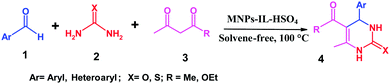
Brønsted acidic ionic liquid based magnetic nanoparticles: a new promoter for the Biginelli synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones
Javad
Safari
* and
Zohre
Zarnegar
Laboratory of Organic Compound Research, Department of Organic Chemistry, College of Chemistry, University of Kashan, P.O. Box: 87317-51167, Kashan, I.R., Iran. E-mail: Safari@kashanu.ac.ir; Fax: +98 (0)361 591 2397; Tel: +98 (0)361 591 2320
First published on 21st November 2013
Abstract
The Brønsted acidic ionic liquid 1-methyl-3-(3-trimethoxysilylpropyl) imidazolium hydrogen sulfate was immobilized on magnetic Fe3O4 nanoparticles (MNPs-IL-HSO4). The properties of the magnetic nanocatalyst were characterized by FT-IR, EDX, SEM, elemental analysis, XRD, VSM, and TGA. The results showed that the ionic liquid had been successfully immobilized onto the magnetic support, and the immobilized ionic liquid catalysts exhibited high catalytic activity in the Biginelli reaction under mild conditions for the synthesis of a diverse range of 3,4-dihydropyrimidin-2(1H)-ones. It also maintained satisfactory catalytic activity for the synthesis of Biginelli compounds after 8 rounds of recycling. The separation and reuse of the novel solid catalyst were simple, effective and economical.
1. Introduction
Green chemistry is joint research in chemistry and environmental protection, involving either new eco-friendly reagents and catalysts, selected media such as water, ionic liquids (ILs) or solvent-free conditions, or non-classical modes of activation such as ultrasound or microwave techniques.1 Enhanced environmental consciousness has promoted the efficiency of chemical reactions under benign conditions with recycling and reuse of the catalysts, a trait that has become an integral part of chemistry research worldwide.2 ILs have attracted interest as environmentally benign media for catalytic applications because of their favorable properties such as high thermal stability, negligible vapor pressure, tunable acidity and selective dissolvability. Though ILs possessed such promising advantages, their widespread practical application was still hampered by several drawbacks: (i) homogeneous reaction, which made product separation and catalyst recovery difficult, (ii) high viscosity, which resulted in only a minor part of the ILs taking part in the catalyzed reaction, and consequently (iii) the high cost of using relatively large amounts of ILs.3 Therefore, in order to solve these problems, one of the most promising alternative solutions is the development of IL-based heterogeneous catalysts. IL-based heterogeneous catalysts are generally prepared by immobilization of ILs onto various solid supports. They not only retain the important physical and chemical features of ILs, such as nonvolatility, nonflammability, and good thermal stability, but also possess a number of advantages in terms of separation, reusability, and particularly, the ability to provide practical conveniences in a continuous system that is valued in industry.4Magnetic nanoparticles (MNPs) have recently appeared as a new type of catalyst support for ILs because of their good stability, easy synthesis and functionalization, high surface area and facile separation by magnetic forces, as well as low toxicity and price.5 These attractive features have made MNPs a promising alternative type of catalyst support.
In recent years, Brønsted acidic ILs (BAILs), as environmentally-friendly catalysts, have received great attention from researchers, for many organic syntheses. BAILs, which possess the advantageous characteristics of solid acids and liquid acids, have been designed to replace traditional mineral liquid acids, such as hydrochloric acid and sulfuric acid.6 Among these, ILs possessing HSO4− as a counter anion find a broad application in organic reactions, acting as catalysts.6 The first synthesis of 1-butyl-3-methylimidazolium hydrogen sulfate7 ([bmim]HSO4) and its application in the Friedel–Crafts alkylation process8 were described by Wasserscheid et al. The acidic nature of these ILs as catalysts has been exploited for many other important organic syntheses, which proceed with excellent yields and selectivities and demonstrate the great potential of these ILs in catalytic technologies for chemical production.9 Also, immobilisation processes involving acidic ILs on solid supports have been designed. Immobilised acidic ILs have been used as novel solid catalysts, e.g., for esterification, nitration reactions and acetal formation.10
Recently, interest in the synthesis of 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) and their derivatives via the Biginelli reaction has increased tremendously because of their therapeutic and pharmacological properties and also because of the interesting biological activities of several marine alkaloids that contain the dihydropyrimidine nucleus.11 The medicinal importance of DHPMs has been recognized for many decades and many of these compounds exhibit antiviral, antibacterial, antitumor, and anti-inflammatory properties.12 Nitractin was first reported in the 1960s as an agent against the trachoma group of viruses.13 Monastrol is currently known as a specific inhibitor of mitotic kinesin Eg5 and is considered as a lead compound to develop new anticancer drugs.14
The synthesis of DHPMs via the Biginelli reaction, a three-component condensation reaction between an aldehyde, urea or a thiourea, and an easily enolizable carbonyl compound, was originally described by the Italian chemist Pietro Biginelli in 1891.15 The development of efficient and versatile catalytic systems for the synthesis of Biginelli compounds is an active ongoing research area and thus, there is scope for further improvement toward milder reaction conditions, variations of substituents in all three components and better yields.16
In view of this, we have utilized a magnetic nanoparticle supported hydrogen sulfate ionic liquid as an efficient Brønsted acid catalyst for the Biginelli condensation reaction (Scheme 1). The main objective of this work is to replace polluting traditional mineral acidic catalysts with other catalysts that are capable and stable. Brønsted acidic ionic liquids immobilized on Fe3O4 nanoparticles as heterogeneous catalysts are considered to be clean chemicals with marvellous properties over the traditional properties. There is an effort focused on designing acidic ILs to replace homogeneous and heterogeneous acids in some wide chemical applications.
2. Results and discussion
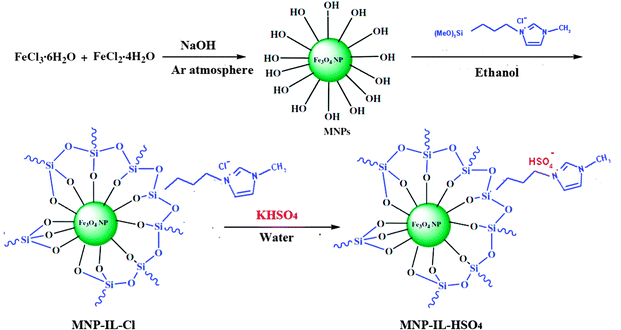
Our concept of supported acidic ionic liquid catalysis involves the surface of a support material that is modified with a monolayer of covalently attached ionic liquid moieties. Efforts to immobilize ILs on solid supports have already been reported, e.g., by Hölderich and co-workers.17 For our purpose, we have chosen the immobilisation of the Brønsted acidic ionic liquid 1-methyl-3-(3-trimethoxysilylpropyl) imidazolium hydrogen sulfate on magnetic Fe3O4 nanoparticles (MNPs-IL-HSO4). This is the first attempt to immobilise an ionic liquid with the HSO4− anion on a magnetic solid surface.2.1. Structural characterization of MNPs-IL-HSO4 as a catalyst
The magnetic nanoparticle supported hydrogen sulfate ionic liquid catalyst (MNPs-IL-HSO4) was prepared following the procedure shown in Scheme 2. 1-Methyl-3-(3-trimethoxysilylpropyl) imidazolium chloride (IL), was prepared from the reaction of N-methyl imidazole with (3-chloropropyl)trimethoxysilane. Then, the external surface of MNPs was coated with the IL to obtain MNPs-IL-Cl.18 In the second step, immobilized hydrogen sulfate ionic liquid on Fe3O4 nanoparticles was obtained by anion exchange in the presence of an excess amount of KHSO4. The characterization of the catalyst was carried out by FT-IR, EDX, SEM, elemental analysis, XRD, VSM, and TGA.The elemental analysis results showed that the carbon, hydrogen, nitrogen, and sulphur contents of MNPs-IL-HSO4 were 4.47, 1.42, 1.23, and 1.41 (wt%), respectively, equivalent to a loading of 0.44 mmol of hydrogen sulfate groups per gram of support, indicative of the successful functionalization of IL groups. As shown in Fig. 1(a), the SEM image shows that the Fe3O4 nanoparticles have a mean diameter of about 20 nm and a nearly spherical shape. The SEM image of MNPs-IL-HSO4 (Fig. 1(b)) confirmed that the catalyst was made up of uniform nanometer sized particles less than 70 nm in diameter.
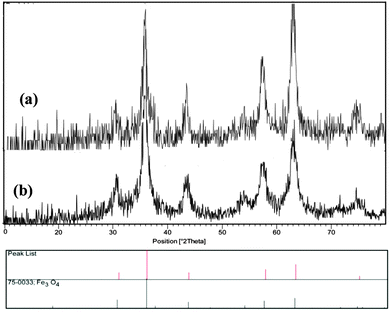
The positions and relative intensities of all the peaks in the XRD pattern of MNPs-IL-HSO4 conform well with the standard XRD pattern of Fe3O4 (Fig. 2), indicating retention of the crystalline cubic spinel structure during functionalization of the MNPs.17 It is implied that the resultant nanoparticles are pure Fe3O4 with a spinel structure and that the grafting process did not induce any phase change of the Fe3O4 nanoparticles.
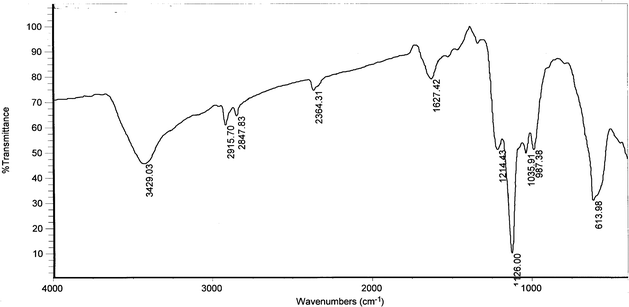
Successful functionalization of the MNPs can be inferred from FT-IR techniques. Fig. 3 shows the FT-IR pattern of the MNPs-IL-HSO4. The Fe–O stretching vibration near 614 cm−1, O–H stretching vibration near 3429 cm−1 and O–H deformation vibration near 1627 cm−1 were observed.17 The significant features observed in Fig. 3 are the appearance of the peaks at 1036 cm−1 (Si–O stretching), 2915 and 2848 cm−1 (–CH2 stretching) and at 1214 and 1126 cm−1 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching). These results provide evidence that IL moieties were successfully attached to the surface of the Fe3O4 nanoparticles.19
O stretching). These results provide evidence that IL moieties were successfully attached to the surface of the Fe3O4 nanoparticles.19
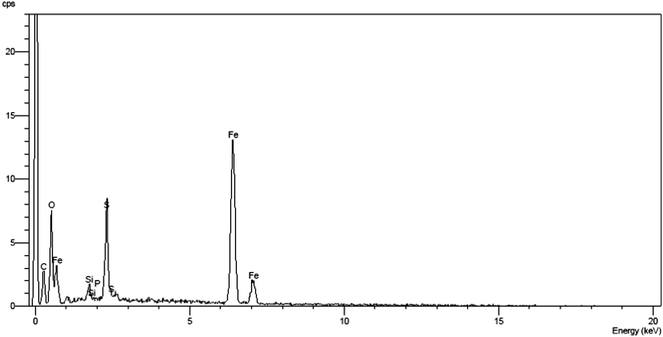
The components of the MNPs-IL-HSO4 were analysed by using energy dispersive spectroscopy (EDX). The EDX spectrum in Fig. 4 shows the elemental composition (S, Si and Fe) of the MNPs-IL-HSO4.
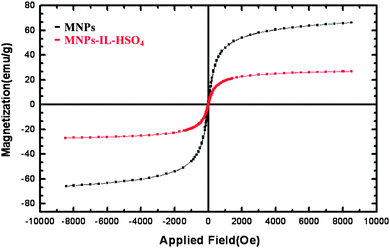
Fig. 5 presents the magnetization curves for the Fe3O4 nanoparticles and MNPs-IL-HSO4. Room temperature specific magnetization (M) versus applied magnetic field (H) curve measurements of the MNPs-IL-HSO4 sample indicate a saturation magnetization value (Ms) of 27.7 emu g−1, lower than that of the bare magnetic nanoparticles (66.6 emu g−1) due to the silica coating and the layer of grafted catalyst.17
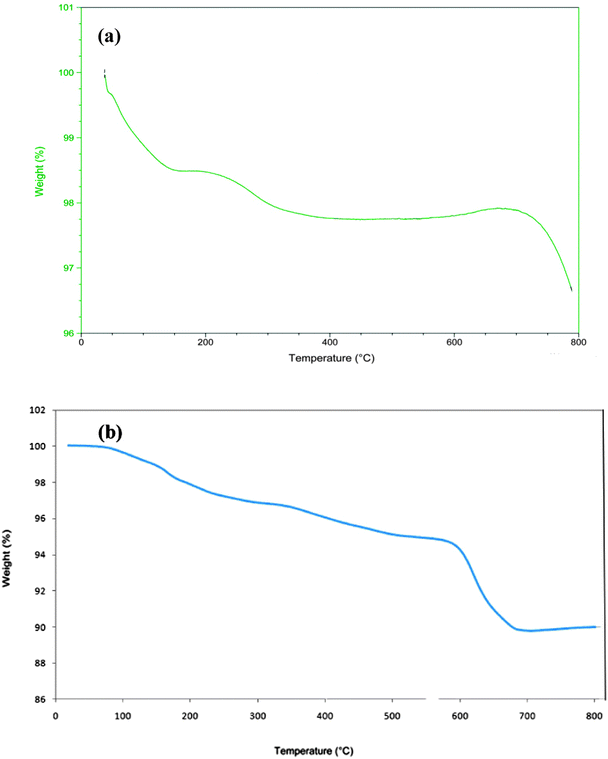
TGA was used to determine the percent of organic functional groups chemisorbed onto the surface of the magnetic nanoparticles. The TGA curve of the MNPs-IL-HSO4 shows a weight loss of about 10% from 200 to 680 °C, resulting from the decomposition of organic materials grafted to the MNP surface (Fig. 6). On the basis of this result, the good grafting of IL groups on the MNPs is verified.
2.2. Evaluation of the catalytic activity of MNPs-IL-HSO4 through the synthesis of dihydropyrimidinones (thiones)
In parallel to the development of ILs for catalytic applications, strategies for immobilizing ILs on a solid support have been addressed. IL-based heterogeneous catalyst systems offer several attractive advantages: (i) concomitant use of ILs, (ii) high catalytic activity owing to a uniform distribution of catalytic active species within the supported ILs and (iii) they can be easily separated from the reaction products for further reusability.20 Further, solvent-free conditions are especially important for providing an eco-friendly system. One advantage of solvent-free reactions, in comparison to reactions in solvents, is that the compounds formed are often pure enough to circumvent the need for purification using chromatography.21Although, some applications of magnetic catalysts for the Biginelli reactions are reported in the literature,22 to the best of our knowledge, there is no report focusing on the development of one pot Biginelli condensation under Brønsted acidic ionic liquid based magnetic nanoparticles as a highly effective catalytic system under solvent-free conditions.
Traditional conditions for the Biginelli reaction commonly require a large excess of one of the reagents, large amounts of catalyst, high temperatures, several hours of reaction, and occasionally the presence of a co-catalyst.22 In an attempt to find milder conditions, the synthesis of compound 4a was used as a model reaction. The reaction was carried out by heating a mixture of benzaldehyde, ethyl acetoacetate, and urea in the presence of MNPs-IL-HSO4. The efficiency of the reaction was affected by the amount of MNPs-IL-HSO4 (Table 1, entries 1–6). The optimal amount of the nanocatalyst was 0.05 g (entry 4). Larger amounts of the magnetic nanocatalyst were found to have an inhibitory effect on the formation of the product (entries 5 and 6). The catalytic activity of MNPs-IL-HSO4 was evident when trace product was obtained in the absence of the catalyst (entry 1). The results clearly show that Fe3O4 supported acidic ILs are effective magnetic catalysts for the syntheses of 3,4-dihydropyrimidin-2(1H)-ones. The use of acidic ionic liquid catalyst allows a clean conversion and the possibility of easy catalyst removal from the reaction mixture using an external magnet and several rounds of reuse.
| Entry | Catalyst (g) | Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | — | 120 | 180 | 20 |
| 2 | 0.02 | 100 | 30 | 50 |
| 3 | 0.04 | 100 | 30 | 75 |
| 4 | 0.05 | 100 | 30 | 95 |
| 5 | 0.06 | 100 | 30 | 95 |
| 6 | 0.07 | 100 | 30 | 93 |
| 7 | 0.05 | 25 | 30 | 10 |
| 8 | 0.05 | 40 | 30 | 45 |
| 9 | 0.05 | 60 | 30 | 65 |
| 10 | 0.05 | 80 | 30 | 80 |
| 11 | 0.05 | 110 | 30 | 94 |
| 12 | 0.05 | 120 | 40 | 94 |
| 13 | 0.05 (MNP) | 100 | 35 | 55 |
| 14 | 0.05 (MNPs-IL-Cl) | 100 | 35 | 70 |
| 15 | 0.05 (MNPs-IL-Cl) | 120 | 30 | 71 |
The same reaction in the presence of 0.05 g of MNPs-IL-HSO4 was carried out at different temperatures under solvent-free conditions to assess the effect of temperature on the reaction yield. The yield increased as the reaction temperature was raised. At 100 °C, product 4a was obtained in high yield within 30 min. A further increase in temperature and time did not improve the product yield (entries 11 and 12). Different molar ratios of reagents were examined and the best result was obtained with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 ratio of aldehyde, ethyl acetoacetate, and urea in the presence of 0.05 g of nanocatalyst.
1.5 ratio of aldehyde, ethyl acetoacetate, and urea in the presence of 0.05 g of nanocatalyst.
For showing the effect of the solvent, the same reaction was also carried out in different solvents, including EtOH, CHCl3, CH3CN, THF and H2O, in the presence of 0.05 g of MNPs-IL-HSO4. As shown in Table 2, it was found that ethanol was the optimum solvent, since in the cases of the other solvents, the reaction completed with lower yield. The non-occurrence of the reaction in other solvents may be due to the poor solubility of the products in these solvents and the low polarity of the solvents.23 There are also more efficient, as well as solvent-free conditions. Presently, organic reactions in solvent-free media have attracted the attention of researchers. Because of the toxicity of organic solvents, we considered green media. When the reactions were conducted in solvent-free conditions, the expected products were obtained with excellent yields in low reaction times.
| Solvent | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|
| EtOH | Reflux | 3 | 80 |
| CH3CN | Reflux | 5 | 50 |
| CHCl3 | Reflux | 7 | 35 |
| THF | Reflux | 8 | 20 |
| H2O | Reflux | 10 | 5 |
| Solvent-free | 100 | 0.5 | 95 |
In order to examine the substrate scope of this Biginelli reaction, various aromatic and heteroaromatic aldehydes with different substituents were used under the above-optimized reaction conditions. The results are shown in Table 3. From the results shown in Table 3, it could be seen that the reactions proceeded successfully and gave the expected products in good to high yields in short reaction times. The type of β-ketoester or aromatic aldehyde had no significant effect on the reaction time and yield.
| Entry | R | X | R1 | Product | Time (min) | Yield (%) | M.p. (°C) | TOFa (h−1) | |
|---|---|---|---|---|---|---|---|---|---|
| Found | Reported | ||||||||
| a TOF = turn over frequency = moles of substrate converted per mole of hydrogen sulfate per h. | |||||||||
| 1 | Ph | O | OEt | 4a | 30 | 95 | 205–206 | 204–20622 | 9500 |
| 2 | 3-Cl-C6H4 | O | OEt | 4b | 25 | 97 | 195–196 | 194–19624 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640 640 |
| 3 | 3-NO2-C6H4 | O | OEt | 4c | 30 | 97 | 222–224 | 222–22424 | 9700 |
| 4 | 2-Tiophen | O | OEt | 4d | 25 | 98 | 215–217 | 216–21825 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
| 5 | 2-F-C6H4 | O | OEt | 4e | 35 | 92 | 239–241 | 240–24126 | 7885 |
| 6 | 4-OMe-C6H4 | S | OEt | 4f | 40 | 90 | 151–152 | 150–15222 | 6750 |
| 7 | Ph | S | OEt | 4g | 35 | 96 | 205–206 | 206–20722 | 8228 |
| 8 | 2-Tiophen | S | OEt | 4h | 25 | 95 | 215–217 | 215–21627 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| 9 | Ph | O | Me | 4i | 30 | 95 | 213–214 | 212–21522 | 9500 |
| 10 | Ph | S | Me | 4j | 30 | 93 | 220–221 | 220–222b22 | 9300 |
| 11 | 4-OMe-C6H4 | S | Me | 4k | 35 | 92 | 169–170 | 168–17022 | 7885 |
| 12 | 3-NO2-C6H4 | S | Me | 4l | 35 | 96 | 210–212 | 210–212b22 | 8228 |
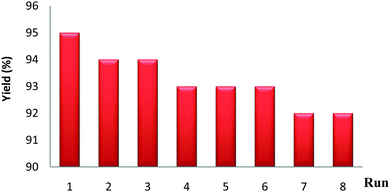
The nanocatalyst was recovered from the reaction mixture by using a simple bar magnet and it was washed with ethyl acetate and acetone, oven dried and then directly used for subsequent experiments under similar reaction conditions. As shown in Fig. 7, the catalyst could be reused at least 8 times with only a slight reduction in activity.
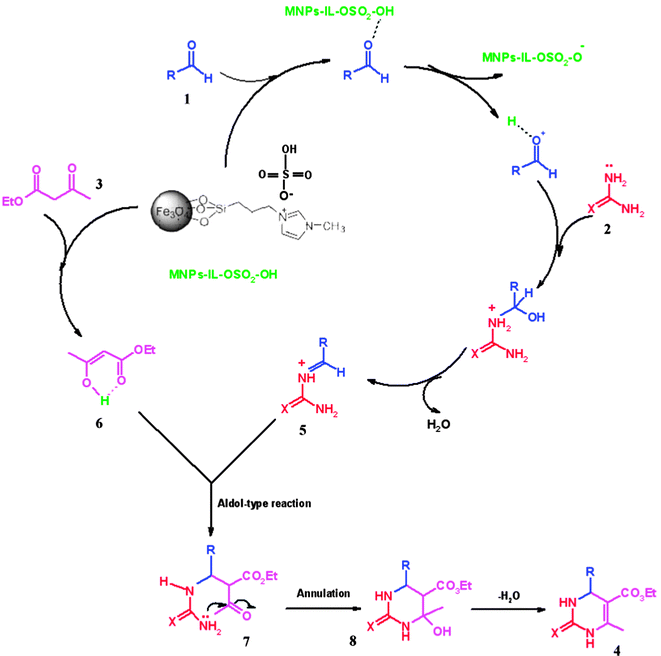
The Biginelli mechanism is still under debate, with no consensus reached about the preferred pathway. Three mechanisms have been proposed for the Biginelli reaction, including iminium, enamine, and Knoevenagel mechanisms. A reasonable mechanism in the presence of IL catalyst is proposed by Neto et al. The MNPs-IL-HSO4 as a Brønsted acidic catalyst participate in the reaction by activating the aldehyde (1). This is followed by nucleophilic addition of urea (2) or thiourea forming the intermediate (5). Then, this intermediate interacts with ethyl acetoacetate enolate (6) to produce an open chain intermediate ureide (7), which is followed by cyclization and dehydration to afford dihydropyrimidinones (thiones) (Scheme 3).28
It can be supposed that the catalytically active species is the hydrogen sulfate, as it is an acidic site. Also, another possible reason may be that the strength of the anion–cation interaction inside the ILs is less than the electrostatic interaction between the HSO4− anion and the imidazolium cation.29,30 For confirmation, we compared the catalytic activities of MNPs-IL-Cl and MNPs-IL-HSO4 (Table 1, entries 4 and 14). These experiments showed that the structure of the cationic species is important. Therefore, MNPs-IL-HSO4 is a better Brønsted and Lewis acid in this reaction and exhibited the highest catalytic activity among the catalysts employed. It exhibits great potential in replacing conventional homogenous and heterogeneous acidic catalysts because of its advantages, such as uniform acid sites, stability in different reaction conditions, and easy separation and reusability, as well as its flexibility and non-volatility.
3. Conclusions
In summary, we have developed an efficient procedure for the synthesis of dihydropyrimidinones (thiones). This involves the use of MNPs-IL-HSO4, where an ionic liquid is “grafted” onto the surface of a magnetic support as an inexpensive, stable and easily available Brønsted acidic catalyst. The use of a powerful, easily accessible and recyclable solid catalyst makes this method quite simple and convenient. The high yield of Biginelli compounds (90–98%), the use of relatively inexpensive nanocatalyst and the straightforward isolation of the products are the advantages of this protocol over the other procedures reported. The utilisation of solid acids is preferred in research laboratories and the chemical industry because they are nonvolatile and less noxious than traditional liquid mineral acids, which can also corrode the equipment.4. Experimental
4.1. Chemicals and apparatus
Chemical reagents in high purity were purchased from Merck and Aldrich and were used without further purification. Melting points were determined in open capillaries using an Electrothermal Mk3 apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded with a Bruker DRX-400 spectrometer at 400 and 100 MHz, respectively. FT-IR spectra were obtained with potassium bromide pellets in the range 400–4000 cm−1 with a Perkin-Elmer 550 spectrometer. The element analyses (C, H, N) were obtained from a Carlo ERBA Model EA 1108 analyzer carried out on a Perkin-Elmer 240c analyzer. Nanostructures were characterized using a Holland Philips Xpert X-ray powder diffraction (XRD) diffractometer (CuK radiation, λ = 0.154056 nm), at a scanning speed of 2° min−1 from 10° to 100° (2θ). Scanning electron microscopy (SEM) was performed on a FEI Quanta 200 scanning electron microscope operated at a 20 kV accelerating voltage. The samples for SEM were prepared by spreading a small drop containing nanoparticles onto a silicon wafer and drying almost completely in air at room temperature for 2 h, and then were transferred onto SEM conductive tapes. The transferred sample was coated with a thin layer of gold before measurement. The purity of the compounds synthesized was monitored by TLC, visualizing with ultraviolet light. The products were characterized by comparison of their spectral (1H NMR, and 13C NMR) and physical data with those of authentic samples. All yields refer to isolated products after purification.4.2. Preparation of catalyst
Fe3O4-MNPs were prepared using chemical coprecipitation as described in the literature.17 Then, 1-methyl-3-(3-trimethoxysilylpropyl) imidazolium chloride (IL), was prepared from the reaction of N-methyl imidazole with (3-chloropropyl)trimethoxysilane at 80 °C.17 Then, chloride ionic liquid immobilized on magnetic nanoparticles and an excess amount of KHSO4 were added into deionized water and stirred for 24 h at room temperature. NaCl, which was prepared during the exchange of chloride anions with HSO4, was removed by washing with deionized water. Hydrogen sulfate ionic liquid immobilized on Fe3O4 nanoparticles (MNPs-IL-HSO4) was obtained as a brownish black powder.4.3. General procedure of Biginelli reactions
A mixture of aromatic aldehyde (2.0 mmol), ethyl acetoacetate/acetylacetone (2 mmol), and urea/thiourea (3 mmol) was stirred at 100 °C utilizing MNPs-IL-HSO4 (0.05 g) for the appropriate time until the reaction was complete. The reaction was monitored by thin layer chromatography (TLC) [6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 hexane
4 hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate]. After the completion of the reaction, the reaction mixture was cooled and washed with ice cooled water. The resultant solid was dissolved in ethyl acetate and the catalyst was separated by an external magnet and reused as such for the next experiment. Organic solvent was evaporated under reduced pressure and the resulting solid product was then crystallized from hot ethanol.
ethyl acetate]. After the completion of the reaction, the reaction mixture was cooled and washed with ice cooled water. The resultant solid was dissolved in ethyl acetate and the catalyst was separated by an external magnet and reused as such for the next experiment. Organic solvent was evaporated under reduced pressure and the resulting solid product was then crystallized from hot ethanol.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of the University of Kashan for supporting this work by Grant NO. 256722/X.References
- A. Loupy, C. R. Chim., 2004, 7, 103–112 CrossRef CAS PubMed.
- R. B. Nasir Baig and R. S. Varma, Green Chem., 2013, 15, 1839–1843 RSC.
- (a) J. Miao, H. Wan and G. Guan, Catal. Commun., 2011, 12, 353–356 CrossRef CAS PubMed; (b) J. Safari and Z. Zarnegar, Monatsh. Chem., 2013, 144, 1389–1396 CrossRef CAS PubMed; (c) S. Sahoo, P. Kumar, F. Lefebvre and S. B. Halligudi, Appl. Catal., A, 2009, 354, 17–25 CrossRef CAS PubMed; (d) L. L. Zhu, Y. H. Liu and J. Chen, Ind. Eng. Chem. Res., 2009, 48, 3261–3267 CrossRef CAS.
- (a) C. Yuan, Z. Huang and J. Chen, Catal. Commun., 2012, 24, 56–60 CrossRef CAS PubMed; (b) J. Safari and Z. Zarnegar, C. R. Chim., 2013, 16, 821–828 CrossRef CAS PubMed.
- (a) X. Zheng, S. Luo, L. Zhang and J. P. Cheng, Green Chem., 2009, 11, 455–458 RSC; (b) Y. Jiang, C. Guo, H. Xia, I. Mahmood, C. Liu and H. Liu, J. Mol. Catal. B: Enzym., 2009, 58, 103–109 CrossRef CAS PubMed.
- (a) H. Song, Z. Li, J. Chen and C. Xia, Catal. Lett., 2012, 142, 81–86 CrossRef CAS PubMed; (b) D. Fang, J. Yang and C. Jiao, ACS Catal., 2011, 1, 42–47 CrossRef CAS; (c) R. Kore, T. J. D. Kumar and R. Srivastava, J. Mol. Catal. A: Chem., 2012, 360, 61–70 CrossRef CAS PubMed; (d) A. Chrobok, S. Baj, W. Pudło and A. Jarzebski, Appl. Catal., A, 2009, 366, 22–28 CrossRef CAS PubMed; (e) W. Chen, Y. Y. Zhang, L. B. Zhu, J. B. Lan, R. G. Xie and J. S. You, J. Am. Chem. Soc., 2007, 129, 13879–13886 CrossRef CAS PubMed.
- W. Keim, W. Korth and P. Wasserscheid, WIPO Pat., WO/2000/016902, 2000 Search PubMed.
- P. Wasserscheid, M. Sesing and W. Korth, Green Chem., 2002, 4, 134–138 RSC.
- (a) N. Gupta, Sonu, G. L. Kad and J. Singh, Catal. Commun., 2007, 8, 1323–1328 CrossRef CAS PubMed; (b) A. R. Hajipour, A. Rajaei and A. E. Ruoho, Tetrahedron Lett., 2009, 50, 708–711 CrossRef CAS PubMed; (c) A. R. Khosropour, Can. J. Chem., 2008, 86, 264–269 CrossRef CAS; (d) D. Q. Xu, W. L. Yang, S. P. Luo, B. T. Wang, M. Wu and Z. Y. Xu, Eur. J. Org. Chem., 2007, 1007–1012 CrossRef CAS; (e) W. Wang, W. Cheng, L. Shao and J. Yang, Catal. Lett., 2008, 121, 77–80 CrossRef CAS; (f) Y. Du, F. Tian and W. Zhao, Synth. Commun., 2006, 36, 1661–1669 CrossRef CAS.
- (a) K. Qiao, H. Hagiwara and C. Yokoyama, J. Mol. Catal. A: Chem., 2006, 246, 65–69 CrossRef CAS PubMed; (b) R. Sugimura, K. Qiao, D. Tomida and C. Yokoyama, Catal. Commun., 2007, 8, 770–772 CrossRef CAS PubMed.
- (a) K. S. Atwal, G. C. Rovnyak, B. C. O’Reilly and J. Schwartz, J. Org. Chem., 1989, 54, 5898–5907 CrossRef CAS; (b) C. O. Kappe, W. M. F. Fabian and M. A. Semones, Tetrahedron, 1997, 53, 2803–2816 CrossRef CAS; (c) C. Blackburn, B. Guan, J. Brown, C. Cullis, S. M. Condon, T. J. Jenkins, S. Peluso, Y. Ye, R. E. Gimeno, S. Punreddy, Y. Sun, H. Wu, B. Hubbard, V. Kaushik, P. Tummino, P. Sanchetti, D. Yu Sun, T. Daniels, E. Tozzo, S. K. Balanic and P. Raman, Bioorg. Med. Chem. Lett., 2006, 16, 3504–3509 CrossRef CAS PubMed.
- (a) K. S. Atwal, B. N. Swanson, S. E. Unger, D. M. Floyd, S. Moreland, A. Hedberg and B. C. O. Reilly, J. Med. Chem., 1991, 34, 806–811 CrossRef CAS; (b) C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043–1052 CrossRef CAS.
- E. W. Hurst, Ann. N. Y. Acad. Sci., 1962, 98, 275–286 CrossRef CAS.
- (a) Z. Maliga, T. M. Kapoor and T. J. Mitchison, Chem. Biol., 2002, 9, 989–996 CrossRef CAS; (b) S. Debonis, J. P. Simorre, I. Crevel, L. Lebeau, D. A. Skoufias, A. Blangy, C. Ebel, P. Gans, R. Cross, D. D. Hackney, R. H. Wade and F. Kozielski, Biochem., 2003, 42, 338–349 CrossRef CAS PubMed.
- (a) P. Biginelli, Ber. Dtsch. Chem. Ges., 1891, 24, 1317–1319 CrossRef; (b) P. Biginelli, Ber. Dtsch. Chem. Ges., 1891, 24, 2962–2967 CrossRef.
- R. Fazaeli, S. Tangestaninejad, H. Aliyan and M. Moghadam, Appl. Catal., A, 2006, 309, 44–51 CrossRef CAS PubMed.
- M. H. Valkenberg, C. deCastro and W. F. Hölderich, Green Chem., 2002, 4, 88–93 RSC.
- J. Safari and Z. Zarnegar, C. R. Chim., 2013, 16, 920–928 CrossRef CAS PubMed.
- J. Safari and Z. Zarnegar, Ultrason. Sonochem., 2013, 20, 740–746 CrossRef CAS PubMed.
- (a) Y. A. Elsheikh, Z. Man, M. A. Bustam, S. Yusup and C. D. Wilfred, Energy Convers. Manage., 2011, 52, 804–809 CrossRef CAS PubMed; (b) T. Selvam, A. Machoke and W. Schwieger, Appl. Catal., A, 2012, 445–446, 92–101 CrossRef CAS PubMed.
- (a) M. Moghaddasi, A. Davoodniai, M. M. Heravi and N. T. Hoseini, Chin. J. Catal., 2012, 33, 706–710 CrossRef; (b) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machad, Chem. Rev., 2009, 109, 4140–4182 CrossRef CAS PubMed.
- (a) J. Mondal, T. Sen and A. Bhaumik, Dalton Trans., 2012, 41, 6173–6181 RSC; (b) M. Nasr-Esfahani, S. J. Hoseini and F. Mohammadi, Chin. J. Catal., 2011, 32, 1484–1489 CrossRef CAS.
- J. H. Clark, D. J. Macquarrie and J. Sherwood, Chem.–Eur. J., 2013, 19, 5174–5182 CrossRef CAS PubMed.
- A. Kuraitheerthakumaran, S. Pazhamalai and M. Gopalakrishnan, Arabian J. Chem., 2011 DOI:10.1016/j.arabjc.2011.06.005.
- J. Azizian, A. A. Mohammadi, A. R. Karimi and M. R. Mohammadizadeh, Appl. Catal., A, 2006, 300, 85–88 CrossRef CAS PubMed.
- H. X. Lin, Q. J. Zhao, B. Xu and X. H. Wang, Chin. Chem. Lett., 2007, 18, 502–504 CrossRef CAS PubMed.
- N. Y. Fu, Y. F. Yuan, Z. Cao, S. W. Wang, J. T. Wang and C. Peppe, Tetrahedron, 2002, 58, 4801–4807 CrossRef CAS.
- L. M. Ramos, B. C. Guido, C. C. Nobrega, J. R. Corrêa, R. G. Silva, H. C. B. de Oliveira, A. F. Gomes, F. C. Gozzo and B. A. D. Neto, Chem.–Eur. J., 2013, 19, 4156–4168 CrossRef CAS PubMed.
- W. Cheng, X. Chen, J. Sun, J. Wang and S. Zhang, Catal. Today, 2013, 200, 117–124 CrossRef CAS PubMed.
- H. O. Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1–2 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |