossRef CAS.
- B. K. Rubio, S. J. Robinson, C. E. Avalos, F. A. Valeriote, N. J. de Voogd and P. Crews, J. Nat. Prod., 2008, 71, 1475–1478 CrossRef CAS PubMed.
- J. M. Garcia, S. S. Curzon, K. R. Watts and J. P. Konopelski, Org. Lett., 2012, 14, 2054–2057 CrossRef CAS PubMed.
- P. Cheruku, A. Plaza, G. Lauro, J. Keffer, J. R. Lloyd, G. Bifulco and C. A. Bewley, J. Med. Chem., 2012, 55, 735–742 CrossRef CAS PubMed.
- M. Kimura, T. Wakimoto, Y. Egami, K. C. Tan, Y. Ise and I. Abe, J. Nat. Prod., 2012, 75, 290–294 CrossRef CAS PubMed.
- M. Arai, Y. Yamano, M. Fujita, A. Setiawan and M. Kobayashi, Bioorg. Med. Chem. Lett., 2012, 22, 1818–1821 CrossRef CAS PubMed.
- C. Festa, S. De Marino, M. V. D'Auria, M. C. Monti, M. Bucci, V. Vellecco, C. Debitus and A. Zampella, Tetrahedron, 2012, 68, 2851–2857 CrossRef CAS PubMed.
- T. D. Tran, N. B. Pham, G. Fechner, D. Zencak, H. T. Vu, J. N. A. Hooper and R. J. Quinn, J. Nat. Prod., 2012, 75, 2200–2208 CrossRef CAS PubMed.
- Y. Yamano, M. Arai and M. Kobayashi, Bioorg. Med. Chem. Lett., 2012, 22, 4877–4881 CrossRef CAS PubMed.
- J. Sorres, M.-T. Martin, S. Petek, H. Levaique, T. Cresteil, S. Ramos, O. Thoison, C. Debitus and A. Al-Mourabit, J. Nat. Prod., 2012, 75, 759–763 CrossRef CAS PubMed.
- I. Paterson, S. M. Dalby, J. C. Roberts, G. J. Naylor, E. A. Guzmán, R. Isbrucker, T. P. Pitts, P. Linley, D. Divlianska, J. K. Reed and A. E. Wright, Angew. Chem., Int. Ed., 2011, 50, 3219–3223 CrossRef CAS PubMed.
- A. Zampella, M. V. D'Auria, L. Minale and C. Debitus, Tetrahedron, 1997, 53, 3243–3248 CrossRef CAS.
- J. Willwacher, N. Kausch-Busies and A. Fürstner, Angew. Chem., Int. Ed., 2012, 51, 12041–12046 CrossRef CAS PubMed.
- J. R. Frost, C. M. Pearson, T. N. Snaddon, R. A. Booth and S. V. Ley, Angew. Chem., Int. Ed., 2012, 51, 9366–9371 CrossRef CAS PubMed.
- A. Bishara, A. Rudi, M. Aknin, D. Neumann, N. Ben-Califa and Y. Kashman, Tetrahedron Lett., 2008, 49, 4355–4358 CrossRef CAS PubMed.
- N. Ben-Califa, A. Bishara, Y. Kashman and D. Neumann, Invest. New Drugs, 2012, 30, 98–104 CrossRef CAS PubMed.
- D. E. Williams, M. Roberge, R. Van Soest and R. J. Andersen, J. Am. Chem. Soc., 2003, 125, 5296–5297 CrossRef CAS PubMed.
- D. E. Williams, M. Lapawa, X. Feng, T. Tarling, M. Roberge and R. J. Andersen, Org. Lett., 2004, 6, 2607–2610 CrossRef CAS PubMed.
- K. Warabi, D. E. Williams, B. O. Patrick, M. Roberge and R. J. Andersen, J. Am. Chem. Soc., 2007, 129, 508–509 CrossRef CAS PubMed.
- M. Suzuki, R. Ueoka, K. Takada, S. Okada, S. Ohtsuka, Y. Ise and S. Matsunaga, J. Nat. Prod., 2012, 75, 1192–1195 CrossRef CAS PubMed.
- Y. Hirata and D. Uemura, Pure Appl. Chem., 1986, 58, 701–710 CrossRef CAS.
- A. Yamamoto, A. Ueda, P. Bremond, P. S. Tiseni and Y. Kishi, J. Am. Chem. Soc., 2012, 134, 893–896 CrossRef CAS PubMed.
- N. K. Utkina and V. B. Krasokhin, Chem. Nat. Compd., 2012, 48, 715–716 CrossRef CAS PubMed.
- S. Shen, D. Liu, C. Wei, P. Proksch and W. Lin, Bioorg. Med. Chem., 2012, 20, 6924–6928 CrossRef CAS PubMed.
- L.-M. Zheng, Y. Zhu, S.-J. Yan and J.-Y. Su, Heterocycles, 1998, 14, 426–427 Search PubMed.
- Y.-W. Liang, X.-J. Liao, C.-J. Wang, J.-Z. Guo, S. Li and S.-H. Xu, J. Chem. Res., 2012, 12, 736–737 CrossRef.
- C. Cychon, G. Schmidt, T. Mordhorst and M. Köck, Z. Naturforsch., B: J. Chem. Sci., 2012, 67, 944–950 CAS.
- M. Ilias, M. A. Ibrahim, S. I. Khan, M. R. Jacob, B. L. Tekwani, L. A. Walker and V. Samoylenko, Planta Med., 2012, 78, 1690–1697 CrossRef CAS PubMed.
- S. J. Wratten, D. J. Faulkner, K. Hirotsu and J. Clardy, Tetrahedron Lett., 1978, 4345–4348 CrossRef CAS.
- M. L. Ciavatta, A. Fontana, R. Puliti, G. Scognamiglio and G. Cimino, Tetrahedron, 1999, 55, 12629–12636 CrossRef CAS.
- A. M. S. Mayer, E. Avilés and A. D. Rodríguez, Bioorg. Med. Chem., 2012, 20, 279–282 CrossRef CAS PubMed.
- E. Quiñoà, M. Adamczeski, P. Crews and G. J. Bakus, J. Org. Chem., 1986, 51, 4494–4497 CrossRef.
- T. A. Johnson, J. Sohn, Y. M. Vaske, K. N. White, T. L. Cohen, H. C. Vervoort, K. Tenney, F. A. Valeriote, L. F. Bjeldanes and P. Crews, Bioorg. Med. Chem., 2012, 20, 4348–4355 CrossRef CAS PubMed.
- A. E. Wahba, Y. Fromentin, Y. Zou and M. T. Hamann, Tetrahedron Lett., 2012, 53, 6329–6331 CrossRef CAS PubMed.
- B. S. Hwang, J. S. Oh, E. J. Jeong, C. J. Sim and J.-R. Rho, Org. Lett., 2012, 14, 6154–6157 CrossRef CAS PubMed.
- N. Saito, M. Yoshino, K. Charupant and K. Suwanborirux, Heterocycles, 2012, 84, 309–314 CrossRef CAS PubMed.
- M. Tatsukawa, L. L. C. Punzalan, H. D. S. Magpantay, I. M. Villaseñor, G. P. Concepcion, K. Suwanborirux, M. Yokoya and N. Saito, Tetrahedron, 2012, 68, 7422–7428 CrossRef CAS PubMed.
- M. Menna, A. Aiello, F. D'Aniello, E. Fattorusso, C. Imperatore, P. Luciano and R. Vitalone, Mar. Drugs, 2012, 10, 2509–2518 CrossRef CAS PubMed.
- T. Nishi, T. Kubota, J. Fromont, T. Sasaki and J. Kobayashi, Tetrahedron, 2008, 64, 3127–3132 CrossRef CAS PubMed.
- S. G. Davies, J. A. Lee, P. M. Roberts, R. S. Shah and J. E. Thomson, Chem. Commun., 2012, 48, 9236–9238 RSC.
- Y. Lee, K. H. Jang, J.-E. Jeon, W.-Y. Yang, C. J. Sim, K.-B. Oh and J. Shin, Mar. Drugs, 2012, 10, 2126–2137 CrossRef CAS PubMed.
- G. Schmidt, C. Timm and M. Köck, Org. Biomol. Chem., 2009, 7, 3061–3064 CAS.
- A. Grube, C. Timm and M. Köck, Eur. J. Org. Chem., 2006, 1285–1295 CrossRef CAS.
- G. Schmidt, C. Timm, A. Grube, C. A. Volk and M. Köck, Chem.–Eur. J., 2012, 18, 8180–8189 CrossRef CAS PubMed.
- M. Lunder, G. Drevenšek, S. Hawlina, K. Sepčić and L. Žiberna, Toxicon, 2012, 60, 1041–1048 CrossRef CAS PubMed.
- R.-P. Wang, H.-W. Lin, L.-Z. Li, P.-Y. Gao, Y. Xu and S.-J. Song, Biochem. Syst. Ecol., 2012, 43, 210–213 CrossRef CAS PubMed.
- H. Prawat, C. Mahidol, W. Kaweetripob, S. Wittayalai and S. Ruchirawat, Tetrahedron, 2012, 68, 6881–6886 CrossRef CAS PubMed.
- M. Tsuda, N. Kawasaki and J. Kobayashi, Tetrahedron Lett., 1994, 35, 4387–4388 CrossRef CAS.
- H. Ishiyama, Y. Mori, T. Matsumoto and J. Kobayashi, Heterocycles, 2012, 86, 1009–1014 CrossRef CAS PubMed.
- R. Yamanokuchi, K. Imada, M. Miyazaki, H. Kato, T. Watanabe, M. Fujimuro, Y. Saeki, S. Yoshinaga, H. Terasawa, N. Iwasaki, H. Rotinsulu, F. Losung, R. E. P. Mangindaan, M. Namikoshi, N. J. de Voogd, H. Yokosawa and S. Tsukamoto, Bioorg. Med. Chem., 2012, 20, 4437–4442 CrossRef CAS PubMed.
- T. Abe, A. Kukita, K. Akiyama, T. Naito and D. Uemura, Chem. Lett., 2012, 41, 728–729 CrossRef CAS.
- E. G. Lyakhova, S. A. Kolesnikova, A. I. Kalinovsky, S. S. Afiyatullov, S. A. Dyshlovoy, V. B. Krasokhin, C. V. Minh and V. A. Stonik, Tetrahedron Lett., 2012, 53, 6119–6122 CrossRef CAS PubMed.
- Y. Takahashi, N. Tanaka, T. Kubota, H. Ishiyama, A. Shibazaki, T. Gonoi, J. Fromont and J. Kobayashi, Tetrahedron, 2012, 68, 8545–8550 CrossRef CAS PubMed.
- C. Liu, X. Tang, P. Li and G. Li, Org. Lett., 2012, 14, 1994–1997 CrossRef CAS PubMed.
- F. Yang, M. T. Hamann, Y. K. Zou, M.-Y. Zhang, X.-B. Gong, J.-R. Xiao, W.-S. Chen and H.-W. Lin, J. Nat. Prod., 2012, 75, 774–778 CrossRef CAS PubMed.
- T. D. Nguyen, X. C. Nguyen, A. Longeon, A. Keryhuel, M. H. Le, Y. H. Kim, V. M. Chau and M.-L. Bourguet-Kondracki, Tetrahedron, 2012, 68, 9256–9259 CrossRef CAS PubMed.
- M. A. Fouad, A. Debbab, V. Wray, W. E. G. Müller and P. Proksch, Tetrahedron, 2012, 68, 10176–10179 CrossRef CAS PubMed.
- R. G. Linington, D. E. Williams, A. Tahir, R. van Soest and R. J. Andersen, Org. Lett., 2003, 5, 2735–2738 CrossRef CAS PubMed.
- G. W. Carlile, R. A. Keyzers, K. A. Teske, R. Robert, D. E. Williams, R. G. Linington, C. A. Gray, R. M. Centko, L. Yan, S. M. Anjos, H. M. Sampson, D. Zhang, J. Liao, J. W. Hanrahan, R. J. Andersen and D. Y. Thomas, Chem. Biol., 2012, 19, 1288–1299 CrossRef CAS PubMed.
- M. Fujita, Y. Nakao, S. Matsunaga, M. Seiki, Y. Itoh, J. Yamashita, R. W. M. van Soest and N. Fusetani, J. Am. Chem. Soc., 2003, 125, 15700–15701 CrossRef CAS PubMed.
- U. Bickmeyer, Mar. Drugs, 2012, 10, 223–233 CrossRef CAS PubMed.
- D. C. Radisky, E. S. Radisky, L. R. Barrows, B. R. Copp, R. A. Kramer and C. M. Ireland, J. Am. Chem. Soc., 1993, 115, 1632–1638 CrossRef CAS.
- T. Oshiyama, T. Satoh, K. Okano and H. Tokuyama, Tetrahedron, 2012, 68, 9376–9383 CrossRef CAS PubMed.
- R. A. Davis, M. S. Buchanan, S. Duffy, V. M. Avery, S. A. Charman, W. N. Charman, K. L. White, D. M. Shackleford, M. D. Edstein, K. T. Andrews, D. Camp and R. J. Quinn, J. Med. Chem., 2012, 55, 5851–5858 CrossRef CAS PubMed.
- S. Bondu, G. Genta-Jouve, M. Leiròs, C. Vale, J. M. Guigonis, L. M. Botana and O. P. Thomas, RSC Adv., 2012, 2, 2828–2835 RSC.
- S. Takishima, A. Ishiyama, M. Iwatsuki, K. Otoguro, H. Yamada, S. Omura, H. Kobayashi, R. W. M. van Soest and S. Matsunaga, Org. Lett., 2009, 11, 2655–2658 CrossRef CAS PubMed.
- N. R. Babij and J. P. Wolfe, Angew. Chem., Int. Ed., 2012, 51, 4128–4130 CrossRef CAS PubMed.
- T. N. Makarieva, K. M. Tabakmaher, A. G. Guzii, V. A. Denisenko, P. S. Dmitrenok, A. S. Kuzmich, H.-S. Lee and V. A. Stonik, Tetrahedron Lett., 2012, 53, 4228–4231 CrossRef CAS PubMed.
- W. Hassan, R. Edrada, R. Ebel, V. Wray, A. Berg, R. van Soest, S. Wiryowidagdo and P. Proksch, J. Nat. Prod., 2004, 67, 817–822 CrossRef CAS PubMed.
- J. Das, P. B. Koswatta, J. D. Jones, M. Yousufuddin and C. J. Lovely, Org. Lett., 2012, 14, 6210–6213 CrossRef CAS PubMed.
- J. B. Gibbons, K. M. Gligorich, B. E. Welm and R. E. Looper, Org. Lett., 2012, 14, 4734–4737 CrossRef CAS PubMed.
- H. Zhang, M. M. Conte, X.-C. Huang, Z. Khalil and R. J. Capon, Org. Biomol. Chem., 2012, 10, 2656–2663 CAS.
- H. Zhang, M. M. Conte, Z. Khalil, X.-C. Huang and R. J. Capon, RSC Adv., 2012, 2, 4209–4214 RSC.
- T. Kubota, A. Araki, T. Yasuda, M. Tsuda, J. Fromont, K. Aoyama, Y. Mikami, M. R. Wälchli and J. Kobayashi, Tetrahedron Lett., 2009, 50, 7268–7270 CrossRef CAS PubMed.
- T. Endo, M. Tsuda, T. Okada, S. Mitsuhasi, H. Shima, K. Kikuchi, Y. Mikami, J. Fromont and J. Kobayashi, J. Nat. Prod., 2004, 67, 1262–1267 CrossRef CAS PubMed.
- S. Forenza, L. Minale, R. Riccio and E. Fattorusso, J. Chem. Soc. Chem. Commun., 1971, 1129–1130 RSC.
- E. P. Stout, B. I. Morinaka, Y.-G. Wang, D. Romo and T. F. Molinski, J. Nat. Prod., 2012, 75, 527–530 CrossRef CAS PubMed.
- E. P. Stout, Y.-G. Wang, D. Romo and T. F. Molinski, Angew. Chem., Int. Ed., 2012, 51, 4877–4881 CrossRef CAS PubMed.
- J. Kobayashi, M. Tsuda, T. Murayama, H. Nakamura, Y. Ohizumi, M. Ishibashi, M. Iwamura, T. Ohta and S. Nozoe, Tetrahedron, 1990, 46, 5579–5586 CrossRef CAS.
- X. Wang, X. Wang, X. Tan, J. Lu, K. W. Cormier, Z. Ma and C. Chen, J. Am. Chem. Soc., 2012, 134, 18834–18842 CrossRef CAS PubMed.
- H. Zhang, Z. Khalil, M. M. Conte, F. Plisson and R. J. Capon, Tetrahedron Lett., 2012, 53, 3784–3787 CrossRef CAS PubMed.
- J. Munoz, C. Moriou, J.-F. Gallard, P. D. Marie and A. Al-Mourabit, Tetrahedron Lett., 2012, 53, 5828–5832 CrossRef CAS PubMed.
- M. D'Ambrosio, A. Guerriero, P. Traldi and F. Pietra, Tetrahedron Lett., 1982, 23, 4403–4406 CrossRef CAS.
- G. M. Sharma and P. R. Burkholder, Tetrahedron Lett., 1976, 17, 4147–4150 CrossRef.
- E. A. Santalova, Nat. Prod. Commun., 2012, 7, 617–619 CAS.
- L. A. Shaala, D. T. A. Youssef, M. Sulaiman, F. A. Behery, A. I. Foudah and K. A. El Sayed, Mar. Drugs, 2012, 10, 2492–2508 CrossRef CAS PubMed.
- Y. Feng, B. F. Bowden and V. Kapoor, Bioorg. Med. Chem. Lett., 2012, 22, 3398–3401 CrossRef CAS PubMed.
- T. Kubota, S. Watase, H. Mukai, J. Fromont and J. Kobayashi, Chem. Pharm. Bull., 2012, 60, 1599–1601 CAS.
- M. Xu, R. A. Davis, Y. Feng, M. L. Sykes, T. Shelper, V. M. Avery, D. Camp and R. J. Quinn, J. Nat. Prod., 2012, 75, 1001–1005 CrossRef CAS PubMed.
- S. Yin, R. A. Davis, T. Shelper, M. L. Sykes, V. M. Avery, M. Elofsson, C. Sundin and R. J. Quinn, Org. Biomol. Chem., 2011, 9, 6755–6760 CAS.
- J. M. Hillgren, C. T. Öberg and M. Elofsson, Org. Biomol. Chem., 2012, 10, 1246–1254 CAS.
- C. Jimenez and P. Crews, Tetrahedron, 1991, 47, 2097–2102 CrossRef CAS.
- N. Fusetani, Y. Masuda, Y. Nakao, S. Matsunaga and R. W. M. van Soest, Tetrahedron, 2001, 57, 7507–7511 CrossRef CAS.
- S. K. Kottakota, M. Benton, D. Evangelopoulos, J. D. Guzman, S. Bhakta, T. D. McHugh, M. Gray, P. W. Groundwater, E. C. L. Marrs, J. D. Perry and J. J. Harburn, Org. Lett., 2012, 14, 6310–6313 CrossRef CAS PubMed.
- R. Xynas and R. J. Capon, Aust. J. Chem., 1989, 42, 1427–1433 CrossRef CAS.
- T. Evan, A. Rudi, M. Ilan and Y. Kashman, J. Nat. Prod., 2001, 64, 226–227 CrossRef CAS.
- M. R. Kernan, R. C. Cambie and P. R. Bergquist, J. Nat. Prod., 1990, 53, 720–723 CrossRef CAS.
- S. Tilvi, C. Rodrigues, C. G. Naik, P. S. Parameswaran and S. Wahidhulla, Tetrahedron, 2004, 60, 10207–10215 CrossRef CAS PubMed.
- M. Tsuda, Y. Sakuma and J. Kobayashi, J. Nat. Prod., 2001, 64, 980–982 CrossRef CAS PubMed.
- S. K. Kottakota, D. Evangelopoulos, A. Alnimr, S. Bhakta, T. D. McHugh, M. Gray, P. W. Groundwater, E. C. L. Marrs, J. D. Perry, C. D. Spilling and J. J. Harburn, J. Nat. Prod., 2012, 75, 1090–1101 CrossRef CAS PubMed.
- J. Kobayashi, K. Honma, T. Sasaki and M. Tsuda, Chem. Pharm. Bull., 1995, 43, 403–407 CrossRef CAS.
- G. Cimino, S. De Rosa, S. De Stefano, R. Self and G. Sodano, Tetrahedron Lett., 1983, 24, 3029–3032 CrossRef CAS.
- A. A. Salim, Z. G. Khalil and R. J. Capon, Tetrahedron, 2012, 68, 9802–9807 CrossRef CAS PubMed.
- A. D. Wright, P. J. Schupp, J.-P. Schrör, A. Engemann, S. Rohde, D. Kelman, N. de Voogd, A. Carroll and C. A. Motti, J. Nat. Prod., 2012, 75, 502–506 CrossRef CAS PubMed.
- L. Mani, V. Jullian, B. Mourkazel, A. Valentin, J. Dubois, T. Cresteil, E. Folcher, J. N. A. Hooper, D. Erpenbeck, W. Aalbersberg and C. Debitus, Chem. Biodiversity, 2012, 9, 1436–1451 CAS.
- I. W. Mudianta, T. Skinner-Adams, K. T. Andrews, R. A. Davis, T. A. Hadi, P. Y. Hayes and M. J. Garson, J. Nat. Prod., 2012, 75, 2132–2143 CrossRef CAS PubMed.
- L. Calcul, W. D. Inman, A. A. Morris, K. Tenney, J. Ratnam, J. H. McKerrow, F. A. Valeriote and P. Crews, J. Nat. Prod., 2010, 73, 365–372 CrossRef CAS PubMed.
- S. Pérez-Rodriguez, R. Pereira-Cameselle and Á. R. de Lera, Org. Biomol. Chem., 2012, 10, 6945–6950 Search PubMed.
- L. K. Shubina, A. I. Kalinovsky, T. N. Makarieva, S. N. Fedorov, S. A. Dyshlovoy, P. S. Dmitrenok, I. I. Kapustina, E. Mollo, N. K. Utkina, V. B. Krasokhin, V. A. Denisenko and V. A. Stonik, Nat. Prod. Commun., 2012, 7, 487–490 CAS.
- W.-H. Jiao, X.-J. Huang, J.-S. Yang, F. Yang, S.-J. Piao, H. Gao, J. Li, W.-C. Ye, X.-S. Yao, W.-S. Chen and H.-W. Lin, Org. Lett., 2012, 14, 202–205 CrossRef CAS PubMed.
- V. J. R. V. Mukku, R. A. Edrada, F. J. Schmitz, M. K. Shanks, B. Chaudhuri and D. Fabbro, J. Nat. Prod., 2003, 66, 686–689 CrossRef CAS PubMed.
- E. Alvarez-Manzaneda, R. Chahboun, E. Alvarez, A. Fernández, R. Alvarez-Manzaneda, A. Haidour, J. M. Ramos and A. Akhaouzan, Chem. Commun., 2012, 48, 606–608 RSC.
- H.-S. Lee, Y.-J. Lee, J. W. Lee, H. J. Shin, J. S. Lee, K.-N. Kim, W.-J. Yoon, S.-J. Heo and H.-K. Kim, Heterocycles, 2012, 85, 1437–1446 CrossRef CAS.
- A. Fukami, Y. Ikdea, S. Kondo, H. Naganawa, T. Takeuchi, S. Furuya, Y. Hirabashi, K. Shimoike, S. Hosaka, Y. Watanabe and K. Umezawa, Tetrahedron Lett., 1997, 38, 1201–1202 CrossRef CAS.
- H. Hosoi, N. Kawai, H. Hagiwara, T. Suzuki, A. Nakazaki, K. Takao, K. Umezawa and S. Kobayashi, Chem. Pharm. Bull., 2012, 60, 137–143 CrossRef CAS.
- D. E. Williams, A. Steino, N. J. de Voogd, A. G. Mauk and R. J. Andersen, J. Nat. Prod., 2012, 75, 1451–1458 CrossRef CAS PubMed.
- R. J. Clark, B. L. Stapleton and M. J. Garson, Tetrahedron, 2000, 56, 3071–3076 CrossRef CAS.
- A. D. Wright, Comp. Biochem. Physiol., 2003, 134A, 307–313 CrossRef CAS.
- H. Mitome, N. Shirato, H. Miyaoka, Y. Yamada and R. W. M. van Soest, J. Nat. Prod., 2004, 67, 833–837 CrossRef CAS PubMed.
- K. Nishikawa, T. Umezawa, M. J. Garson and F. Matsuda, J. Nat. Prod., 2012, 75, 2232–2235 CrossRef CAS PubMed.
- N. Tanaka, S. Suto, H. Ishiyama, T. Kubota, A. Yamano, M. Shiro, J. Fromont and J. Kobayashi, Org. Lett., 2012, 14, 3498–3501 CrossRef CAS PubMed.
- M. S. Butler and R. J. Capon, Aust. J. Chem., 1992, 45, 1705–1743 CrossRef CAS.
- S. De Rosa, R. Puliti, A. Crispino, A. De Giulio, C. De Sena, C. Iodice and C. A. Mattia, Tetrahedron, 1995, 51, 10731–10736 CrossRef CAS.
- P. S. Deore and N. P. Argade, J. Org. Chem., 2012, 77, 739–746 CrossRef CAS PubMed.
- Y. Xu, J.-H. Lang, W.-H. Jiao, R.-P. Wang, Y. Peng, S.-J. Song, B.-H. Zhang and H.-W. Lin, Mar. Drugs, 2012, 10, 1445–1458 CrossRef CAS PubMed.
- Y. Xu, N. Li, W.-H. Jiao, R.-P. Wang, Y. Peng, S.-H. Qi, S.-J. Song, W.-S. Chen and H.-W. Lin, Tetrahedron, 2012, 68, 2876–2883 CrossRef CAS PubMed.
- C. W. J. Chang, A. Patra, D. M. Roll, P. J. Scheuer, G. K. Matsumoto and J. Clardy, J. Am. Chem. Soc., 1984, 106, 4644–4646 CrossRef CAS.
- H. Miyako, M. Shimomura, H. Kimura and Y. Yamada, Tetrahedron, 1998, 54, 13467–13474 CrossRef.
- C. W. J. Chang, A. Patra, J. A. Baker and P. J. Scheuer, J. Am. Chem. Soc., 1987, 109, 6119–6123 CrossRef CAS.
- H. Miyaoka, Y. Abe, N. Sekiya, H. Mitome and E. Kawashima, Chem. Commun., 2012, 48, 901–903 RSC.
- H. Miyaoka, Y. Abe and E. Kawashima, Chem. Pharm. Bull., 2012, 60, 1224–1226 CrossRef CAS PubMed.
- R. Kazlauskas, P. T. Murphy, R. J. Wells and J. F. Blount, Tetrahedron Lett., 1980, 21, 315–318 CrossRef CAS.
- S. V. Pronin and R. A. Shenvi, J. Am. Chem. Soc., 2012, 134, 19604–19606 CrossRef CAS PubMed.
- N. Chanthathamrongsiri, S. Yuenyongsawad, C. Wattanapiromsakul and A. Plubrukarn, J. Nat. Prod., 2012, 75, 789–792 CrossRef CAS PubMed.
- S. Tang, R. Xu, W. Lin and H. Duan, Rec. Nat. Prod., 2012, 6, 398–401 CAS.
- M. H. Uddin, M. K. Hossain, M. Nigar, M. C. Roy and J. Tanaka, Chem. Nat. Compd., 2012, 48, 412–415 CrossRef CAS.
- P. L. Katavic, P. Jumaryatno, J. N. A. Hooper, J. T. Blanchfield and M. J. Garson, Aust. J. Chem., 2012, 65, 531–538 CrossRef CAS.
- P. Gupta, U. Sharma, T. C. Schulz, A. B. McLean, A. J. Robins and L. M. West, J. Nat. Prod., 2012, 75, 1223–1227 CrossRef CAS PubMed.
- E. P. Stout, L. C. Yu and T. F. Molinski, Eur. J. Org. Chem., 2012, 27, 5131–5135 CrossRef.
- T. Kubota, T. Iwai, A. Takahashi-Nakaguchi, J. Fromont, T. Gonoi and J. Kobayashi, Tetrahedron, 2012, 68, 9738–9744 CrossRef CAS PubMed.
- W. He, X. Lin, T. Xu, J. H. Jung, H. Yin, B. Yang and Y. Liu, Chem. Nat. Compd., 2012, 48, 208–210 CrossRef CAS.
- S. R. M. Ibrahim, Nat. Prod. Commun., 2012, 7, 9–12 CAS.
- R. Forestieri, C. E. Merchant, N. J. de Voogd, T. Matainaho, T. J. Kieffer and R. J. Andersen, Org. Lett., 2009, 11, 5166–5169 CrossRef CAS PubMed.
- J. Huang, J. R. Yang, J. Zhang and J. Yang, J. Am. Chem. Soc., 2012, 134, 8806–8809 CrossRef CAS PubMed.
- M. Xuan, I. Paterson and S. M. Dalby, Org. Lett., 2012, 14, 5492–5495 CrossRef CAS PubMed.
- W. Wang, Y. Lee, T. G. Lee, B. Mun, A. G. Giri, J. Lee, H. Kim, D. Hahn, I. Yang, J. Chin, H. Choi, S.-J. Nam and H. Kang, Org. Lett., 2012, 14, 4486–4489 CrossRef CAS PubMed.
- T. Diyabalanage, R. Ratnayake, H. R. Bokesch, T. T. Ransom, C. J. Henrich, J. A. Beutler, J. B. McMahon and K. R. Gustafson, J. Nat. Prod., 2012, 75, 1490–1494 CrossRef CAS PubMed.
- Y.-C. Chang, S.-W. Tseng, L.-L. Liu, Y. Chou, Y.-S. Ho, M.-C. Lu and J.-H. Su, Mar. Drugs, 2012, 10, 987–997 CrossRef CAS PubMed.
- G. Cimino, S. De Stefano and L. Minale, Experientia, 1974, 30, 846–847 Search PubMed.
- L. Margarucci, M. C. Monti, M. G. Chini, A. Tosco, R. Riccio, G. Bifulco and A. Casapullo, ChemBioChem, 2012, 13, 2259–2264 CrossRef CAS PubMed.
- C. Festa, S. De Marino, M. V. D'Auria, G. Bifulco, B. Renga, S. Fiorucci, S. Petek and A. Zampella, J. Med. Chem., 2011, 54, 401–405 CrossRef CAS PubMed.
- V. Sepe, R. Ummarino, M. V. D'Auria, B. Renga, S. Fiorucci and A. Zampella, Eur. J. Org. Chem., 2012, 27, 5187–5194 CrossRef.
- R. Teta, G. Della Sala, B. Renga, A. Mangoni, S. Fiorucci and V. Costantino, Mar. Drugs, 2012, 10, 1383–1390 CrossRef CAS PubMed.
- S. V. S. Govindam, B.-K. Choi, Y. Yoshioka, A. Kanamoto, T. Fujiwara, T. Okamoto and M. Ojika, Biosci., Biotechnol., Biochem., 2012, 76, 999–1002 CrossRef CAS.
- V. Sepe, R. Ummarino, M. V. D'Auria, M. G. Chini, G. Bifulco, B. Renga, C. D'Amore, C. Debitus, S. Fiorucci and A. Zampella, J. Med. Chem., 2012, 55, 84–93 CrossRef CAS PubMed.
- M. G. Chini, C. R. Jones, A. Zampella, M. V. D'Auria, B. Renga, S. Fiorucci, C. P. Butts and G. Bifulco, J. Org. Chem., 2012, 77, 1489–1496 CrossRef CAS PubMed.
- S. De Marino, R. Ummarino, M. V. D'Auria, M. G. Chini, G. Bifulco, C. D'Amore, B. Renga, A. Mencarelli, S. Petek, S. Fiorucci and A. Zampella, Steroids, 2012, 77, 484–495 CrossRef CAS PubMed.
- J.-K. Guo, C.-Y. Chiang, M.-C. Lu, W.-B. Chang and J.-H. Su, Mar. Drugs, 2012, 10, 1536–1544 CrossRef CAS PubMed.
- F. Berrué, M. W. B. McCulloch, P. Boland, S. Hart, M. K. Harper, J. Johnston and R. Kerr, J. Nat. Prod., 2012, 75, 2094–2100 CrossRef PubMed.
- S. Ushiyama, H. Umaoka, H. Kato, Y. Suwa, H. Morioka, H. Rotinsulu, F. Losung, R. E. P. Mangindaan, N. J. de Voogd, H. Yokosawa and S. Tsukamoto, J. Nat. Prod., 2012, 75, 1495–1499 CrossRef CAS PubMed.
- W. Zhang and C. Che, J. Nat. Prod., 2001, 64, 1489–1492 CrossRef CAS PubMed.
- F. W. K. Cheung, J. Guo, Y.-H. Ling, C.-T. Che and W.-K. Liu, Mar. Drugs, 2012, 10, 465–476 CrossRef CAS PubMed.
- J.-H. Lee, J.-E. Jeon, Y.-J. Lee, H.-S. Lee, C. J. Sim, K.-B. Oh and J. Shin, J. Nat. Prod., 2012, 75, 1365–1372 CrossRef CAS PubMed.
- J. Li, H. J. Zhu, J. Ren, Z. Deng, N. J. de Voogd, P. Proksch and W. Lin, Tetrahedron, 2012, 68, 559–565 CrossRef CAS PubMed.
- V. Costantino, G. D. Sala, A. Mangoni, C. Perinu and R. Teta, Eur. J. Org. Chem., 2012, 27, 5171–5176 CrossRef.
- M. Y. Putra, A. Ianaro, E. Panza, G. Bavestrello, C. Cerrano, E. Fattorusso and O. Taglialatela-Scafati, Bioorg. Med. Chem. Lett., 2012, 22, 2723–2725 CrossRef CAS PubMed.
- M. Y. Putra, A. Ianaro, E. Panza, G. Bavestrello, C. Cerrano, E. Fattorusso and O. Taglialatela-Scafati, Tetrahedron Lett., 2012, 53, 3937–3939 CrossRef CAS PubMed.
- J. G. L. Almeida, A. I. V. Maia, D. V. Wilke, E. R. Silveira, R. Braz-Filho, J. J. La Clair, L. V. Costa-Lotufo and O. D. L. Pessoa, Mar. Drugs, 2012, 10, 2846–2860 CrossRef PubMed.
- A. Alfonso, A. Fernandez-Araujo, C. Alfonso, B. Carames, A. Tobio, M. C. Louzao, M. R. Vieytes and L. M. Botana, Anal. Biochem., 2012, 424, 64–70 CrossRef CAS PubMed.
- S.-K. Wang, Y.-S. Lee and C.-Y. Duh, Mar. Drugs, 2012, 10, 1528–1535 CrossRef CAS PubMed.
- H. Shi, S. Yu, D. Liu, L. van Ofwegen, P. Proksch and W. Lin, Mar. Drugs, 2012, 10, 1331–1344 CrossRef CAS PubMed.
- D. Chen, S. Yu, L. van Ofwegen, P. Proksch and W. Lin, J. Agric. Food Chem., 2012, 60, 112–123 CrossRef CAS PubMed.
- N. Gören, A. Ulubelen, J. Jakupovic, F. Bohlmann and M. Grenz, Phytochemistry, 1984, 23, 2281–2284 CrossRef.
- C.-H. Lee, C.-Y. Kao, S.-Y. Kao, C.-H. Chang, J.-H. Su, T.-L. Hwang, Y.-H. Kuo, Z.-H. Wen and P.-J. Sung, Mar. Drugs, 2012, 10, 427–438 CrossRef CAS PubMed.
- P.-J. Sung, S.-Y. Kao, C.-Y. Kao, Y.-C. Chang, Y.-H. Chen and Y.-D. Su, Biochem. Syst. Ecol., 2012, 40, 53–55 CrossRef CAS PubMed.
- L.-S. Huang, F. He, H. Huang, X.-Y. Zhang and S.-H. Qi, Beilstein J. Org. Chem., 2012, 8, 170–176 CrossRef CAS PubMed.
- J.-H. Su, C.-Y. Huang, P.-J. Li, Y. Lu, Z.-H. Wen, Y.-H. Kao and J.-H. Sheu, Arch. Pharmacal Res., 2012, 35, 779–784 CrossRef CAS PubMed.
- Y.-J. Tseng, K.-P. Shen, H.-L. Lin, C.-Y. Huang, C.-F. Dai and J.-H. Sheu, Mar. Drugs, 2012, 10, 1572–1581 CrossRef CAS PubMed.
- J.-R. Zhang, P.-L. Li, X.-L. Tang, X. Qi and G.-Q. Li, Chem. Biodiversity, 2012, 9, 2218–2224 CAS.
- R. A. Keyzers, C. A. Gray, M. H. Schleyer, C. E. Whibley, D. T. Hendricks and M. T. Davies-Coleman, Tetrahedron, 2006, 62, 2200–2206 CrossRef CAS PubMed.
- H. Sorek, A. Rudi, Y. Benayahu, N. Ben-Califa, D. Neumann and Y. Kashman, J. Nat. Prod., 2007, 70, 1104–1109 CrossRef CAS PubMed.
- L.-G. Yao, H.-Y. Zhang, L.-F. Liang, X.-J. Guo, S.-C. Mao and Y.-W. Guo, Helv. Chim. Acta, 2012, 95, 235–239 CrossRef CAS.
- E. Tello, L. Castellanos, C. Arévalo-Ferro and C. Duque, J. Nat. Prod., 2012, 75, 1637–1642 CrossRef CAS PubMed.
- Y.-S. Lin, N.-L. Lee, M.-C. Lu and J.-H. Su, Molecules, 2012, 17, 5422–5429 CrossRef CAS PubMed.
- S.-K. Wang, M.-K. Hsieh and C.-Y. Duh, Mar. Drugs, 2012, 10, 1433–1444 CrossRef CAS PubMed.
- M. Qin, X. Li and B. Wang, Chin. J. Chem., 2012, 30, 1278–1282 CrossRef CAS.
- W.-H. Yen, L.-C. Hu, J.-H. Su, M.-C. Lu, W.-H. Twan, S.-Y. Yang, Y.-C. Kuo, C.-F. Weng, C.-H. Lee, Y.-H. Kuo and P.-J. Sung, Molecules, 2012, 17, 14058–14066 CrossRef CAS PubMed.
- B. Yang, X. Zhou, H. Huang, X.-W. Yang, J. Liu, X. Lin, X. Li, Y. Peng and Y. Liu, Mar. Drugs, 2012, 10, 2023–2032 CrossRef CAS PubMed.
- R. Kazlauskas, P. T. Murphy, R. J. Wells, P. Schönholzer and J. C. Coll, Aust. J. Chem., 1978, 31, 1817–1824 CrossRef CAS.
- H.-J. Shih, Y.-J. Tseng, C.-Y. Huang, Z.-H. Wen, C.-F. Dai and J.-H. Sheu, Tetrahedron, 2012, 68, 244–249 CrossRef CAS PubMed.
- N. P. Thao, N. H. Nam, N. X. Cuong, T. H. Quang, P. T. Tung, B. H. Tai, B. T. T. Luyen, D. Chae, S. Kim, Y.-S. Koh, P. V. Kiem, C. V. Minh and Y. H. Kim, Chem. Pharm. Bull., 2012, 60, 1581–1589 Search PubMed.
- M.-E. F. Hegazy, A. M. G. Eldeen, A. A. Shahat, F. F. Abdel-Latif, T. A. Mohamed, B. R. Whittlesey and P. W. Pare, Mar. Drugs, 2012, 10, 209–222 CrossRef CAS PubMed.
- J.-y. Su, S.-j. Yan and L.-m. Zeng, Chem. J. Chin. Univ., 2001, 22, 1515–1517 CAS.
- D. Czarkie, S. Carmely, A. Groweiss and Y. Kashman, Tetrahedron, 1985, 41, 1049–1056 CrossRef CAS.
- S.-K. Wang and C.-Y. Duh, Mar. Drugs, 2012, 10, 306–318 CrossRef CAS PubMed.
- W.-Y. Lin, Y. Lu, B.-W. Chen, C.-Y. Huang, J.-H. Su, Z.-H. Wen, C.-F. Dai, Y.-H. Kuo and J.-H. Sheu, Mar. Drugs, 2012, 10, 617–626 CrossRef CAS PubMed.
- S.-P. Chen, B.-W. Chen, C.-F. Dai, P.-J. Sung, Y.-C. Wu and J.-H. Sheu, Bull. Chem. Soc. Jpn., 2012, 85, 920–922 CrossRef CAS.
- M. Kobayashi and T. Hirase, Chem. Pharm. Bull., 1990, 38, 2442–2445 CrossRef CAS.
- S.-T. Lin, S.-K. Wang and C.-Y. Duh, Mar. Drugs, 2011, 9, 2705–2716 CrossRef CAS PubMed.
- S. Shen, H. Zhu, D. Chen, D. Liu, L. van Ofwegen, P. Proksch and W. Lin, Tetrahedron Lett., 2012, 53, 5759–5762 CrossRef CAS PubMed.
- J.-Y. Chang, A. E. Fazary, Y.-C. Lin, T.-L. Hwang and Y.-C. Shen, Chem. Biodiversity, 2012, 9, 654–661 CAS.
- D. Chen, D. Liu, S. Shen, W. Cheng and W. Lin, Chin. J. Chem., 2012, 30, 1459–1463 CrossRef CAS.
- A. D. Wright, J. L. Nielson, D. M. Tapiolas, C. H. Liptrot and C. A. Motti, Mar. Drugs, 2012, 10, 1619–1630 CrossRef CAS PubMed.
- D. H. Marchbank and R. G. Kerr, Tetrahedron, 2011, 67, 3053–3061 CrossRef CAS PubMed.
- D. H. Marchbank, F. Berrue and R. G. Kerr, J. Nat. Prod., 2012, 75, 1289–1293 CrossRef CAS PubMed.
- S. V. S. Govindam, Y. Yoshioka, A. Kanamoto, T. Fujiwara, T. Okamoto and M. Ojika, Bioorg. Med. Chem., 2012, 20, 687–692 CrossRef CAS PubMed.
- R. W. Dunlop and R. J. Wells, Aust. J. Chem., 1979, 32, 1345–1351 CrossRef CAS.
- X. Wei, K. Nieves and A. D. Rodríguez, Pure Appl. Chem., 2012, 84, 1847–1855 CrossRef CAS.
- S. A. Look, W. Fenical, Z. Qi-tai and J. Clardy, J. Org. Chem., 1984, 49, 1417–1423 CrossRef CAS.
- H.-J. Su, N.-L. Lee, M.-C. Lu and J.-H. Su, Nat. Prod. Commun., 2012, 7, 479–480 CAS.
- C.-H. Cheng, Y.-S. Lin, Z.-H. Wen and J.-H. Su, Molecules, 2012, 17, 10072–10078 CrossRef CAS PubMed.
- P. K. Roy, W. Maarisit, S. R. Roy, J. Taira and K. Ueda, Heterocycles, 2012, 85, 2465–2472 CrossRef CAS PubMed.
- P. K. Roy, W. Maarisit, M. C. Roy, J. Taira and K. Ueda, Mar. Drugs, 2012, 10, 2741–2748 CrossRef CAS PubMed.
- D. Lai, D. Liu, Z. Deng, L. van Ofwegen, P. Proksch and W. Lin, J. Nat. Prod., 2012, 75, 1595–1602 CrossRef CAS PubMed.
- S.-L. Wu, J.-H. Su, C.-Y. Huang, C.-J. Tai, P.-J. Sung, C.-C. Liaw and J.-H. Sheu, Mar. Drugs, 2012, 10, 1203–1211 CrossRef CAS PubMed.
- B.-W. Chen, Y.-C. Wu, M. Y. Chiang, J.-H. Su, W.-H. Wang, T.-Y. Fan and J.-H. Sheu, Tetrahedron, 2009, 65, 7016–7022 CrossRef CAS PubMed.
- Y.-H. Chen, T.-L. Hwang, Y.-D. Su, Y.-C. Chang, Y.-H. Chen, P.-H. Hong, L.-C. Hu, W.-H. Yen, H.-Y. Hsu, S.-J. Huang, Y.-H. Kuo and P.-J. Sung, Chem. Pharm. Bull., 2012, 60, 160–163 CrossRef CAS.
- Y.-H. Chen, C.-Y. Tai, T.-L. Hwang and P.-J. Sung, Nat. Prod. Commun., 2012, 7, 481–484 CAS.
- J. F. Gómez-Reyes, A. Salazar, H. M. Guzmán, Y. González, P. L. Fernández, A. Ariza-Castolo and M. Gutiérrez, Mar. Drugs, 2012, 10, 2608–2617 CrossRef PubMed.
- S.-T. Ishigami, Y. Goto, N. Inoue, S.-I. Kawazu, Y. Matsumoto, Y. Imahara, M. Tarumi, H. Nakai, N. Fusetani and Y. Nakao, J. Org. Chem., 2012, 77, 10962–10966 CrossRef CAS PubMed.
- H.-M. Chung, L.-C. Hu, W.-H. Yen, J.-H. Su, M.-C. Lu, T.-L. Hwang, W.-H. Wang and P.-J. Sung, Mar. Drugs, 2012, 10, 2246–2253 CrossRef CAS PubMed.
- C.-H. Cheng, H.-M. Chung, T.-L. Hwang, M.-C. Lu, Z.-H. Wen, Y.-H. Kuo, W.-H. Wang and P.-J. Sung, Molecules, 2012, 17, 9443–9450 CrossRef CAS PubMed.
- H.-M. Chung, P.-H. Hong, J.-H. Su, T.-L. Hwang, M.-C. Lu, L.-S. Fang, Y.-C. Wu, J.-J. Li, J.-J. Chen, W.-H. Wang and P.-J. Sung, Mar. Drugs, 2012, 10, 1169–1179 CrossRef CAS PubMed.
- K. Ota, N. Okamoto, H. Mitome and H. Miyaoka, Heterocycles, 2012, 85, 135–145 CrossRef CAS.
- P.-H. Hong, Y.-D. Su, N.-C. Lin, Y.-H. Chen, Y.-H. Kuo, T.-L. Hwang, W.-H. Wang, J.-J. Chen, J.-H. Sheu and P.-J. Sung, Tetrahedron Lett., 2012, 53, 1710–1712 CrossRef CAS PubMed.
- P.-H. Hong, Y.-D. Su, J.-H. Su, Y.-H. Chen, T.-L. Hwang, C.-F. Weng, C.-H. Lee, Z.-H. Wen, J.-H. Sheu, N.-C. Lin, Y.-H. Kuo and P.-J. Sung, Mar. Drugs, 2012, 10, 1156–1168 CrossRef CAS PubMed.
- Y.-D. Su, T.-L. Hwang, N.-C. Lin, Y.-H. Chen, Y.-C. Wu, J.-H. Sheu and P.-J. Sung, Bull. Chem. Soc. Jpn., 2012, 85, 1031–1036 CrossRef CAS.
- T.-T. Yeh, S.-K. Wang, C.-F. Dai and C.-Y. Duh, Mar. Drugs, 2012, 10, 1019–1026 CrossRef CAS PubMed.
- S.-K. Wang, T.-T. Yeh and C.-Y. Duh, Mar. Drugs, 2012, 10, 2103–2110 CrossRef CAS PubMed.
- C. Li, M.-P. La, H. Tang, W.-H. Pan, P. Sun, K. Krohn, Y.-H. Yi, L. Li and W. Zhang, Bioorg. Med. Chem. Lett., 2012, 22, 4368–4372 CrossRef CAS PubMed.
- J.-Y. Chang, C.-C. Liaw, A. E. Fazary, T.-L. Hwang and Y.-C. Shen, Mar. Drugs, 2012, 10, 1321–1330 CrossRef CAS PubMed.
- S.-H. Qi, S. Zhang, P.-Y. Qian, Z.-H. Xiao and M.-Y. Li, Tetrahedron, 2006, 62, 9123–9130 CrossRef CAS PubMed.
- R. J. Meginley, P. Gupta, T. C. Schulz, A. B. McLean, A. J. Robins and L. M. West, Mar. Drugs, 2012, 10, 1662–1670 CrossRef CAS PubMed.
- B. S. Mootoo, R. Ramsewak, R. Sharma, W. F. Tinto, A. J. Lough, S. McLean, W. F. Reynolds, J.-P. Yang and M. Yu, Tetrahedron, 1996, 52, 9953–9962 CrossRef CAS.
- C.-H. Chao, K.-J. Chou, C.-Y. Huang, Z.-H. Wen, C.-H. Hsu, Y.-C. Wu, C.-F. Dai and J.-H. Sheu, Mar. Drugs, 2012, 10, 439–450 CrossRef CAS PubMed.
- X.-Y. Chai, J.-F. Sun, L.-Y. Tang, X.-W. Yang, Y.-Q. Li, H. Huang, X.-F. Zhou, B. Yang and Y. Liu, Chem. Pharm. Bull., 2010, 58, 1391–1394 CrossRef CAS.
- P. Sun, Y. Xu, T. Liu, H. Tang, Y. Yi, K. Krohn, L. Li and W. Zhang, Helv. Chim. Acta, 2012, 95, 522–527 CrossRef CAS.
- X.-J. Liao, L.-D. Tang, Y.-W. Liang, S.-L. Jian, S.-H. Xu and Y.-H. Liu, Chem. Biodiversity, 2012, 9, 370–375 CAS.
- K. Shin, J. Chin, D. Hahn, J. Lee, H. Hwang, D. H. Won, J. Ham, H. Choi, E. Kang, H. Kim, M. K. Ju, S.-J. Nam and H. Kang, Steroids, 2012, 77, 355–359 CrossRef CAS PubMed.
- D. Lai, S. Yu, L. van Ofwegen, F. Totzke, P. Proksch and W. Lin, Bioorg. Med. Chem., 2011, 19, 6873–6880 CrossRef CAS PubMed.
- M. Y. Putra, G. Bavestrello, C. Cerrano, B. Renga, C. D'Amore, S. Fiorucci, E. Fattorusso and O. Taglialatela-Scafati, Steroids, 2012, 77, 433–440 CrossRef CAS PubMed.
- S.-K. Wang, S.-Y. Puu and C.-Y. Duh, Mar. Drugs, 2012, 10, 1288–1296 CrossRef CAS PubMed.
- R. Li, C.-L. Shao, X. Qi, X.-B. Li, J. Li, L.-L. Sun and C.-Y. Wang, Mar. Drugs, 2012, 10, 1422–1432 CrossRef CAS PubMed.
- J.-H. Su, Y.-J. Tseng, H.-H. Huang, A. F. Ahmed, C.-K. Lu, Y.-C. Wu and J.-H. Sheu, J. Nat. Prod., 2006, 69, 850–852 CrossRef CAS PubMed.
- C.-Y. Huang, J.-H. Su, C.-Y. Duh, B.-W. Chen, Z.-H. Wen, Y.-H. Kuo and J.-H. Sheu, Bioorg. Med. Chem. Lett., 2012, 22, 4373–4376 CrossRef CAS PubMed.
- P. Sun, L.-Y. Meng, H. Tang, B.-S. Liu, L. Li, Y. Yi and W. Zhang, J. Nat. Prod., 2012, 75, 1656–1659 CrossRef CAS PubMed.
- H.-Y. Fang, C.-C. Liaw, C.-H. Chao, Z.-H. Wen, Y.-C. Wu, C.-H. Hsu, C.-F. Dai and J.-H. Sheu, Tetrahedron, 2012, 68, 9694–9700 CrossRef CAS PubMed.
- K. O. Hanssen, B. Schuler, A. J. Williams, T. B. Demissie, E. Hansen, J. H. Anderson, J. Svenson, K. Blinov, M. Repisky, F. Mohn, G. Meyer, J.-S. Svendsen, K. Ruud, M. Elyashberg, L. Gross, M. Jaspars and J. Isaksson, Angew. Chem., Int. Ed., 2012, 51, 12238–12241 CrossRef CAS PubMed.
- T. Řezanka and V. M. Dembitsky, Tetrahedron, 2001, 57, 8743–8749 CrossRef.
- B. M. Trost and A. Quintard, Org. Lett., 2012, 14, 4698–4700 CrossRef CAS PubMed.
- J.-H. Su, Y. Lu, W.-Y. Lin, W.-H. Wang, P.-J. Sung and J.-H. Sheu, Chem. Lett., 2010, 39, 172–173 CrossRef CAS.
- A. Groweiss, Y. Kashman, D. J. Vanderah, B. Tursch, P. Cornet, J. C. Braekman and D. Daloze, Bull. Soc. Chim. Belg., 1978, 87, 277–283 CrossRef CAS.
- J.-H. Su, Y. Lu, W.-Y. Lin, W.-H. Wang, P.-J. Sung and J.-H. Sheu, Chem. Lett., 2012, 41, 340–340 CrossRef CAS.
- H.-M. Chung, Y.-H. Chen, M.-R. Lin, J.-H. Su, W.-H. Wang and P.-J. Sung, Tetrahedron Lett., 2010, 51, 6025–6027 CrossRef CAS PubMed.
- H.-M. Chung, Y.-H. Chen, T.-L. Hwang, L.-F. Chuang, W.-H. Wang and P.-J. Sung, Tetrahedron Lett., 2010, 51, 2734–2736 CrossRef CAS PubMed.
- T. Hirokawa, T. Nagasawa and S. Kuwahara, Tetrahedron Lett., 2012, 53, 705–706 CrossRef CAS PubMed.
- T. Hirokawa and S. Kuwahara, Tetrahedron, 2012, 68, 4581–4587 CrossRef CAS PubMed.
- G. G. de Souza, T. S. Oliveira, J. A. Takahashi, I. G. Collado, A. J. Macias-Sanchez and R. Hernandez-Galan, Org. Biomol. Chem., 2012, 10, 3315–3320 Search PubMed.
- A. J. Weinheimer, J. A. Matson, M. B. Hossain and D. van der Helm, Tetrahedron Lett., 1977, 2923–2926 CrossRef CAS.
- S.-Y. Huang, N.-F. Chen, W.-F. Chen, H.-C. Hung, H.-P. Lee, Y.-Y. Lin, H.-M. Wang, P.-J. Sung, J.-H. Sheu and Z.-H. Wen, Mar. Drugs, 2012, 10, 1899–1919 CrossRef CAS PubMed.
- M. Herin and B. Tursch, Bull. Soc. Chim. Belg., 1976, 85, 707–719 CrossRef CAS.
- T.-R. Su, F.-J. Tsai, J.-J. Lin, H. H. Huang, C.-C. Chiu, J.-H. Su, Y.-T. Yang, J. Y.-F. Chen, B.-S. Wong and Y.-J. Wu, Mar. Drugs, 2012, 10, 1883–1898 CrossRef CAS PubMed.
- W.-F. Chen, C. Chakraborty, C.-S. Sung, C.-W. Feng, Y.-H. Jean, Y.-Y. Lin, H.-C. Hung, T.-Y. Huang, S.-Y. Huang, T.-M. Su, P.-J. Sung, J.-H. Sheu and Z.-H. Wen, Naunyn-Schmiedebergs Arch. Pharmacol., 2012, 385, 265–275 CrossRef CAS PubMed.
- B. Tursch, J. C. Braekman, D. Daloze, M. Herin, R. Karlsson and D. Losman, Tetrahedron, 1975, 31, 129–133 CrossRef CAS.
- C.-A. Neoh, R. Y.-L. Wang, Z.-H. Din, J.-H. Su, Y.-K. Chen, F.-J. Tsai, S.-H. Weng and Y.-J. Wu, Mar. Drugs, 2012, 10, 2893–2911 CrossRef PubMed.
- Y. Uchio, S. Eguchi, M. Nakayama and T. Hase, Chem. Lett., 1982, 3, 277–278 CrossRef.
- J.-Y. Hong, H.-J. Boo, J.-I. Kang, M.-K. Kim, E.-S. Yoo, J.-W. Hyun, Y.-S. Koh, G. Y. Kim, Y. H. Maeng, C. L. Hyun, W. Y. Chang, Y. H. Kim, Y. R. Kim and H.-K. Kang, Biol. Pharm. Bull., 2012, 35, 1054–1063 CAS.
- P. T. Szymanski, B. Kuppast, S. A. Ahmed, S. Khalifa and H. Fahmy, Mar. Drugs, 2012, 10, 1–19 CrossRef CAS PubMed.
- W. F. Tinto, R. S. Laydoo, S. L. Miller, W. F. Reynolds and S. McLean, J. Nat. Prod., 1995, 58, 1975–1977 CrossRef CAS.
- A. D. Rodríguez and J. J. Soto, Chem. Pharm. Bull., 1996, 44, 91–94 CrossRef.
- A. S. Kate, K. Richard, B. Ramanathan and R. G. Kerr, Can. J. Chem., 2010, 88, 318–322 CrossRef CAS.
- H. Weinstabl, T. Gaich and J. Mulzer, Org. Lett., 2012, 14, 2834–2837 CrossRef CAS PubMed.
- R. Jia, Y.-W. Guo, P. Chen, Y.-M. Yang, E. Mollo, M. Gavagnin and G. Cimino, J. Nat. Prod., 2007, 70, 1158–1166 CrossRef CAS PubMed.
- Y. Li, A.-H. Gao, H. Huang, J. Li, E. Mollo, M. Gavagnin, G. Cimino, Y.-C. Gu and Y.-W. Guo, Helv. Chim. Acta, 2009, 92, 1341–1348 CrossRef CAS.
- A. Bishara, A. Rudi, Y. Benayahu and Y. Kashman, J. Nat. Prod., 2007, 70, 1951–1954 CrossRef CAS PubMed.
- A. C. Coleman and R. G. Kerr, Tetrahedron, 2000, 56, 9569–9574 CrossRef CAS.
- M. Nasuda, M. Ohmori, K. Ohyama and Y. Fujimoto, Chem. Pharm. Bull., 2012, 60, 681–685 CAS.
- H. Correa, P. Zorro, C. Arevalo-Ferro, M. Puyana and C. Duque, J. Chem. Ecol., 2012, 38, 1190–1202 CrossRef CAS PubMed.
- A. D. Rodríguez, C. Ramirez, I. I. Rodríguez and E. González, Org. Lett., 1999, 1, 527–530 CrossRef.
- I. I. Rodríguez and A. D. Rodríguez, J. Nat. Prod., 2003, 66, 855–857 CrossRef PubMed.
- M. W. B. McCulloch, B. Haltli, D. H. Marchbank and R. G. Kerr, Mar. Drugs, 2012, 10, 1711–1728 CrossRef CAS PubMed.
- M. J. Martín, R. Fernández, A. Francesch, P. Amade, S. S. de Matos-Pita, F. Reyes and C. Cuevas, Org. Lett., 2010, 12, 912–914 CrossRef PubMed.
- V. Sio, J. G. Harrison and D. J. Tantillo, Tetrahedron Lett., 2012, 53, 6919–6922 CrossRef CAS PubMed.
- J. Li, Z. Zhang, Z. Xia, C. Ni and Y. Wu, Acta Chim. Sinica, 1987, 45, 558–561 CAS.
- Z.-Y. Yang, H.-Z. Liao, K. Sheng, Y.-F. Chen and Z.-J. Yao, Angew. Chem., Int. Ed., 2012, 51, 6484–6487 CrossRef CAS PubMed.
- C. Li, M.-P. La, L. Li, X.-B. Li, H. Tang, B.-S. Liu, K. Krohn, P. Sun, Y.-H. Yi and W. Zhang, J. Nat. Prod., 2011, 74, 1658–1662 CrossRef CAS PubMed.
- C. Li, M.-P. La, L. Li, X.-B. Li, H. Tang, B.-S. Liu, K. Krohn, P. Sun, Y.-H. Yi and W. Zhang, J. Nat. Prod., 2012, 75, 2047–2047 CrossRef CAS PubMed.
- Y.-C. Chang, T.-L. Hwang, S.-K. Huang, L.-W. Huang, M.-R. Lin and P.-J. Sung, Heterocycles, 2010, 81, 991–996 CrossRef CAS.
- X. Huang, Z. Deng, X. Zhu, L. van Ofwegen, P. Proksch and W. Lin, Helv. Chim. Acta, 2006, 89, 2020–2026 CrossRef CAS.
- L.-Q. Shen, S.-Y. Huang, Y. Tang and F.-H. Lei, Steroids, 2012, 77, 1398–1402 CrossRef CAS PubMed.
- W. Wang, J.-S. Lee, T. Nakazawa, K. Ukai, R. E. P. Mangindaan, D. S. Wewengkang, H. Rotinsulu, H. Kobayashi, S. Tsukamoto and M. Namikoshi, Steroids, 2009, 74, 758–760 CrossRef CAS PubMed.
- G. G. Mellado, E. Zubía, M. J. Ortega and P. J. López-González, J. Nat. Prod., 2005, 68, 1111–1115 CrossRef CAS PubMed.
- R. Saini, S. Boland, O. Kataeva, A. W. Schmidt, T. V. Kurzchalia and H.-J. Knolker, Org. Biomol. Chem., 2012, 10, 4159–4163 CAS.
- J. S. Oliveira, D. Fuentes-Silva and G. F. King, Toxicon, 2012, 60, 539–550 CrossRef CAS PubMed.
- I. Urbarova, B. O. Karlsen, S. Okkenhaug, O. M. Seternes, S. D. Johansen and A. Emblem, Mar. Drugs, 2012, 10, 2265–2279 CrossRef CAS PubMed.
- N. Cachet, G. Genta-Jouve, E. L. Regalado, R. Mokrini, P. Amade, G. Culioli and O. P. Thomas, J. Nat. Prod., 2009, 72, 1612–1615 CrossRef CAS PubMed.
- E. Manzo, D. Pagano, G. Nuzzo, M. Gavagnin and M. L. Ciavatta, Tetrahedron Lett., 2012, 53, 7083–7084 CrossRef CAS PubMed.
- A. R. Carroll, S. J. Wild, S. Duffy and V. M. Avery, Tetrahedron Lett., 2012, 53, 2873–2875 CrossRef CAS PubMed.
- A. J. Blackman and R. D. Green, Aust. J. Chem., 1987, 40, 1655–1662 CrossRef CAS.
- A. R. Carroll, S. Duffy, M. Sykes and V. M. Avery, Org. Biomol. Chem., 2011, 9, 604–609 CAS.
- F. A. Khan and S. Ahmad, J. Org. Chem., 2012, 77, 2389–2397 CrossRef CAS PubMed.
- C. K. Narkowicz, A. J. Blackman, E. Lacey, J. H. Gill and K. Heiland, J. Nat. Prod., 2002, 65, 938–941 CrossRef CAS PubMed.
- M. Watanabe, H. Fuda, S. Jin, T. Sakurai, F. Ohkawa, S.-P. Hui, S. Takeda, T. Watanabe, T. Koike and H. Chiba, J. Agric. Food Chem., 2012, 60, 830–835 CrossRef CAS PubMed.
- X.-L. Xu, X. Fan, F.-H. Song and J.-G. Shi, Haiyang Kexue, 2004, 28, 40–43 CAS.
- L. Bigot, C. Zatylny-Gaudin, F. Rodet, B. Bernay, P. Boudry and P. Favrel, Peptides, 2012, 34, 303–310 CrossRef CAS PubMed.
- P. McCarron, J. Kilcoyne, C. O. Miles and P. Hess, J. Agric. Food Chem., 2009, 57, 160–169 CrossRef CAS PubMed.
- J. Kilcoyne, A. Keogh, G. Clancy, P. LeBlanc, I. Burton, M. A. Quilliam, P. Hess and C. O. Miles, J. Agric. Food Chem., 2012, 60, 2447–2455 CrossRef CAS PubMed.
- P. McCarron, W. A. Rourke, W. Hardstaff, B. Pooley and M. A. Quilliam, J. Agric. Food Chem., 2012, 60, 1437–1446 CrossRef CAS PubMed.
- M. Silva, J. Azevedo, P. Rodriguez, A. Alfonso, L. M. Botana and V. Vasconcelos, Mar. Drugs, 2012, 10, 712–726 CrossRef CAS PubMed.
- A. Franco, S. N. Kompella, K. B. Akondi, C. Melaun, N. L. Daly, C. W. Luetje, P. F. Alewood, D. J. Craik, D. J. Adams and F. Mari, Biochem. Pharmacol., 2012, 83, 419–426 CrossRef CAS PubMed.
- A. Van Der Haegen, S. Peigneur, N. Dyubankova, C. Möller, F. Marí, E. Diego-García, R. Naudé, E. Lescrinier, P. Herdewijn and J. Tytgat, Peptides, 2012, 34, 106–113 CrossRef CAS PubMed.
- M. Ye, K. K. Khoo, S. Xu, M. Zhou, N. Boonyalai, M. A. Perugini, X. Shao, C. Chi, C. A. Galea, C. Wang and R. S. Norton, J. Biol. Chem., 2012, 287, 14973–14983 CrossRef CAS PubMed.
- P. Favreau, E. Benoit, H. G. Hocking, L. Carlier, D. D'hoedt, E. Leipold, R. Markgraf, S. Schlumberger, M. A. Córdova, H. Gaertner, M. Paolini-Bertrand, O. Hartley, J. Tytgat, S. H. Heinemann, D. Bertrand, R. Boelens, R. Stöcklin and J. Molgó, Br. J. Pharmacol., 2012, 166, 1654–1668 CrossRef CAS PubMed.
- I. Vetter, Z. Dekan, O. Knapp, D. J. Adams, P. F. Alewood and R. J. Lewis, Biochem. Pharmacol., 2012, 84, 540–548 CrossRef CAS PubMed.
- A. Violette, D. Biass, S. Dutertre, D. Koua, D. Piquemal, F. Pierrat, R. Stocklin and P. Favreau, J. Proteomics, 2012, 75, 5215–5225 CrossRef CAS PubMed.
- H. Safavi-Hemami, D. G. Gorasia, A. M. Steiner, N. A. Williamson, J. A. Karas, J. Gajewiak, B. M. Olivera, G. Bulaj and A. W. Purcell, J. Biol. Chem., 2012, 287, 34288–34303 CrossRef CAS PubMed.
- C. L. Bromley, W. L. Popplewell, S. C. Pinchuck, A. N. Hodgson and M. T. Davies-Coleman, J. Nat. Prod., 2012, 75, 497–501 CrossRef CAS PubMed.
- A. San-Martín, J. Rovirosa, M. Bacho, K. Gaete and J. Ampuero, Bol. Latinoam. Caribe Plant. Med. Aromat., 2012, 11, 520–525 Search PubMed.
- J. T. Blanchfield, D. J. Brecknell, I. M. Brereton, M. J. Garson and D. D. Jones, Aust. J. Chem., 1994, 47, 2255–2269 CrossRef CAS.
- F. Becerril-Jimenez and D. E. Ward, Org. Lett., 2012, 14, 1648–1651 CrossRef CAS PubMed.
- M. Carbone, M. Gavagnin, C. A. Mattia, C. Lotti, F. Castelluccio, B. Pagano, E. Mollo, Y.-W. Guo and G. Cimino, Tetrahedron, 2009, 65, 4404–4409 CrossRef CAS PubMed.
- J.-R. Wang, M. Carbone, M. Gavagnin, A. Mandi, S. Antus, L.-G. Yao, G. Cimino, T. Kurtan and Y.-W. Guo, Eur. J. Org. Chem., 2012, 1107–1111 CrossRef CAS.
- M. L. Ciavatta, M. Gavagnin, R. Puliti, G. Cimino, E. Martinez, J. Ortea and C. A. Mattia, Tetrahedron, 1996, 52, 12831–12838 CrossRef CAS.
- C. Jimenez-Romero, K. Gonzalez and A. D. Rodriguez, Tetrahedron Lett., 2012, 53, 6641–6645 CrossRef CAS PubMed.
- R. H. Currie and J. M. Goodman, Angew. Chem., Int. Ed., 2012, 51, 4695–4697 CrossRef CAS PubMed.
- M. Tori, K. Nakashima and Y. Asakawa, Phytochemistry, 1995, 38, 651–653 CrossRef CAS.
- A. R. Diaz-Marrero, J. M. de la Rosa, I. Brito, J. Darias and M. Cueto, J. Nat. Prod., 2012, 75, 115–118 CrossRef CAS PubMed.
- M. J. Somerville, P. L. Katavic, L. K. Lambert, G. K. Pierens, J. T. Blanchfield, G. Cimino, E. Mollo, M. Gavagnin, M. G. Banwell and M. J. Garson, J. Nat. Prod., 2012, 75, 1618–1624 CrossRef CAS PubMed.
- M. Ojika, H. Kigoshi, K. Suenaga, Y. Imamura, K. Yoshikawa, T. Ishigaki, A. Sakakura, T. Mutou and K. Yamada, Tetrahedron, 2012, 68, 982–987 CrossRef CAS PubMed.
- K. Yamada, M. Ojika, T. Ishigaki, Y. Yoshida, H. Ekimoto and M. Arakawa, J. Am. Chem. Soc., 1993, 115, 11020–11021 CrossRef CAS.
- M. Kita, Y. Hirayama, K. Yamagishi, K. Yoneda, R. Fujisawa and H. Kigoshi, J. Am. Chem. Soc., 2012, 134, 20314–20317 CrossRef CAS PubMed.
- N. Fusetani, K. Yasumuro, S. Matsunaga and K. Hashimoto, Tetrahedron Lett., 1989, 30, 2809–2812 CrossRef CAS.
- K. Kobayashi, Y. Fujii, Y. Hirayama, S. Kobayashi, I. Hayakawa and H. Kigoshi, Org. Lett., 2012, 14, 1290–1293 CrossRef CAS PubMed.
- R. R. Vardaro, V. Di Marzo, A. Marin and G. Cimino, Tetrahedron, 1992, 48, 9561–9566 CrossRef CAS.
- A. Cutignano, G. Villani and A. Fontana, Org. Lett., 2012, 14, 992–995 CrossRef CAS PubMed.
- M. S. C. Pedras and P. B. Chumala, Phytochemistry, 2005, 66, 81–87 CrossRef CAS PubMed.
- Y. Takada, M. Umehara, Y. Nakao and J. Kimura, Tetrahedron Lett., 2008, 49, 1163–1165 CrossRef CAS PubMed.
- Y. Nakao, W. Y. Yoshida, Y. Takada, J. Kimura, L. Yang, S. L. Mooberry and P. J. Scheuer, J. Nat. Prod., 2004, 67, 1332–1340 CrossRef CAS PubMed.
- Y. Takada, M. Umehara, R. Katsumata, Y. Nakao and J. Kimura, Tetrahedron, 2012, 68, 659–669 CrossRef CAS PubMed.
- M. Umehara, T. Negishi, T. Tashiro, Y. Nakao and J. Kimura, Bioorg. Med. Chem. Lett., 2012, 22, 7422–7425 CrossRef CAS PubMed.
- M. L. Ciavatta, E. Manzo, G. Nuzzo, G. Villani, G. Cimino, J. L. Cervera, M. A. E. Malaquias and M. Gavagnin, Tetrahedron Lett., 2009, 50, 527–529 CrossRef CAS PubMed.
- O. Geiseler and J. Podlech, Tetrahedron, 2012, 68, 7280–7287 CrossRef CAS PubMed.
- M. Carbone, Y. Li, C. Irace, E. Mollo, F. Castelluccio, A. Di Pascale, G. Cimino, R. Santamaria, Y.-W. Guo and M. Gavagnin, Org. Lett., 2011, 13, 2516–2519 CrossRef CAS PubMed.
- H.-Y. Lin and B. B. Snider, J. Org. Chem., 2012, 77, 4832–4836 CrossRef CAS PubMed.
- E. Manzo, D. Pagano, M. Carbone, M. L. Ciavatta and M. Gavagnin, ARKIVOC, 2012, 220–228 CrossRef CAS.
- T. Vasskog, J. H. Andersen, E. Hansen and J. Svenson, Mar. Drugs, 2012, 10, 2676–2690 CrossRef CAS.
- G. Cimino, A. Spinella and G. Sodano, Tetrahedron Lett., 1989, 30, 5003–5004 CrossRef CAS.
- G. D. Sala, A. Cutignano, A. Fontana, A. Spinella, G. Calabrese, A. D. Coll, G. d'Ippolito, C. D. Monica and G. Cimino, Tetrahedron, 2007, 63, 7256–7263 CrossRef PubMed.
- A. Cutignano, C. Avila, A. Rosica, G. Romano, B. Laratta, A. Domenech-Coll, G. Cimino, E. Mollo and A. Fontana, ChemBioChem, 2012, 13, 1759–1766 CrossRef CAS PubMed.
- M. Gavagnin, E. Mollo, G. Cimino and J. Ortea, Tetrahedron Lett., 1996, 37, 4259–4262 CrossRef CAS.
- P. Sharma, N. Griffiths and J. E. Moses, Org. Lett., 2008, 10, 4025–4027 CrossRef CAS PubMed.
- K. J. Powell, P. Sharma, J. L. Richens, B. M. Davis, J. E. Moses and P. O'Shea, Phys. Chem. Chem. Phys., 2012, 14, 14489–14491 RSC.
- M. Ronci, D. Rudd, T. Guinan, K. Benkendorff and N. H. Voelcker, Anal. Chem., 2012, 84, 8996–9001 CAS.
- I. Surowiec, W. Nowik and T. Moritz, Dyes Pigm., 2012, 94, 363–369 CrossRef CAS PubMed.
- T. Diyabalanage, K. B. Iken, J. B. McClintock, C. D. Amsler and B. J. Baker, J. Nat. Prod., 2010, 73, 416–421 CrossRef CAS PubMed.
- J. A. Maschek, E. Mevers, T. Diyabalanage, L. Chen, Y. Ren, J. B. McClintock, C. D. Amsler, J. Wu and B. J. Baker, Tetrahedron, 2012, 68, 9095–9104 CrossRef CAS PubMed.
- P. Karuso and W. C. Taylor, Aust. J. Chem., 1986, 39, 1629–1641 CAS.
- G. Cimino, D. De Rosa, S. De Stefano and L. Minale, Tetrahedron, 1974, 30, 645–649 CrossRef CAS.
- P. L. Katavic, P. Jumaryatno, J. N. A. Hooper, J. T. Blanchfield and M. J. Garson, Aust. J. Chem., 2012, 65, 531–538 CrossRef CAS.
- G. Nuzzo, M. L. Ciavatta, R. Kiss, V. Mathieu, H. Leclercqz, E. Manzo, G. Villani, E. Mollo, F. Lefranc, L. D'Souza, M. Gavagnin and G. Cimino, Mar. Drugs, 2012, 10, 1799–1811 CrossRef CAS PubMed.
- B. Carte and D. J. Faulkner, J. Org. Chem., 1983, 48, 2314–2318 CrossRef CAS.
- N. Lindquist and W. Fenical, Experientia, 1991, 47, 504–506 CrossRef CAS.
- A. Franks, P. Haywood, C. Holmström, S. Egan, S. Kjelleberg and N. Kumar, Molecules, 2005, 10, 1286–1291 CrossRef CAS PubMed.
- P. I. Hernandez, D. Moreno, A. A. Javier, T. Torroba, R. Perez-Tomas and R. Quesada, Chem. Commun., 2012, 48, 1556–1558 RSC.
- D. S. Dalisay, E. W. Rogers, A. S. Edison and T. F. Molinski, J. Nat. Prod., 2009, 72, 732–738 CrossRef CAS PubMed.
- D. S. Nielsen, H. N. Hoang, R.-J. Lohman, F. Diness and D. P. Fairlie, Org. Lett., 2012, 14, 5720–5723 CrossRef CAS PubMed.
- E. K. Singh, D. M. Ramsey and S. R. McAlpine, Org. Lett., 2012, 14, 1198–1201 CrossRef CAS PubMed.
- A. Fontana, P. Cavaliere, S. Wahidulla, C. G. Naik and G. Cimino, Tetrahedron, 2000, 56, 7305–7308 CrossRef CAS.
- W. Liu, X. Liao, W. Dong, Z. Yan, N. Wang and Z. Liu, Tetrahedron, 2012, 68, 2759–2764 CrossRef CAS PubMed.
- C. Imperatore, A. Aiello, F. D'Aniello, P. Luciano, R. Vitalone, R. Meli, G. M. Raso and M. Menna, Molecules, 2012, 17, 12642–12650 CrossRef CAS PubMed.
- A. Aiello, E. Fattorusso, C. Imperatore, P. Luciano, M. Menna and R. Vitalone, Mar. Drugs, 2012, 10, 51–63 CrossRef CAS PubMed.
- S. Miao and R. J. Andersen, J. Org. Chem., 1991, 56, 6275–6280 CrossRef CAS.
- J. Sikorska, S. Parker-Nance, M. T. Davies-Coleman, O. B. Vining, A. E. Sikora and K. L. McPhail, J. Nat. Prod., 2012, 75, 1824–1827 CrossRef CAS PubMed.
- W. Wang, H. Kim, S.-J. Nam, B. J. Rho and H. Kang, J. Nat. Prod., 2012, 75, 2049–2054 CrossRef CAS PubMed.
- C. J. Smith, R. L. Hettich, J. Jompa, A. Tahir, M. V. Buchanan and C. M. Ireland, J. Org. Chem., 1998, 63, 4147–4150 CrossRef CAS.
- T. H. Won, J.-E. Jeon, S.-H. Kim, S.-H. Lee, B. J. Rho, D.-C. Oh, K.-B. Oh and J. Shin, J. Nat. Prod., 2012, 75, 2055–2061 CrossRef CAS PubMed.
- T. H. Won, J. Jeon, S.-H. Lee, B. J. Rho, K.-B. Oh and J. Shin, Bioorg. Med. Chem., 2012, 20, 4082–4087 CrossRef CAS PubMed.
- N. P. Tale, A. V. Shelke, G. B. Tiwari, P. B. Thorat and N. N. Karade, Helv. Chim. Acta, 2012, 95, 852–857 CrossRef CAS.
- J. Sikorska, A. M. Hau, C. Anklin, S. Parker-Nance, M. T. Davies-Coleman, J. E. Ishmael and K. L. McPhail, J. Org. Chem., 2012, 77, 6066–6075 CrossRef CAS PubMed.
- J. Sikorska, A. M. Hau, C. Anklin, S. Parker-Nance, M. T. Davies-Coleman, J. E. Ishmael and K. L. McPhail, J. Org. Chem., 2013, 78, 2812–2812 CrossRef CAS.
- K. L. McPhail, personal communication.
- M. Carbone, L. Núñez-Pons, M. Paone, F. Castelluccio, C. Avila and M. Gavagnin, Tetrahedron, 2012, 68, 3541–3544 CrossRef CAS PubMed.
- M. Carbone, L. Núñez-Pons, M. Paone, F. Castelluccio, C. Avila and M. Gavagnin, Tetrahedron, 2012, 68, 8515–8515 CrossRef CAS PubMed.
- D. R. Appleton, C. S. Chuen, M. V. Berridge, V. L. Webb and B. R. Copp, J. Org. Chem., 2009, 74, 9195–9198 CrossRef CAS PubMed.
- L. Núñez-Pons, M. Carbone, J. Vázquez, J. Rodríguez, R. M. Nieto, M. M. Varela, M. Gavagnin and C. Avila, Mar. Drugs, 2012, 10, 1741–1764 CrossRef PubMed.
- A. R. Carroll, B. D. Nash, S. Duffy and V. M. Avery, J. Nat. Prod., 2012, 75, 1206–1209 CrossRef CAS PubMed.
- A. R. Carroll and V. M. Avery, J. Nat. Prod., 2009, 72, 696–699 CrossRef CAS PubMed.
- H. Yamazaki, D. S. Wewengkang, T. Nishikawa, H. Rotinsulu, R. E. P. Mangindaan and M. Namikoshi, Mar. Drugs, 2012, 10, 349–357 CrossRef CAS PubMed.
- J. L. Li, S. C. Han, E. S. Yoo, S. Shin, J. Hong, Z. Cui, H. Li and J. H. Jung, J. Nat. Prod., 2011, 74, 1792–1797 CrossRef CAS PubMed.
- J. L. Li, B. Xiao, M. Park, E. S. Yoo, S. Shin, J. Hong, H. Y. Chung, H. S. Kim and J. H. Jung, J. Nat. Prod., 2012, 75, 2082–2087 CrossRef CAS PubMed.
- J. L. Li, B. Xiao, M. Park, E. S. Yoo, S. Shin, J. Hong, H. Y. Chung, H. S. Kim and J. H. Jung, J. Nat. Prod., 2013, 76, 815–815 CrossRef CAS.
- Z. Lu, M. K. Harper, C. D. Pond, L. R. Barrows, C. M. Ireland and R. M. van Wagoner, J. Nat. Prod., 2012, 75, 1436–1440 CrossRef CAS PubMed.
- M. S. Donia, W. F. Fricke, J. Ravel and E. W. Schmidt, PLoS One, 2011, 6(3), e17897 CAS.
- M. D. B. Tianero, M. S. Donia, T. S. Young, P. G. Schultz and E. W. Schmidt, J. Am. Chem. Soc., 2012, 134, 418–425 CrossRef CAS PubMed.
- F. Plisson, X.-C. Huang, H. Zhang, Z. Khalil and R. J. Capon, Chem.–Asian J., 2012, 7, 1616–1623 CrossRef CAS PubMed.
- F. Plisson, M. Conte, Z. Khalil, X.-C. Huang, A. M. Piggott and R. J. Capon, ChemMedChem, 2012, 7, 983–990 CrossRef CAS PubMed.
- P. C. Jimenez, D. V. Wilke, E. G. Ferreira, R. Takeara, M. O. de Moraes, E. R. Silveira, T. M. C. Lotufo, N. P. Lopes and L. V. Costa-Lotufo, Mar. Drugs, 2012, 10, 1092–1102 CrossRef CAS PubMed.
- B. Baker, T. Diyabalanage, J. B. McClintock and C. D. Amsler, WO 200703573 A2, 2007.
- G. J. Florence and J. Wlochal, Chem.–Eur. J., 2012, 18, 14250–14254 CrossRef CAS PubMed.
- L. Garrido, E. Zubía, M. J. Ortega and J. Salvá, J. Org. Chem., 2003, 68, 293–299 CrossRef CAS PubMed.
- M. Matveenko, G. Liang, E. M. W. Lauterwasser, E. Zubía and D. Trauner, J. Am. Chem. Soc., 2012, 134, 9291–9295 CrossRef CAS PubMed.
- J. F. Biard, S. Guyot, C. Roussakis, J. F. Verbist, J. Vercauteren, J. F. Weber and K. Boukef, Tetrahedron Lett., 1994, 35, 2691–2694 CrossRef CAS.
- M.-P. Sauviat, J. Vercauteren, N. Grimaud, M. Jugé, M. Nabil, J.-Y. Petit and J.-F. Biard, J. Nat. Prod., 2006, 69, 558–562 CrossRef CAS PubMed.
- M. A. Perry, M. D. Morin, B. W. Slafer and S. D. Rychnovsky, J. Org. Chem., 2012, 77, 3390–3400 CrossRef CAS PubMed.
- M. Tadesse, M. B. Strøm, J. Svenson, M. Jaspars, B. F. Milne, V. Tørfoss, J. H. Andersen, E. Hansen, K. Stensvåg and T. Haug, Org. Lett., 2010, 12, 4752–4755 CrossRef CAS PubMed.
- M. Tadesse, J. Svenson, M. Jaspars, M. B. Strøm, M. H. Abdelrahman, J. H. Andersen, E. Hansen, P. E. Kristiansen, K. Stensvåg and T. Haug, Tetrahedron Lett., 2011, 52, 1804–1806 CrossRef CAS PubMed.
- K. H. Hopmann, J. Šebestík, J. Novotná, W. Stensen, M. Urbanová, J. Svenson, J. S. Svendsen, P. Bouř and K. Ruud, J. Org. Chem., 2012, 77, 858–869 CrossRef CAS PubMed.
- Y. Takahasi, H. Ishiyama, T. Kubota and J. Kobayashi, Bioorg. Med. Chem. Lett., 2010, 20, 4100–4103 CrossRef PubMed.
- T. Suzuki, T. Kubota and J. Kobayashi, Bioorg. Med. Chem. Lett., 2011, 21, 4220–4223 CrossRef CAS PubMed.
- H. Ishiyama, K. Yoshizawa and J. Kobayashi, Tetrahedron, 2012, 68, 6186–6192 CrossRef CAS PubMed.
- J. Sun, Y. Dou, H. Ding, R. Yang, Q. Sun and Q. Xiao, Mar. Drugs, 2012, 10, 881–889 CrossRef CAS PubMed.
- P. Margiastuti, T. Ogi, T. Teruya, J. Taira, K. Suenaga and K. Ueda, Chem. Lett., 2008, 37, 448–449 CrossRef CAS.
- P. Margiastuti, T. Ogi, T. Teruya, J. Taira, K. Suenaga and K. Ueda, Chem. Lett., 2008, 37, 670–670 CrossRef CAS.
- A. Aiello, E. Fattorusso, P. Luciano, A. Macho, M. Menna and E. Munoz, J. Med. Chem., 2005, 48, 3410–3416 CrossRef CAS PubMed.
- A. Carbone, C. L. Lucas and C. J. Moody, J. Org. Chem., 2012, 77, 9179–9189 CrossRef CAS PubMed.
- I. M. Khalil, D. Barker and B. R. Copp, J. Nat. Prod., 2012, 75, 2256–2260 CrossRef CAS PubMed.
- A. R. Carroll, S. Duffy and V. M. Avery, J. Org. Chem., 2010, 75, 8291–8294 CrossRef CAS PubMed.
- J. P. Mahajan, Y. R. Suryawanshi and S. B. Mhaske, Org. Lett., 2012, 14, 5804–5807 CrossRef CAS PubMed.
- J. D. Panarese and C. W. Lindsley, Org. Lett., 2012, 14, 5808–5810 CrossRef CAS PubMed.
- B. C. M. Potts, D. J. Faulkner, J. A. Chan, G. C. Simolike, P. Offen, M. E. Hemling and T. A. Francis, J. Am. Chem. Soc., 1991, 113, 6321–6322 CrossRef CAS.
- F.-M. Zhang, L. Peng, H. Li, A.-J. Ma, J.-B. Peng, J.-J. Guo, D. T. Yang, S.-H. Hou, Y.-Q. Tu and W. Kitching, Angew. Chem., Int. Ed., 2012, 51, 10846–10850 CrossRef CAS PubMed.
- L.-P. La, C. Li, L. Li, P. Sun, H. Tang, B.-S. Liu, W. Gong, H. Han, Y.-H. Yi and W. Zhang, Chem. Biodiversity, 2012, 9, 1166–1171 Search PubMed.
- M.-P. La, J.-J. Shao, J. Jiao and Y.-H. Yi, Chin. J. Nat. Med., 2012, 10, 105–109 CrossRef CAS.
- F. Kisa, K. Yamada, M. Kaneko, M. Inagaki and R. Higuchi, Chem. Pharm. Bull., 2005, 53, 382–386 CrossRef CAS.
- L. Du, Z.-J. Li, J.-F. Wang, Y. Xue, C.-H. Xue, K. Takahashi and Y.-M. Wang, J. Oleo Sci., 2012, 61, 321–330 CrossRef CAS.
- K. Pan, C. Tanaka, M. Inagaki, R. Higuchi and T. Miyamoto, Mar. Drugs, 2012, 10, 2467–2480 CrossRef CAS PubMed.
- E. V. Levina, A. I. Kalinovskii, S. P. Ermakova and P. S. Dmitrenok, Russ. J. Bioorg. Chem., 2012, 38, 520–525 CrossRef CAS.
- A. O. Kaihil, M. S. Diop and A. Samb, Chem. Nat. Compd., 2012, 47, 932–934 CrossRef CAS PubMed.
- A. A. Kicha, N. V. Ivanchina, T. V. Malyarenko, A. I. Kalinovskii and P. S. Dmitrenok, Chem. Nat. Compd., 2012, 48, 806–809 CrossRef CAS.
- N. V. Ivanchina, A. I. Kalinovsky, A. A. Kicha, T. V. Malyarenko, P. S. Dmitrenok, S. P. Ermakova and V. A. Stonik, Nat. Prod. Commun., 2012, 7, 853–858 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Martyyas and V. I. Kalinin, Nat. Prod. Commun., 2012, 7, 1157–1162 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Martyyas and V. I. Kalinin, Nat. Prod. Commun., 2012, 7, 517–525 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Martyyas and V. I. Kalinin, Nat. Prod. Commun., 2012, 7, 845–852 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjashchenko, P. S. Dmitrenok, V. I. Kalinin and V. A. Stonik, Biochem. Syst. Ecol., 2012, 44, 53–60 CrossRef CAS PubMed.
- S. S. Afiyatullov, L. Y. Tishenko, V. A. Stonik, A. I. Kalinovskii and G. B. Elyakov, Khim. Prir. Soedin., 1985, 244–248 CAS.
- V. I. Kalinin, S. A. Avilov, A. I. Kalinovskii and V. A. Stonik, Khim. Prir. Soedin., 1992, 729–730 CAS.
- S. A. Avilov, V. I. Kalinin, T. N. Makarieva, V. A. Stonik, A. I. Kalinovsky, Y. W. Rashkes and Y. M. Milgrom, J. Nat. Prod., 1994, 57, 1166–1171 CrossRef CAS.
- V. I. Kalinin, S. A. Avilov, A. I. Kalinovskii, V. A. Stonik, Y. M. Mil'grom and Y. V. Rashkes, Khim. Prir. Soedin., 1992, 691–694 CAS.
- Y.-C. Zhan, Y. Sun, W. Li, Y. Lin, Y. Sha and Y.-H. Pei, J. Asian Nat. Prod. Res., 2006, 8, 631–636 CrossRef CAS PubMed.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Yurchenko and V. I. Kalinin, Nat. Prod. Res., 2012, 26, 1765–1774 CrossRef CAS PubMed.
- Z. Wang, H. Zhang, W. Yuan, W. Gong, H. Tang, B. Liu, K. Krohn, L. Li, Y. Yi and W. Zhang, Food Chem., 2012, 132, 295–300 CrossRef CAS PubMed.
- Z. Wang, W. Gong, G. Sun, H. Tang, B. Liu, L. Li, Y. Yi and W. Zhang, Nat. Prod. Commun., 2012, 7, 1431–1434 CAS.
- I. Kitagawa, M. Kobayashi, M. Hori and Y. Kyogoku, Chem. Pharm. Bull., 1989, 37, 61–67 CrossRef CAS.
- V. Lakshmi, S. Srivastava, S. K. Mishra and P. K. Shukla, Nat. Prod. Res., 2012, 26, 913–918 CrossRef CAS PubMed.
- I. Kitagawa, T. Inamoto, M. Fuchida, S. Okada, M. Kobayashi, T. Nishino and Y. Kyogoku, Chem. Pharm. Bull., 1980, 28, 1651–1653 CrossRef CAS.
- F. R. Melek, M. M. Tadros, F. Yousif, M. A. Selim and M. H. Hassan, Pharm. Biol., 2012, 50, 490–496 CrossRef CAS PubMed.
- Q. Zhao, Y. Xue, J.-F. Wang, H. Li, T.-T. Long, Z. Li, Y.-M. Wang, P. Dong and C.-H. Xue, J. Sci. Food Agric., 2012, 92, 965–974 CrossRef CAS PubMed.
- S. A. Avilov, A. I. Kalinovskii and V. A. Stonik, Khim. Prir. Soedin., 1990, 53–57 CAS.
- P. Yibmantasiri, D. C. Leahy, B. P. Busby, S. A. Angermayr, A. G. Sorgo, K. Boeger, R. Heathcott, J. M. Barber, G. Moraes, J. H. Matthews, P. T. Northcote, P. H. Atkinson and D. S. Bellows, Mol. BioSyst., 2012, 8, 902–912 RSC.
- H.-L. Liu, X.-Y. Huang, J. Li, G.-R. Xin and Y.-W. Guo, Chirality, 2012, 24, 459–462 CrossRef CAS PubMed.
- M. G. Ponnapalli, S. C. V. A. R. Annam, S. Ravirala, S. Sukki, M. Ankireddy and V. R. Tuniki, J. Nat. Prod., 2012, 75, 275–279 CrossRef CAS PubMed.
- Y. Li, S. Yu, D. Liu, P. Proksch and W. Lin, Bioorg. Med. Chem. Lett., 2012, 22, 1099–1102 CrossRef CAS PubMed.
- D. A. Okorie and D. A. H. Taylor, Phytochemistry, 1968, 7, 1683–1686 CrossRef CAS.
- J. Li, M.-Y. Li, G. Feng, J. Zhang, M. Karonen, J. Sinkkonen, T. Satyanandamurty and J. Wu, J. Nat. Prod., 2012, 75, 1277–1283 CrossRef CAS PubMed.
- J. Li, M.-Y. Li, T. Bruhn, D. C. G. Götz, Q. Xiao, T. Satyanandamurty, J. Wu and G. Bringmann, Chem.–Eur. J., 2012, 18, 14342–14351 CrossRef CAS PubMed.
- D. Noutsias and G. Vassilikogiannakis, Org. Lett., 2012, 14, 3565–3567 CrossRef CAS PubMed.
- X.-L. Chen, H.-L. Liu, J. Li, G.-R. Xin and Y.-W. Guo, Org. Lett., 2011, 13, 5032–5035 CrossRef CAS PubMed.
- K. Mondo, N. Hammerschlag, M. Basile, J. Pablo, S. A. Banack and D. C. Mash, Mar. Drugs, 2012, 10, 509–520 CrossRef CAS PubMed.
- J. Henry, C. Zatylny and E. Boucaud-Camou, Peptides, 1999, 20, 1061–1070 CrossRef CAS.
- M. Laurencin, B. Legrand, E. Duval, J. Henry, M. Baudy-Floc'h, C. Zatylny-Gaudin and A. Bondon, J. Med. Chem., 2012, 55, 2025–2034 CrossRef CAS PubMed.
- http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml Accessed 28 October 2013.
- Email: E-mail: marinlit@rsc.org.
| This journal is © The Royal Society of Chemistry 2014 |