The performance of mesoporous organosilicas with phenyl groups in Heme protein immobilization
Yu
Xiao
,
Buyuan
Guan
,
Xue
Wang
,
Zhuofu
Wu
,
Yunling
Liu
and
Qisheng
Huo
*
State Key Lab of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun, China. E-mail: huoqisheng@jlu.edu.cn; Fax: +86-431-85168602; Tel: +86-431-85168602
First published on 6th November 2014
Abstract
A series of mesoporous organosilicas with different phenyl group content have been synthesized for the immobilization of Heme proteins. A higher number of phenyl groups are conducive to immobilization of Heme proteins and improvement of the activity of the immobilized enzymes. The amount of immobilized horseradish peroxidase (HRP), myoglobin (Mb) and hemoglobin (Hb) is 35 mg, 51 mg and 244 mg, respectively, with 1 g of mesoporous organosilica. In particular, the immobilization efficiency of Mb can reach 100%. A sensor utilizing HRP immobilized in mesoporous organosilica is constructed on a glassy carbon electrode. In buffer solution (pH 6.0), the modified electrode shows an electrochemical response towards catechol.
Introduction
Enzyme catalysts can be used in many fields such as the pharmaceutical,1 agriculture and food,2,3 and bioremediation4,5 industries. Enzyme-catalysed reactions have seen rapid growth because they conform to the trend of the development of green and sustainable chemistry.6,7 Nonetheless, the application of enzymes in industry is still limited by their low manipulative stability (denaturation and deactivation) in extreme environments (high temperature, low or high pH values or the presence of organic solvents) and difficulties in recycling.8,9 Thus, the immobilisation of enzymes onto solid supports has gained wide attention.10–14 Adsorption, covalent attachment, encapsulation and cross-linked enzyme aggregates are several normal methods for immobilization of enzymes onto supports.15 Physical adsorption is the most cost-effective and simple method as a consequence of the interactions between the support surface and the outer shell of the enzyme.16 Adsorption on the support may not impact the active site of the enzyme or the activity of the enzyme.17 The host–guest interaction during adsorption is based on van der Waals forces, hydrogen bonding and hydrophobic interactions so the surface state of the materials plays a key role for successful immobilization and retention of activity.18Because HRP is utilized as a reagent for biotransformation and organic synthesis as well as in wastewater treatment, immunoassays and coupled enzyme assays,19,20 it is a promising candidate for industrial application. Nevertheless, it costs a lot to recycle and reuse after utilizing the HRP as an enzymic catalyst. Additionally, HRP exhibits a short catalytic lifetime because of the influence from the environment during the reaction process.21 Hence, HRP has been immobilized into many kinds of materials, including iron oxide nanoparticles,22,23 phospholipid bilayers,24 sol–gel and glass beads. Barbosa et al. reported that immobilized HRP on magnetite-modified polyaniline can be used for 13 cycles.25 Yu et al. used a facile vapour deposition method to form a titania sol–gel thin film to immobilize HRP on a glassy carbon electrode surface for production of an amperometric hydrogen peroxide biosensor.26 Gómez et al. immobilized HRP on glutaraldehyde-activated aminopropyl glass beads to remove phenol.27 Wang et al. successfully immobilized HRP on silane-modified ceramics with the method of covalent bonding and cross-linking to remove oil from wastewater.28 Ju et al. prepared an electrochemical biosensor for phenol, based on immobilization of tyrosinase-peroxidase on mesoporous silica.29
Recently, mesoporous organosilicas have been recognised as novel types of hybrid mesoporous materials with the dispersity of organic moieties in their framework.30,31 Periodic mesoporous organosilicas (PMOs) not only have an ordered structure, high surface area, and large pore volume, but also maintain their hydrophobicity due to the organic groups in the framework.32 The types and the content of organic groups in the framework are also adjustable. All of these characteristics are conducive to immobilization of enzymes. However, there are only a few reports about the immobilization of HRP onto mesoporous organosilica materials. Zhu et al. studied the mesoporous organosilicas with –OH, –O– and –S– organic groups as supports for immobilization of HRP.33–35 They stated that the influence of the organic groups is important for enzyme immobilization.
In this paper, our aim is to find simple and convenient supports for Heme protein immobilization. We investigate the influence of different phenyl content in the mesoporous organosilicas on the Heme protein immobilization. The results elucidate that these supports have high Heme protein loading and keep the enzymatic activity of immobilized HRP. The viability of using an immobilized HRP modified electrode as a catechol sensor is demonstrated.
Experimental
Chemicals and reagents
Triblock co-polymer P123, myoglobin (Mb) and 3,3′,5,5-tetramethylbenzidine (TMB) were purchased from Sigma-Aldrich. Bis(triethoxysilyl)ethane and diphenyldimethoxysilane were obtained from Gelest. Horseradish peroxidase (HRP) was purchased from Roche. 1,3,5-trimethyl benzene, hydrogen peroxide (30%) and hemoglobin (Hb) were purchased from Sinopharm.Synthesis of PMO materials
The cage-like pore mesoporous organosilicas were synthesized by using 1,3,5-trimethyl benzene as a swelling agent according to the literature.36 In a typical preparation, the block copolymer P123 was dissolved in a solution containing deionized water and concentrated HCl (37 wt%) at 38 °C under stirring for 2 h. After addition of TMB, the mixture was stirred at 38 °C for another 2 h and the organosilane was added. After further stirring for 24 h at 38 °C, the reaction mixture was aged at 98 °C for 48 h under static conditions. The mixture was allowed to cool to room temperature and the white precipitate was filtered off and dried in air for at least 2 days. The template was removed by means of extraction with ethanol. The PMO that was synthesized by using bis(triethoxysilyl)ethane (BTEE) as a bridged organosilane source was named MOS0 and those synthesized by using a mixture of BTEE and diphenyldimethoxysilane (DPDM) as two source precursors were named MOS-n (n is the mass percentage of DPDM/(DPDM + BTEE) in the initial sol mixture, as listed in Table 1).| Sample | MOS0 | MOS1 | MOS2.5 | MOS5 | MOS10 |
|---|---|---|---|---|---|
| a S BET, BET surface area; dP, pore diameter calculated from adsorption branch with improved KJS method; VP, pore volume at P/P0 = 0.92. | |||||
| Mass percentage of DPDM in the initial sol mixture | 0% | 1% | 2.5% | 5% | 10% |
| S BET/m2 g−1 | 868 | 844 | 781 | 734 | 663 |
| V P/cm3 g−1 | 1.24 | 1.21 | 1.06 | 0.88 | 0.72 |
| d P/nm | 9.2 | 9.5 | 9.3 | 7.6; 18 | 4.7; >50 |
| Mass percentage of DPDM in the framework | 0% | 0.8% | 1.3% | 5.3% | 9.5% |
| Zeta-potential in pH 6.0 solution/mV | −6.8 | −6.3 | −6.0 | −8.3 | −10.6 |
Characterization
The nitrogen adsorption–desorption isotherms were obtained at −196 °C on a Micromeritics ASAP 2420 analyzer, and the sample was degassed at 120 °C for at least 10 h before measurement. The BET specific surface area was calculated in a relative pressure range of P/P0 = 0.05–0.3. The FT-IR spectra of samples were obtained on a Bruker IFS 66/vS FTIR spectrometer (the samples were mixed with KBr). Thermo-gravimetric analysis of the extracted samples was performed on a NETZSCH STA 449C analyzer in air from 30 to 800 °C with a heating rate of 10 °C min−1. The morphology and structure of the materials were analyzed by transmission electron microscopy (TEM) using a FEI Tecnai G2 F20 s-twin D573 operated at 200 kV. Cyclic voltammograms were obtained from the CHI660C (CHI Instruments) using a standard three-electrode cell.Enzyme immobilization and enzyme leaching
The immobilization of enzyme was performed according to the literature:33 the powder support (50 mg) was added into a centrifuge tube with 5 mL buffer solution (pH 6.0) containing HRP at a concentration of 2 mg mL−1, and the mixture was shaken at 4 °C for 16 h. The mixture was separated by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min and the supports containing the encapsulated HRP were washed three times with 5 mL buffer solution. The amount of immobilized HRP was determined by calculation from the difference in the supernatant absorbance at 401 nm before and after the addition of the support. The immobilization of Hb and Mb was implemented in a similar way. To ensure that the number of enzyme molecules in the solution was consistent, the initial concentrations of Hb and Mb were 3.2 mg mL−1 and 0.84 mg mL−1, respectively.
000 rpm for 5 min and the supports containing the encapsulated HRP were washed three times with 5 mL buffer solution. The amount of immobilized HRP was determined by calculation from the difference in the supernatant absorbance at 401 nm before and after the addition of the support. The immobilization of Hb and Mb was implemented in a similar way. To ensure that the number of enzyme molecules in the solution was consistent, the initial concentrations of Hb and Mb were 3.2 mg mL−1 and 0.84 mg mL−1, respectively.
To check the stability of immobilized enzyme, the immobilized enzyme was washed with the same buffer solution and deionized water under stirring and then vacuum-dried. The suspensions were collected and measured to calculate the amount of enzyme leaching.33
Assay of free and immobilized enzyme
TMB and hydrogen peroxide were used as substrates to evaluate the activity of free HRP and immobilized HRP. The reaction began through the addition of 2 μL hydrogen peroxide (0.2 M) to the mixture of 5 mL phosphate buffer solution (pH 6.0) containing TMB and the same content of either free HRP or immobilized HRP. After reacting at room temperature for a period, the reaction was stopped by 2 M H2SO4 and the absorbance of the product was measured at 450 nm using a UV-Vis spectrometer. The absorbance at 450 nm is defined as the specific activity of free HRP or immobilized HRP. All the samples were analysed in triplicate.Electrode modification
The working electrodes were fabricated as follows: 5 μL of a certain suspension was dropped onto a glassy carbon electrode. After drying, a 0.5 wt% Nafion solution was used to coat the sample.37Results and discussion
Characterization of the mesoporous organosilica materials
Five mesoporous organosilica materials with different contents of phenyl groups were explored for their capacity in the immobilization of enzyme. A series of mesoporous materials were synthesised using Pluronic P123 as a template, 1,3,5-trimethylbenzene as a pore expander and BTEE and DPDM as source precursors.Fig. 1a–e show TEM images of samples MOS0, MOS1, MOS2.5, MOS5 and MOS10. The samples MOS0, MOS1 and MOS2.5 exhibit uniform mesoporous structures. The samples MOS5 and MOS10 are composed of large hollow cage-like pores with multiple walls36 with the content of phenyl groups increasing.
Fig. 2 shows N2 adsorption–desorption isotherms of the samples MOS0, MOS1, MOS2.5, MOS5 and MOS10. The sorption isotherms of samples MOS0, MOS1, MOS2.5 have the characteristics of type IV isotherms suggesting mesoporous systems.36 The sorption isotherms of the samples MOS5 and MOS10 have the characteristics of both type IV and type II isotherms. The increasing adsorption of nitrogen at a higher relative pressure demonstrates the larger pores in these materials. A small capillary condensation step at relative pressure 0.6 < P/P0 < 0.8 indicates the mesoporosity.38
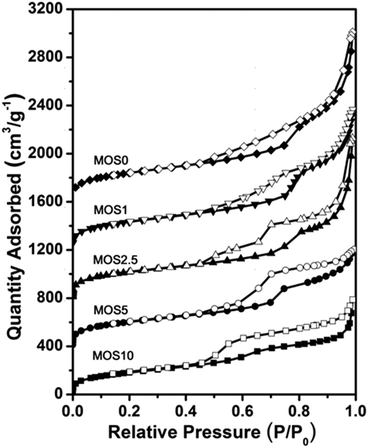 | ||
| Fig. 2 N2 adsorption–desorption isotherms of supports. Isotherms are offset by 400 cm3 g−1 along the vertical axis for clarity. | ||
As listed in Table 1, these five materials have high BET surface areas. The samples MOS5 and MOS10 show two pore size distributions. The smaller values are due to the existence of multilamellar pores in the walls of the large cages and the larger values are due to the existence of cage-like pores. These results are consistent with the TEM images.
Existence of organic functional groups in these materials was proven by FT-IR spectra and TG plots. As is shown in Fig. 3, the absorption bands at 1594 and 1430 cm−1 are ascribed to the skeletal vibrations of the benzene ring.39
TG analysis of materials was conducted from 30 °C to 800 °C in air. From the profiles displayed in Fig. 4, the samples all exhibit high thermal stability. Between 250 °C and 700 °C, the plots indicate the degradation of organic groups in the mesoporous wall. The different data obtained from TG analysis can be attributed to the different organic content in the samples. Based on the different weight loss values, the mass percentage of DPDM/(DPDM + BTEE) in the framework can be calculated (as shown in Table 1). The phenyl group content in the samples shows the following trend: MOS0 < MOS1 < MOS2.5 < MOS5 < MOS10. The calculative result is roughly consistent with the starting materials ratio.
 | ||
| Fig. 4 TG plots of supports MOS0 (black), MOS1 (blue), MOS2.5 (magenta), MOS5 (olive) and MOS10 (red). | ||
In conclusion, we have successfully synthesised five supports with different phenyl group content. These mesoporous materials have been used in enzyme immobilization.
HRP immobilization
Our exploratory experiment adopted an initial concentration of 2 mg mL−1 HRP for immobilization of enzyme on the supports. Further increasing the initial concentration of enzyme could not improve the immobilization of enzyme. The result is consistent with the literature.35Fig. 5a shows that the support MOS5 adsorbs the largest amount of HRP (35 mg g−1). The HRP immobilization capacity of supports is as follows: MOS10 (16 mg g−1) < MOS0 (17 mg g−1) < MOS1 (21 mg g−1) < MOS2.5 (25 mg g−1) < MOS5 (35 mg g−1). The values indicate that the existence of phenyl groups in the supports is significant for the immobilization.
Because zeta potentials for all supports are negative at pH 6.0 (listed in Table 1) and the isoelectric point of HRP is 7.2, the charge of the supports favors the adsorption of HRP via electrostatic interaction. The hydrophobic interactions between the hydrophobic domain of HRP and phenyl groups of the supports gradually increases with increasing phenyl group content. The support with more phenyl groups tends to adsorb more HRP molecules. The HRP immobilization capacity in our supports is higher than that in the inorganic silica support SBA-15 (12 mg g−1, DBJH 9.1 nm).34 The driving force for HRP adsorption on SBA-15 is mainly electrostatic interactions. Hence, the increasing of the hydrophobic interactions between HRP and the support is significant for the immobilization of more HRP.
As shown in Fig. 5a, the HRP immobilization capacity of the support MOS0 is higher than that of the support MOS10, although the support MOS10 has the most phenyl groups. The existence of multilamellar pores (4.7 nm) gives the support MOS10 its high BET surface area. The large sized HRP molecules (M = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 3.7 nm × 4.3 nm × 6.4 nm)35,40 are hard to adsorb into the small pore. The larger pore which is suitable for immobilization of HRP has a lower contribution to BET surface area. The stronger interactions between support MOS10 and HRP make up for the disadvantages caused by the lower useable BET surface area. Thus, the BET surface area, pore size and the organic group synergistically affect the performance of these supports at adsorbing the HRP molecule.
000, 3.7 nm × 4.3 nm × 6.4 nm)35,40 are hard to adsorb into the small pore. The larger pore which is suitable for immobilization of HRP has a lower contribution to BET surface area. The stronger interactions between support MOS10 and HRP make up for the disadvantages caused by the lower useable BET surface area. Thus, the BET surface area, pore size and the organic group synergistically affect the performance of these supports at adsorbing the HRP molecule.
To test the potential for our materials as general supports for Heme proteins, two other enzymes with different sizes were selected for the immobilization study. Myoglobin (Mb) (M = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700)41 has a smaller molecular size and Hemoglobin (Hb) (M = 64
700)41 has a smaller molecular size and Hemoglobin (Hb) (M = 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)42 has a larger molecular size.
000)42 has a larger molecular size.
Fig. 5b shows that adsorbed Mb on MOS1, MOS2.5, and MOS5 is 76 mg g−1, 81 mg g−1, and 84 mg g−1, respectively. It is worth mentioning that the immobilization efficiencies of Mb on these three supports are close to 100%. The influence of phenyl group content in the supports is also important for the immobilization of Mb.
The Mb immobilization capacity of MOS10 (54 mg g−1) is higher than that of MOS0 (23 mg g−1). This phenomenon is different from the one shown in Fig. 5a, because the smaller sized Mb (ca. 17.6 nm3)43 molecule could be adsorbed into both multilamellar pores (4.7 nm) and the cage-like pores driven by the hydrophobic interaction.
Fig. 5c shows that the Hb loading on these supports can reach up to 244 mg g−1. The phenyl group is clearly beneficial to the Hb immobilization. The immobilization behaviour of Hb has a similar tendency to that of HRP. The support MOS10 adsorbs the least amount of Hb, because the large Hb molecule (5.3 nm × 5.4 nm × 6.5 nm)43 has more difficulty in entering into the small pore. This fact further confirms our inferences.
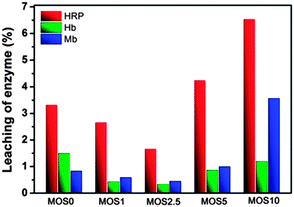
The stability of immobilized Heme protein is estimated by a leaching test. As shown in Fig. 6, the leach ratios of Heme protein are all lower than 6.8%. For immobilized HRP and Mb, the leach ratios are lower than 6.8% and 3.6% respectively, with the larger pore sizes of MOS5 and MOS10 allowing more enzyme molecules to leach from the support and giving the higher values. On the other hand, Mb with its smaller molecule size can be adsorbed into smaller pores in the walls of MOS5 and MOS10. Hence the leach ratios (lower than 1.5%) of Mb from MOS5 and MOS10 are relatively lower. Due to the strength of interactions between the Heme proteins and the support, the leach ratios of different enzymes are different.
The activity of immobilized HRP
Immobilized HRP was chosen to investigate the activity of immobilized proteins. The activities of immobilized HRP on different supports are shown in Fig. 7. HRP-MOS10 has the highest relative activity which is slightly higher than the activity of native HRP with the same amount of HRP in the solution. The relative activities of immobilized HRP gradually increase with increasing content of phenyl groups.An increase in hydrophobic group content affects positively the activity of immobilized HRP. The activity of HRP-MOS10 is almost five times that of HRP-MOS0 although both of them are loaded with a similar amount of enzyme. HRP-MOS5 contains the largest number of HRP molecules, however the average activity of immobilized enzyme molecules in MOS5 is lower than that in MOS10. All the results show that the phenyl moiety in the support is important for the relative activity of the immobilized enzyme.
When HRP is immobilized onto the supports, many parameters affect its activity.33 Firstly, the enlarged pores of the supports reduce the resistance of substance mass-transfer to the enzyme active site.33,44 At the same time, the vesicular multishell structure of MOS10 is beneficial in promoting the mass-transfer and then improving the activity of immobilized HRP. Secondly, the numerous organic groups in these five supports provide suitable host–guest interactions, enabling the HRP to be adsorbed sufficiently strongly while avoiding enzyme rigidity after immobilization.34 The pore size distributions (DKJS) of HRP-MOS0, HRP-MOS1 and HRP-MOS2.5 matrixes are very close. The hydrophobic interaction plays a key role in increasing the activities of HRP-MOS0, HRP-MOS1 and HRP-MOS2.5 matrixes. Thirdly, the dispersion of the HRP molecules in the pore structure is also important for the activity of immobilized HRP.45 Too many enzyme molecules may cause overlaps of the active sites of enzyme molecules. Although HRP-MOS5 contains the most HRP molecules, the activity of HRP-MOS5 is 71% of that of free HRP due to the overlap of HRP molecules.
In conclusion, the activity of immobilized HRP is enhanced with an increasing number of phenyl groups in the support materials. HRP-MOS10 gives slightly improved activity over the free HRP. The presence of both organic groups and a vesicular multishell structure are favourable for improving the activity of immobilized HRP.
Amperometric response of the HRP-MOS10/GCE to catechol
To test the use of HRP in an electrochemical sensor, we used our HRP-MOS10 immobilization matrix to make a simple sensor. The response to catechol shows the possibility of using the HRP-MOS10 immobilization matrix as an electrochemical sensor for the detection of phenolic compounds.Fig. 8 shows the cyclic voltammogram curves of MOS10/GCE and HRP-MOS10/GCE at pH 6.0. When the HRP-MOS10/GCE electrode is in the PBS containing 9 mM of catechol and 20 mM H2O2, it shows redox peaks. Comparing the two electrodes, the presence of HRP increases the reduced current by an order of magnitude. Meanwhile, an obvious oxidized peak can be observed for the HRP-MOS10/GCE electrode and it cannot be observed for the MOS10/GCE. In conclusion, the HRP-MOS10 immobilization matrix modified electrode has the potential for detection of phenolic compounds as an amperometric sensor.
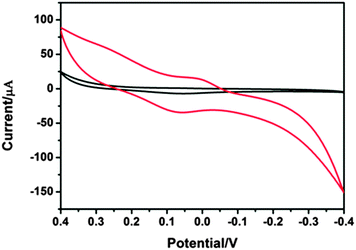 | ||
| Fig. 8 CVs of HRP-MOS10/GCE (red line) and MOS10/GCE (black line) in the presence of H2O2 containing 9 mM catechol at a scan rate of 0.1 V s−1. | ||
Conclusion
The mesoporous organosilica supports with phenyl groups show an excellent ability to immobilise Heme proteins. The hydrophobic interaction between the hydrophobic domain of proteins and the phenyl groups is a key factor in the immobilization of proteins. The immobilized HRP on MOS10 shows higher enzymatic activity than free HRP. Meanwhile, a simple sensor based on the HRP-MOS10 immobilization matrix exhibits an electrochemical response to catechol. This type of support is meaningful for improving the possibility of practical application of the immobilized enzymes.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21371067, 21373095 and 21171064).Notes and references
- M. Magnani and L. Rossi, Expert Opin. Drug Delivery, 2014, 11, 677–687 CrossRef CAS.
- K. Mrizova, E. Holaskova, M. T. Oz, E. Jiskrova, I. Frebort and P. Galuszka, Biotechnol. Adv., 2014, 32, 137–157 CrossRef CAS.
- A. B. Snyder and R. W. Worobo, J. Sci. Food Agric., 2014, 94, 28–44 CrossRef CAS PubMed.
- N. Gaur, G. Flora, M. Yadav and A. Tiwari, Environ. Sci.: Processes Impacts, 2014, 16, 180–193 CAS.
- A. P. Singh and T. Singh, Biomass Bioenergy, 2014, 62, 198–206 CrossRef CAS.
- S. Aguila, R. Vazquez-Duhalt, R. Tinoco, M. Rivera, G. Pecchi and J. B. Alderete, Green Chem., 2008, 10, 647–653 RSC.
- S. J. Sigg, F. Seidi, K. Renggli, T. B. Silva, G. Kali and N. Bruns, Macromol. Rapid Commun., 2011, 32, 1710–1715 CrossRef CAS PubMed.
- V. V. Mozhaev, M. V. Sergeeva, A. B. Belova and Y. L. Khmelnitsky, Biotechnol. Bioeng., 1990, 35, 653–659 CrossRef CAS PubMed.
- S. G. Burton, D. A. Cowan and J. M. Woodley, Nat. Biotechnol., 2002, 20, 37–45 CrossRef CAS.
- S. Jain, S. Chattopadhyay, R. Jackeray, C. Abid and H. Singh, Nanoscale, 2013, 5, 6883–6892 RSC.
- C. H. Lee, T. S. Lin and C. Y. Mou, Nano Today, 2009, 4, 165–179 CrossRef CAS.
- M. Hartmann and X. Kostrov, Chem. Soc. Rev., 2013, 42, 6277–6289 RSC.
- E. Magner, Chem. Soc. Rev., 2013, 42, 6213–6222 RSC.
- F. Secundo, Chem. Soc. Rev., 2013, 42, 6250–6261 RSC.
- C. Lei, Y. Shin, J. Liu and E. J. Ackerman, J. Am. Chem. Soc., 2002, 124, 11242–11243 CrossRef CAS.
- H. H. P. Yiu and P. A. Wright, J. Mater. Chem., 2005, 15, 3690–3700 RSC.
- A. Galarneau, M. Mureseanu, S. Atger, G. Renard and F. Fajula, New J. Chem., 2006, 30, 562–571 RSC.
- M. Hartmann and D. Jung, J. Mater. Chem., 2010, 20, 844–857 RSC.
- O. Ryan, M. R. Smyth and C. O'Fagain, Essays Biochem., 1994, 28, 129–146 CAS.
- N. C. Veitch and A. T. Smith, Adv. Inorg. Chem., 2001, 51, 107–162 CrossRef CAS.
- I. D. Buchanan and J. A. Nicell, Biotechnol. Bioeng., 1997, 54, 251–261 CrossRef CAS.
- E. Cevik, M. Senel, A. Baykal and M. F. Abasiyanik, Sens. Actuators, B, 2012, 173, 396–405 CrossRef CAS.
- H. J. Jin, N. Gan, J. G. Hou, F. T. Hu, Y. T. Cao, L. Zheng and Z. Y. Guo, Sens. Lett., 2012, 10, 886–893 CrossRef CAS.
- M. B. Fritzen-Garcia, V. C. Zoldan, I. Oliveira, V. Soldi, A. A. Pasa and T. B. Creczynski-Pasa, Biotechnol. Bioeng., 2013, 110, 374–382 CrossRef CAS.
- E. F. Barbosa, F. J. Molina, F. M. Lopes, P. A. Garcia-Ruiz, S. S. Caramori and K. F. Fernandes, Sci. World J., 2012, 2012, 716374–716379 Search PubMed.
- J. H. Yu and H. X. Ju, Anal. Chem., 2002, 74, 3579–3583 CrossRef CAS.
- J. L. Gómez, A. Bódalo, E. Gómez, J. Bastida, A. M. Hidalgo and M. Gómez, Enzyme Microb. Technol., 2006, 39, 1016–1022 CrossRef PubMed.
- W. C. Wang, Z. L. Li, W. Liu and J. L. Wu, Sep. Purif. Technol., 2012, 89, 206–211 CrossRef CAS PubMed.
- Z. Dai, X. Xu, L. Wu and H. Ju, Electroanalysis, 2005, 17, 1571–1577 CrossRef CAS.
- J. Li, Y. Wei, W. Li, Y. H. Deng and D. Y. Zhao, Nanoscale, 2012, 4, 1647–1651 RSC.
- S. Parambadath, V. K. Rana, D. Y. Zhao and C. S. Ha, Microporous Mesoporous Mater., 2011, 141, 94–101 CrossRef CAS PubMed.
- S. Fujita and S. Inagaki, Chem. Mater., 2008, 20, 891–908 CrossRef CAS.
- Y. Zhou, M. M. Wan, L. Gao, N. Lin, W. G. Lin and J. H. Zhu, J. Mater. Chem. B, 2013, 1, 1738–1748 RSC.
- N. Lin, L. Gao, Z. Chen and J. H. Zhu, New J. Chem., 2011, 35, 1867–1875 RSC.
- M. M. Wan, L. Gao, Z. Chen, Y. K. Wang, Y. Wang and J. H. Zhu, Microporous Mesoporous Mater., 2012, 155, 24–33 CrossRef CAS PubMed.
- Z. Zhou, R. N. K. Taylor, S. Kullmann, H. Bao and M. Hartmann, Adv. Mater., 2011, 23, 2627–2632 CrossRef CAS.
- W. Chaikittisilp, M. Hu, H. J. Wang, H. S. Huang, T. Fujita, K. C. W. Wu, L. C. Chen, Y. Yamauchi and K. Ariga, Chem. Commun., 2012, 48, 7259–7261 RSC.
- Z. Zhou, A. Inayat, W. Schwieger and M. Hartmann, Microporous Mesoporous Mater., 2012, 154, 133–141 CrossRef CAS.
- Y. Zhang, Z.-A. Qiao, Y. Li, Y. Liu and Q. Huo, J. Mater. Chem., 2011, 21, 17283–17289 RSC.
- J. C. Kendrew, G. Bodo, H. M. Dintzis, R. G. Parrish, H. Wyckoff and D. C. Phillips, Nature, 1958, 181, 662–666 CrossRef CAS.
- S. Q. Liu, Z. H. Dai, H. Y. Chen and H. X. Ju, Biosens. Bioelectron., 2004, 19, 963–969 CrossRef CAS.
- H. Y. Gu, A. M. Yu and H. Y. Chen, J. Electroanal. Chem., 2001, 516, 119–126 CrossRef CAS.
- S. Hudson, J. Cooney and E. Magner, Angew. Chem., Int. Ed., 2008, 47, 8582–8594 CrossRef CAS.
- W. Chouyyok, J. Panpreanot, C. Thanachayanant and S. Prichanont, J. Mol. Catal. B: Enzym., 2009, 56, 246–252 CrossRef CAS.
- H. Takahashi, B. Li, T. Sasaki, C. Miyazaki, T. Kajino and S. Inagaki, Microporous Mesoporous Mater., 2001, 44–45, 755–762 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |