A generalized strategy for controlled synthesis of ternary metal sulfide nanocrystals†
Manjiao
Deng‡
a,
Shuling
Shen‡
b,
Yejun
Zhang
a,
Huarui
Xu
c and
Qiangbin
Wang
*a
aDivision of Nanobiomedicine and i-Lab, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, 215123, P. R. China. E-mail: qbwang2008@sinano.ac.cn; Fax: +86-512-62872620; Tel: +86-512-62872620
bSchool of Materials Science and Engineering, University of Shanghai for Science and Technology, Shanghai, 200093, P. R. China
cSchool of Material Science and Engineering, Guilin University of Electronic Technology, Guilin 541004, P. R. China
First published on 3rd October 2013
Abstract
Ternary metal sulfide nanocrystals (TMS NCs) have drawn intense attention for their wide applications in photovoltaics and nanophotonics, etc. However, a facile and general method for controlled synthesis of TMS NCs with uniform size and pure crystal phase is yet to be realized. Here we report a simple and versatile one-pot method for preparing high quality TMS NCs with controlled morphologies, sizes, crystalline structures and compositions, including orthorhombic Cu3BiS3 nanosheets and nanoparticles, orthorhombic Cu4Bi4S9 nanowires and nanoribbons, wurtzite CuInS2 nanopencils, cubic AgBiS2 nanocubes, orthorhombic Ag8SnS6 nanoparticles, and orthorhombic Cu3SnS4 nanorods, based on the co-thermal decomposition of metal-diethyl dithiocarbamate (metal-DDTC) precursors. We expect that this methodology will be broadly applicable for synthesizing new metal sulfide NCs and play an important role in exploring their new properties for various applications.
1. Introduction
Ternary metal sulfide nanocrystals (TMS NCs), with unique physical and chemical properties such as high optical absorption coefficients and appropriate direct band gaps, have attracted extensive attention in recent studies due to their potential applications in photovoltaics, linear and nonlinear optical devices, superconducting materials, fluorescent materials, photocatalysts, etc.1 It is notable that these unique properties, which are distinct from their bulk materials, are size, shape, crystal structure and composition dependent.1e,2 Therefore, preparation of TMS NCs with fine control over their size, shape, crystal structure and composition is very important for their applications.In recent years, much effort has been afforded to the controlled synthesis of TMS NCs, such as CuInS2, AgInS2 and AgInSe2 NCs, etc., as effective alternative materials in photovoltaic, photoelectric and other optical devices.3–7 A variety of synthetic strategies have been developed. For instance, Qian and co-workers successfully obtained CuMS2 and AgMS2 (M = In or Ga) NCs by using a solvothermal method and synthesized M–Bi–S (M = Cu or Ag) dendrites by a microwave irradiation method.8 Li et al. put forward a facile one-pot route to prepare TMS NCs such as AgInS2 and CuInS2 by employing metal salts and S powders as the reactants.5b However, the versatility of current synthetic methods for TMS NCs is very limited, and a facile and general strategy to synthesize high quality TMS NCs is highly desirable.
The single-molecular precursor method, pioneered and popularized by O'Brien and later extensively investigated by Vittal, has been widely used for the synthesis of metal sulfides in the last two decades.9 For example, binary CdS and ZnS NCs were obtained by thermo-decomposing Cd[S2CN(C2H5)2]29a and Zn[S2CNMe(C6H13)]29d as precursors in the presence of trioctylphosphine (TOP)/trioctylphosphine oxide (TOPO) at high temperature, respectively. Ternary AgInS2 NCs were successfully obtained by employing [(Ph3P)2AgIn(SCOPh)4] as precursor.9e In these studies, phosphorous surfactants such as TOP/TOPO or phosphorous precursors were usually employed to obtain crystalline, defect-free metal sulfide particles. Recently, a metal-diethyl dithiocarbamate (metal-DDTC)-based phosphorus-free single-source precursor method has been demonstrated as a simple, low-cost, and highly efficient method to synthesize binary metal sulfide NCs with great control over the product morphology and size.10,11
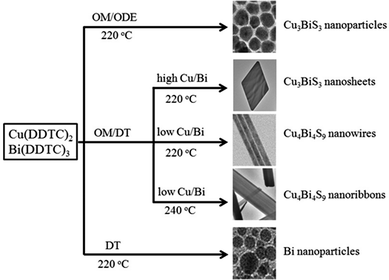
Herein, we report a one-pot approach with metal-DDTCs as precursors to synthesize a wide variety of high quality TMS NCs with controlled size, morphology and structure. Orthorhombic single-crystalline Cu3BiS3 nanosheets, Cu3BiS3 nanoparticles, Cu4Bi4S9 nanowires and Cu4Bi4S9 nanoribbons were selectively synthesized in a one-pot reaction by using Cu-DDTC and Bi-DDTC as reactants and oleylamine (OM) and 1-dodecanethiol (DT) as surfactants. Furthermore, a collection of other TMS NCs including wurtzite CuInS2 nanopencils, cubic AgBiS2 nanocubes, orthorhombic Ag8SnS6 nanoparticles, orthorhombic Cu3SnS4 nanorods, etc., were also obtained using this facile methodology. We expect that this facile and versatile strategy is widely applicable in preparing more multinary semiconductor NCs with desirable morphologies, compositions and properties, and thus benefits their applications.
2. Experimental details
2.1. Chemicals
Sodium diethyl dithiocarbamate trihydrate (Na(DDTC)·3H2O, 99%, Sinopharm Chemical Reagent Company), oleylamine (OM, 83–90%), oleic acid (OA, 90%, Aldrich), octadecylamine (ODA, 90%, Fluka), 1-octadecene (ODE, 90%, Aldrich), 1-dodecanethiol (DT, 98%, Aladdin), ethanol (AR) and cyclohexane (AR) (Sinopharm Chemical Reagent Company) were all of analytical grade and used as received without further purification.Taking the synthesis of Cu3BiS3 nanosheets as an example, the typical single-source precursor one-pot method following our previously reported procedure is described as follows.
2.2. Synthesis of Cu(DDTC)2 and Bi(DDTC)3 precursors
For synthesizing Cu(DDTC)2, 10 mmol of Cu(NO3)2 was dissolved in 80 mL of water. Then 80 mL aqueous solution of Na(DDTC)·3H2O (20 mmol) was dropped into the Cu(NO3)2 solution under continuous magnetic stirring. After stirring for 30 min, the brown precipitate was kept stationary under ambient conditions for 4 h. The resulting precipitate was filtered and washed four times with pure water and dried in an oven at 60 °C. Bi(DDTC)3 was also prepared by the same method as described above, except the molar ratio of Bi(NO3)3 to Na(DDTC)·3H2O was 1 to 3. The other precursors were prepared by the same method as depicted above and the molar ratio of the metal salt and Na(DDTC)·3H2O was determined by the stoichiometry of the metal and DDTC in the metal-DDTC compounds.2.3. Synthesis of Cu3BiS3 nanosheets
In a typical procedure, 0.054 g of Cu(DDTC)2 and 0.0327 g of Bi(DDTC)3 were added into a mixture of 20 mmol of OM and 20 mmol of DT in a three-necked flask (100 mL) at room temperature. The slurry was degassed for 15 min at room temperature. Then the solution was heated at a rate of 10 °C min−1 and held at 220 °C for one hour under nitrogen. The final solution was cooled down to room temperature naturally. Excess ethanol was poured into the solution and the resultant mixture was centrifugally separated. The precipitates were washed with ethanol twice and the final products were dispersed in cyclohexane. Other experiments were carried out following similar procedures and the detailed experimental conditions are listed in Table 1. By using this simple single-source precursor method, a collection of high quality TMS NCs with high yields above 95% was achieved as listed in Table 1.| Samples | Precursor 1 | Precursor 2 | T (°C) | OM (mmol) | DT (mmol) | ODE (mmol) | Yield (%) |
|---|---|---|---|---|---|---|---|
| Cu3BiS3 nanosheets | 0.15 mmol Cu(DDTC)2 | 0.05 mmol Bi(DDTC)3 | 220 | 20 | 20 | — | ∼100 |
| Cu3BiS3 nanoparticles | 0.15 mmol Cu(DDTC)2 | 0.05 mmol Bi(DDTC)3 | 220 | 20 | — | 20 | ∼95 |
| Cu4Bi4S9 nanowires | 0.05 mmol Cu(DDTC)2 | 0.05 mmol Bi(DDTC)3 | 220 | 20 | 20 | — | ∼100 |
| Cu4Bi4S9 nanoribbons | 0.05 mmol Cu(DDTC)2 | 0.05 mmol Bi(DDTC)3 | 240 | 20 | 20 | — | ∼96 |
| CuInS2 nanopencils | 0.05 mmol Cu(DDTC)2 | 0.05 mmol In(DDTC)3 | 240 | — | 40 | — | ∼100 |
| AgBiS2 nanoparticles | 0.05 mmol Ag(DDTC) | 0.05 mmol Bi(DDTC)3 | 240 | —/20 ODA | — | 20 | ∼98 |
| Cu3SnS4 nanorods | 0.15 mmol Cu(DDTC)2 | 0.05 mmol Sn(DDTC)4 | 200 | 20 | 60 | — | ∼98 |
| Ag8SnS6 nanoparticles | 0.40 mmol Ag(DDTC) | 0.05 mmol Sn(DDTC)2Phen | 220 | 20 | 20 | — | ∼100 |
2.4. Characterization methods
The size, shape and chemical composition of the products were examined by scanning electron microscopy (SEM, Quanta 400FEG, FEI, USA) and Tecnai G2 F20 S-Twin transmission electron microscopy (TEM, FEI, USA) operated at 200 kV and equipped with an X-ray energy dispersive spectrometer (EDS). The TEM samples were prepared by dropping 1 or 2 droplets of the cyclohexane solution onto a carbon-coated Cu grid, and the solvent was evaporated at room temperature. The crystalline structures of the ternary metal sulfides were recorded on an X'Pert-Pro MPD diffractometer (Holland) with a slit of 1/2° at a scanning rate of 4° min−1, using Cu Kα radiation (λ = 1.5406 Å). The UV-vis absorption spectra were obtained on a Lambda 25 UV-vis-NIR spectrometer (Perkin Elmer, USA) at room temperature. Diffuse reflectance measurements were performed on a Perkin-Elmer Lambda 750 equipped with a 60 mm integrating sphere in the range of 400–1800 nm.3. Results and discussions
First, the controlled synthesis of Cu–Bi–S TMS NCs was systematically investigated. With Cu(DDTC)2 and Bi(DDTC)3 as precursors and a mixture of DT and OM as solvent (for experimental details, please see Experimental section), well-defined Cu3BiS3 nanosheets were obtained in high yield, as shown in Fig. 1a. The typical scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images in Fig. 1a exhibit the rhombic nanosheet morphology of Cu3BiS3 with nanosheets that are 1.3 μm in length. The thickness of the sheets is around 4 nm based on the atomic force microscopy (AFM) images (Fig. S1a, ESI†). The high resolution TEM (HRTEM) image in Fig. 1b reveals the well-crystalline structure of the nanosheets with an interplanar distance of 0.25 nm, corresponding to the (040) plane of orthorhombic Cu3BiS3, implying the preferential growth direction of the Cu3BiS3 nanosheets along the 〈010〉 direction. Moreover, the selected area electron diffraction pattern (SAED) in the inset of Fig. 1b reveals the single-crystalline nature of the as-prepared Cu3BiS3 nanosheets. | ||
| Fig. 1 SEM image (inset: TEM image) (a), HRTEM image (inset: SAED pattern) (b), XRD pattern (c) and diffuse reflectance spectrum (inset: [F(R)hν]2vs. energy) (d) of Cu3BiS3 nanosheets. | ||
The crystal structure of the nanosheets is further characterized by the X-ray diffraction pattern (XRD) in Fig. 1c. It clearly shows that the obtained nanosheets can be indexed to the orthorhombic structure (JCPDS card No. 71-2115, space group P212121 with the lattice constants a = 7.723 Å, b = 10.395 Å, c = 6.716 Å). The X-ray energy dispersive spectroscopy (EDS) pattern (Fig. S1b, ESI†) also indicates that the three elements of Cu, Bi, and S exist with the atomic ratio of Cu to Bi to S as 3.19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.42. Moreover, in attempting to investigate the optical properties of the Cu3BiS3 nanosheets, diffuse reflectance spectroscopy was performed, as shown in Fig. 1d, indicating the onset of absorption around 800 nm. The direct band gap is estimated by performing Kubelka–Munk transformations.12 A plot of [F(R)hν]2versus energy (inset of Fig. 1d) yields a direct band gap of 1.24 eV.
3.42. Moreover, in attempting to investigate the optical properties of the Cu3BiS3 nanosheets, diffuse reflectance spectroscopy was performed, as shown in Fig. 1d, indicating the onset of absorption around 800 nm. The direct band gap is estimated by performing Kubelka–Munk transformations.12 A plot of [F(R)hν]2versus energy (inset of Fig. 1d) yields a direct band gap of 1.24 eV.
Capping ligands, which adjust the growth kinetics of nanocrystals due to their selective binding affinity to various crystal facets, have been reported to play an important role in the growth of nanocrystals.13 With OM as the sole capping ligand, monodisperse Cu3BiS3 nanoparticles with a diameter of 25 nm were obtained, as shown in Fig. 2a. The HRTEM image in Fig. 2b presents the interplanar distance of 0.28 nm, which is well-matched to the (131) plane of orthorhombic Cu3BiS3, as confirmed by the X-ray diffraction pattern (Fig. 2c). All the diffraction peaks in the XRD pattern can be indexed to the standard orthorhombic Cu3BiS3 crystal structure (JCPDS card No. 71-2115), and no other impurities such as Bi, Bi2S3, or Cu2S are found. The optical properties of the Cu3BiS3 nanoparticles were characterized by diffuse reflectance spectroscopy, as shown in Fig. 2d. The direct band gap was estimated to be 1.35 eV by performing Kubelka–Munk transformations (inset of Fig. 2d).
 | ||
| Fig. 2 TEM (a) and HRTEM (b) images, XRD pattern (c) and UV-vis spectrum (d) of Cu3BiS3 nanoparticles. | ||
Besides the capping ligand, which can affect the morphology of NCs, the precursor concentration is also a decisive factor in determining the morphology and phase structure of the products.4i,5a,b,d By decreasing the precursor molar ratio of Cu(DDTC)2 to Bi(DDTC)3, while keeping other reaction conditions the same as for the Cu3BiS3 nanosheets, Cu3BiS3 nanowires are produced. As shown in the SEM and TEM images in Fig. 3a, uniform nanowires with diameters of about 20 nm are observed. The HRTEM image in Fig. 3b shows the obvious lattice fringes and the growth direction of the nanowires along the (002) facet. Fig. 3c confirms that the as-prepared sample is orthorhombic Cu4Bi4S9 (JCPDS card No. 77-1238, Pnam, with the lattice constants a = 31.68 Å, b = 11.659 Å, c = 3.972 Å). The EDS data (Fig. S2, ESI†) reveal that the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi
Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio of the product is 1
S ratio of the product is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.14
1.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.43, in line with the ratio of 4
2.43, in line with the ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 in Cu4Bi4S9 NCs. The optical properties of the nanowires were characterized by diffuse reflectance spectroscopy (Fig. 3d), and a direct band gap of 0.9 eV was determined, consistent with the reported band gap energy of Cu4Bi4S9 nanowires.14 In addition, by increasing the reaction temperature from 220 °C to 240 °C, Cu4Bi4S9 nanoribbons were obtained (Fig. S3, ESI†).
9 in Cu4Bi4S9 NCs. The optical properties of the nanowires were characterized by diffuse reflectance spectroscopy (Fig. 3d), and a direct band gap of 0.9 eV was determined, consistent with the reported band gap energy of Cu4Bi4S9 nanowires.14 In addition, by increasing the reaction temperature from 220 °C to 240 °C, Cu4Bi4S9 nanoribbons were obtained (Fig. S3, ESI†).
 | ||
| Fig. 3 SEM image (inset: TEM image) (a), HRTEM image (b), XRD pattern (c) and diffuse reflectance spectrum (inset: [F(R)hν]2vs. energy) (d) of Cu4Bi4S9 nanowires. | ||
The relationship between the reaction conditions and morphology and phase structure of the as-prepared Cu–Bi–S samples is summarized in Fig. 4. Various morphologies and phases are obtained by simply tuning the capping ligands, the molar ratio of the precursors and the reaction temperature. As shown in Fig. 4, when OM acts as the capping ligand and ODE as the solvent, Cu3BiS3 nanoparticles are synthesized. If DT is added as a co-capping ligand with OM, while keeping the same reaction temperature, different Cu–Bi–S alloyed compounds (Cu3BiS3 nanosheets and Cu4Bi4S9 nanowires) are produced, which are the results of the synergy effect of the capping ligands.9f It is known that the crystal formation is determined by the kinetic and thermodynamic growth regimes,15 in which the precursor concentration and temperature determine the morphology and phase structure of the product. In this way, orthorhombic Cu3BiS3 nanosheets and Cu4Bi4S9 nanowires can be obtained by changing the molar ratio of Cu and Bi precursors. When the reaction temperature was elevated to 240 °C, the morphology of the Cu4Bi4S9 changed from nanowires to nanoribbons (Fig. S3, ESI†). With DT as the sole capping agent, Bi nanoparticles are synthesized (Fig. S4, ESI†). In light of Wu's work,16 a redox reaction via a radical process of bismuth thiolate is undergone at 90–150 °C. In this study, the Bi(DDTC)3 precursor was easily reduced by the thiolate anion (SR−) provided by DT at 220 °C, which induced the preferential formation of Bi nanoparticles.
As illustrated in Fig. 4, the morphology and the composition of the product can be finely controlled by tuning the capping ligand and the precursor concentration during the thermo-decomposition of the single source precursors. The versatility of this facile strategy is further extended to synthesize other TMS NCs, including CuInS2 nanopencils, AgBiS2 nanoparticles, Ag8SnS6 nanoparticles, and Cu3SnS4 nanorods.
CuInS2 nanopencils are prepared by using Cu(DDTC)2 and In(DDTC)3 as precursors and DT as the capping ligand. These CuInS2 nanopencils are more uniform and highly crystalline compared with the CuInS2 nanorods obtained by thermolysis of CuAc and In(Ac)3 in DT and ODE under similar conditions.17Fig. 5a represents the monodisperse CuInS2 nanopencils, which are ∼10 nm in diameter and ∼55 nm in length. The HRTEM image further indicates the well-crystalline nature of the CuInS2 nanopencils with an interplanar distance of 0.32 nm, which is consistent with the (002) plane of wurtzite CuInS2, indicating the growth direction of CuInS2 nanopencils along the (001) direction. Fig. 5c shows that the XRD pattern of the CuInS2 nanopencils is in line with wurtzite CuInS2.4h,k Furthermore, the optical properties of the CuInS2 nanopencils were investigated as shown in Fig. 5d, which presents strong absorption in the visible light regime, making it promising in photovoltaic devices.18 By replacing Cu(DDTC)2 with Cu(DDTC)2Phen and keeping the other conditions unchanged, tadpole-like CuInS2 nanocrystals with an average length of ∼140 nm and width of ∼25 nm are obtained (Fig. S5, ESI†). Elongated CuInS2 nanocrystals in the presence of alkanethiols have also been observed in the case of nanocrystalline CuGaS219 and Cu2ZnSnS4.20
Similarly, AgBiS2 nanocubes with edges that are 7 nm long are obtained with Bi(DDTC)3 and Ag(DDTC) as precursors in a mixture of ODA and ODE at 240 °C (Fig. 6). The typical TEM and HRTEM images (Fig. 6a and b) present the cubic morphology of the AgBiS2 NCs, and indicate their good monodispersity and single crystalline nature. The lattice distance of 0.33 nm observed in the HRTEM image can be assigned to the (111) plane of cubic AgBiS2 (JCPDS card No. 89-2045, Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, a = b = c = 5.648 Å), which is confirmed by the XRD pattern in Fig. 6c. No characteristic peaks corresponding to Ag2S or Bi2S3 are found in the XRD pattern, showing that pure phase cubic AgBiS2 NCs are obtained. Fig. 6d characterizes the optical absorption spectrum with an absorbance onset around 800 nm, indicative of the future application of AgBiS2 NCs in photonic devices.
m, a = b = c = 5.648 Å), which is confirmed by the XRD pattern in Fig. 6c. No characteristic peaks corresponding to Ag2S or Bi2S3 are found in the XRD pattern, showing that pure phase cubic AgBiS2 NCs are obtained. Fig. 6d characterizes the optical absorption spectrum with an absorbance onset around 800 nm, indicative of the future application of AgBiS2 NCs in photonic devices.
 | ||
| Fig. 6 TEM (a) and HRTEM (b) images, XRD pattern (c) and UV-vis spectrum (d) of AgBiS2 nanoparticles. | ||
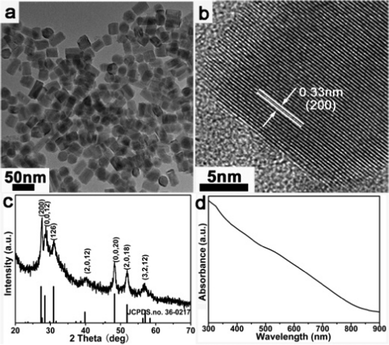
Also, orthorhombic Cu3SnS4 nanorods that are 20 nm in diameter are prepared by this facile and versatile method. Fig. 7a shows the nanorod morphology of the product and the HRTEM image in Fig. 7b clearly demonstrates that the nanorods grow along the (200) direction with a lattice distance of 0.33 nm. The XRD pattern (Fig. 7c) confirms the orthorhombic Cu3SnS4 crystal structure (JCPDS card No. 36-0217, space group Pmn21, a = 6.525 Å, b = 7.523 Å, c = 37.662 Å) of the as-prepared sample. The absorption band edge at about 850 nm (1.46 eV, Fig. 7d) implies the prospective application of Cu3SnS4 nanorods in photovoltaics.
Further, Ag8SnS6 nanoparticles are obtained via co-thermal decomposition of Ag(DDTC) and Sn(DDTC)2Phen in a mixture of OM and DT at 220 °C. Fig. 8 shows the morphology, phase structure and optical properties of the prepared Ag8SnS6 nanoparticles. Fig. 8a illustrates the nanoparticles with sizes of about 15 nm. The HRTEM image in Fig. 8b displays the (424) crystal plane with a lattice spacing of 0.19 nm. The crystalline structure of the as-synthesized sample is illustrated by the XRD pattern, which can be well assigned to orthorhombic Ag8SnS6 (JCPDS Card No. 89-1996). The optical properties were characterized in the UV-vis spectrum in Fig. 8d, which illustrates that the absorption band edge of Ag8SnS6 is at 950 nm (1.31 eV), which is consistent with the previous report.21
 | ||
| Fig. 8 (a) TEM image, (b) HRTEM image, (c) XRD pattern and (d) UV-vis spectrum of Ag8SnS6 nanoparticles. | ||
4. Conclusions
In summary, we have successfully developed a facile and general one-pot strategy for the controlled synthesis of high quality TMS NCs with metal-DDTCs as the precursors. By optimizing the reaction conditions, such as the mole ratios of different precursors, the compositions of the capping ligands, and the reaction temperature, orthorhombic Cu–Bi–S TMS NCs, including Cu3BiS3 nanosheets, Cu3BiS3 nanoparticles, Cu4Bi4S9 nanowires and Cu4Bi4S9 nanoribbons, and wurtzite CuInS2 nanopencils, cubic AgBiS2 nanocubes, orthorhombic Ag8SnS6 nanoparticles, and orthorhombic Cu3SnS4 nanorods are successfully obtained in more than 95% yield. The effects of precursors, capping ligands, and reaction temperature on the final products are investigated in detail. We expect that this methodology will be ready to synthesize multinary metal sulfide NCs, from binary to ternary and quaternary, with desirable morphologies, tunable compositions and engineered properties, and thus favour the potential application of metal sulfide NCs in nanophotonics, photovoltaics, and so on.Acknowledgements
The authors acknowledge funding by the Chinese Academy of Sciences Bairen Ji Hua Program, the Ministry of Science and Technology of China (No. 2011CB965004), the National Natural Science Foundation of China (No. 21073225, 2110116), the National Natural Science Foundation of Jiangsu Province (No. BK2012007), and the CAS/SAFEA International Partnership Program for Creative Research Teams.Notes and references
- (a) L. Li, N. Coates and D. Moses, J. Am. Chem. Soc., 2010, 132, 22 CrossRef CAS PubMed; (b) H. S. Ra, K. M. Ok and P. S. Halasyamani, J. Am. Chem. Soc., 2003, 125, 7764 CrossRef CAS PubMed; (c) M. Miyakawa, S. W. Kim, M. Hirano, Y. Kohama, H. Kawaji, T. Atake, H. Ikegami, K. Kono and H. Hosono, J. Am. Chem. Soc., 2007, 129, 7270 CrossRef CAS PubMed; (d) T. Torimoto, T. Adachi, K. I. Okazaki, M. Sakuraoka, T. Shibayama, B. Ohtani, A. Kudo and S. Kuwabata, J. Am. Chem. Soc., 2007, 129, 12388 CrossRef CAS PubMed; (e) S. Shen and Q. Wang, Chem. Mater., 2013, 25, 1166 CrossRef CAS.
- J. Qiu, Z. Jin, W. Wu and L. Xiao, Thin Solid Films, 2006, 510, 1 CrossRef CAS.
- (a) J. Zhang and Y. Huang, Cryst. Res. Technol., 2010, 45, 1194 CrossRef CAS; (b) T. Uematsu, T. Doi, T. Torimoto and S. Kuwabata, J. Phys. Chem. Lett., 2010, 1, 3283 CrossRef CAS; (c) L. Tian and J. J. Vittal, New J. Chem., 2007, 31, 2083 RSC; (d) B. Mao, C. Chuang, J. Wang and C. Burda, J. Phys. Chem. C, 2011, 115, 8945 CrossRef CAS; (e) Y. Hamanaka, T. Ogawa and M. Tsuzuki, J. Phys. Chem. C, 2011, 115, 1786 CrossRef CAS; (f) X. Li, J. Z. Niu, H. Shen, W. Xu, H. Wang and L. S. Li, CrystEngComm, 2010, 12, 4410 RSC; (g) G. Zhu and Z. Xu, J. Am. Chem. Soc., 2011, 133, 148 CrossRef CAS PubMed; (h) X. Lu, Z. Zhuang, Q. Peng and Y. Li, CrystEngComm, 2011, 13, 4039 RSC.
- (a) W. C. Huang, C. H. Tseng, S. H. Chang, H. Y. Tuan, C. C. Chiang, L. M. Lyu and M. H. Huang, Langmuir, 2012, 28, 8496 CrossRef CAS PubMed; (b) W. Yue, S. Han, R. Peng, W. Shen, H. Geng, F. Wu, S. Tao and M. Wang, J. Mater. Chem., 2010, 20, 7570 RSC; (c) A. Pein, M. Baghbanzadeh, T. Rath, W. Haas, E. Maier, H. Amenitsch, F. H. Ofer, C. O. Kappe and G. Trimmel, Inorg. Chem., 2011, 50, 193 CrossRef CAS PubMed; (d) K. Nose, Y. Soma, T. Omata and S. Otsuka-Yao-Matsuo, Chem. Mater., 2009, 21, 2607 CrossRef CAS; (e) F. M. Courtel, R. W. Paynter, B. T. Marsan and M. Morin, Chem. Mater., 2009, 21, 3752 CrossRef CAS; (f) S. L. Castro, S. G. Bailey, R. P. Raffaelle, K. K. Banger and A. F. Hepp, Chem. Mater., 2003, 15, 3142 CrossRef CAS; (g) S. T. Connor, C. Hsu, B. D. Wei, S. Aloni and Y. Cui, J. Am. Chem. Soc., 2009, 131, 4962 CrossRef CAS PubMed; (h) D. Pan, L. An, Z. Sun, W. Hou, Y. Yang, Z. Yang and Y. Lu, J. Am. Chem. Soc., 2008, 130, 5620 CrossRef CAS PubMed; (i) W. Han, L. Yi, N. Zhao, A. Tang, M. Gao and Z. Tang, J. Am. Chem. Soc., 2008, 130, 13152 CrossRef CAS PubMed; (j) B. Koo, R. N. Pate and B. A. Korgel, Chem. Mater., 2009, 21, 1962 CrossRef CAS; (k) M. Kruszynska, H. Borchert, J. Parisi and J. Kolny-Olesiak, J. Am. Chem. Soc., 2010, 132, 15976 CrossRef CAS PubMed.
- (a) R. Xie, M. Rutherford and X. Peng, J. Am. Chem. Soc., 2009, 131, 5691 CrossRef CAS PubMed; (b) D. Wang, W. Zheng, C. Hao, Q. Peng and Y. Li, Chem. Commun., 2008, 2556 RSC; (c) I. Tsuji, H. Kato and A. Kudo, Angew. Chem., Int. Ed., 2005, 44, 3565 CrossRef CAS PubMed; (d) M. G. Panthani, V. Akhavan, B. Goodfellow, J. P. Schmidtke, L. Dunn, A. Dodabalapur, P. F. Barbara and B. A. Korgel, J. Am. Chem. Soc., 2008, 130, 6770 CrossRef PubMed.
- (a) T. Surek, J. Cryst. Growth, 2005, 275, 292 CrossRef CAS; (b) K. Zweibel, Sol. Energy Mater. Sol. Cells, 2000, 63, 375 CrossRef CAS; (c) X. Michalet, F. F. Pinaud, A. Bentolila, L. J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS PubMed; (d) S. G. Bailey and D. J. Flood, Prog. Photovoltaics, 1999, 6, 1 CrossRef; (e) R. P. Raffaelle, S. L. Castro, A. F. Hepp and S. G. Bailey, Prog. Photovoltaics, 2002, 10, 433 CrossRef CAS; (f) J. L. Shay, B. Tell, L. M. Schiavone, H. M. Kasper and F. Thiel, Phys. Rev. B: Solid State, 1974, 9, 1719 CrossRef CAS; (g) K. Yoshinoa and T. Ikari, Appl. Phys. Lett., 2001, 78, 742 CrossRef.
- (a) Q. Li, Y. Ding, X. Liu and Y. Qian, Mater. Res. Bull., 2001, 36, 2649 CrossRef CAS; (b) L. Shi, P. Yin, L. Wang and Y. Qian, CrystEngComm, 2012, 14, 7217 RSC; (c) H. Nakamura, W. Kato, M. Uehara, K. Nose, T. Omata, S. Otsuka-Yao-Matsuo, M. Miyazaki and H. Maeda, Chem. Mater., 2006, 18, 3330 CrossRef CAS; (d) G. Shen, D. Chen, K. Tang and Y. Qian, J. Cryst. Growth, 2003, 257, 293 CrossRef CAS; (e) H. Zhong, Y. Zhou, M. Ye, Y. He, J. Ye, C. He, C. Yang and Y. Li, Chem. Mater., 2008, 20, 6434 CrossRef CAS; (f) E. Arici, N. S. Sariciftci and D. Meissner, Adv. Funct. Mater., 2003, 13, 165 CrossRef CAS.
- (a) Q. Lu, J. Hu, K. Tang, Y. Qian, G. Zhou and X. Liu, Inorg. Chem., 2000, 39, 1606 CrossRef CAS PubMed; (b) J. Hu, Q. Lu, K. Tang, Y. Qian, G. Zhou and X. Liu, Chem. Commun., 1999, 1093 RSC; (c) D. Chen, G. Shen, K. Tang, X. Jiang, L. Huang, Y. Jin and Y. Qian, Inorg. Chem. Commun., 2006, 6, 710 CrossRef.
- (a) T. Trindade and P. O'Brien, J. Mater. Chem., 1996, 6, 343 RSC; (b) T. Trindade, P. O'Brien and X. M. Zhang, Chem. Mater., 1997, 9, 523 CrossRef CAS; (c) T. Trindade, P. O'Brien, X. M. Zhang and M. Motevalli, J. Mater. Chem., 1997, 7, 1011 RSC; (d) B. Ludolph, M. A. Malik, P. O'Brien and N. Revaprasadu, Chem. Commun., 1998, 1849 RSC; (e) L. Tian, H. I. Elim, W. Ji and J. J. Vittal, Chem. Commun., 2006, 4276 RSC; (f) M. T. Ng, C. B. Boothroyd and J. J. Vittal, J. Am. Chem. Soc., 2006, 128, 7118 CrossRef CAS PubMed; (g) S. K. Batabyal, L. Tian, N. Venkatram, W. Ji and J. J. Vittal, J. Phys. Chem. C, 2009, 113, 15037 CrossRef CAS; (h) L. Tian, M. T. Ng, N. Venkatram, W. Ji and J. J. Vittal, Cryst. Growth Des., 2010, 10, 1237 CrossRef CAS.
- S. L. Castro, S. G. Bailey, R. P. Raffaelle, K. K. Banger and A. F. Hepp, J. Phys. Chem. B, 2004, 108, 12429 CrossRef CAS.
- (a) Y. Du, B. Xu, T. Fu, M. Cai, F. Li, Y. Zhang and Q. Wang, J. Am. Chem. Soc., 2010, 132, 1470 CrossRef CAS PubMed; (b) Y. Zhang, J. Lu, S. Shen, H. Xu and Q. Wang, Chem. Commun., 2011, 47, 5226 RSC; (c) S. Shen, Y. Zhang, L. Peng, B. Xu, Y. Du, M. Deng, H. Xu and Q. Wang, CrystEngComm, 2011, 13, 4572 RSC; (d) Y. Zhang, H. Xu and Q. Wang, Chem. Commun., 2010, 46, 8941 RSC; (e) Y. Zhang, Y. Du, H. Xu and Q. Wang, CrystEngComm, 2010, 12, 3658 RSC; (f) S. Shen, Y. Zhang, L. Peng, Y. Du and Q. Wang, Angew. Chem., Int. Ed., 2011, 50, 7115 CrossRef CAS PubMed; (g) S. Shen, Y. Zhang, Y. Liu, L. Peng, X. Chen and Q. Wang, Chem. Mater., 2012, 24, 2407 CrossRef CAS; (h) L. Peng, S. Shen, Y. Zhang, H. Xu and Q. Wang, J. Colloid Interface Sci., 2012, 377, 13 CrossRef CAS PubMed.
- A. Hagfeldt and M. Graetzel, Chem. Rev., 1995, 95, 49 CrossRef CAS.
- (a) Y. Jun, S. Lee, N. Kang and J. Cheon, J. Am. Chem. Soc., 2001, 123, 5150 CrossRef CAS PubMed; (b) Y. Jun, Y. Jung and J. Cheon, J. Am. Chem. Soc., 2002, 124, 615 CrossRef CAS PubMed; (c) F. Dumestre, B. Chaudret, C. Amiens, M. Respaud, P. Fejes, P. Renaud and P. Zurcher, Angew. Chem., Int. Ed., 2003, 42, 5213 CrossRef CAS PubMed; (d) Y. Yang, H. Ma, J. Zhuang and X. Wang, Inorg. Chem., 2011, 50, 10143 CrossRef CAS PubMed; (e) Z. A. Peng and X. Peng, J. Am. Chem. Soc., 2001, 123, 1389 CrossRef CAS; (f) X. Peng, M. C. Schlamp, A. V. Kadavanich and A. P. Alivisatos, J. Am. Chem. Soc., 1997, 119, 7019 CrossRef CAS; (g) Z. Zhuang, Q. Peng and Y. Li, Chem. Soc. Rev., 2011, 40, 5492 RSC.
- J. Li, H. Zhong, H. Liu, T. Zhai, X. Wang, M. Liao, Y. Bando, R. Liu and B. Zou, J. Mater. Chem., 2012, 22, 17813 RSC.
- (a) X. G. Peng, Adv. Mater., 2003, 15, 459 CrossRef CAS; (b) Y. Sun and Y. Xia, J. Am. Chem. Soc., 2004, 126, 3892 CrossRef CAS PubMed.
- (a) J. Chen, L. M. Wu and L. Chen, Inorg. Chem., 2007, 46, 586 CrossRef CAS PubMed; (b) Y. Wang, J. Chen, L. Chen, Y. B. Chen and L. M. Wu, Cryst. Growth Des., 2010, 10, 1578 CrossRef CAS.
- H. Zhong, Y. Zhou, M. Ye, Y. He, J. Ye, C. He, C. Yang and Y. Li, Chem. Mater., 2008, 20, 6434 CrossRef CAS.
- (a) R. Scheer, T. Walter, H. W. Schrock, M. L. Fearheiley and H. J. Lewerenz, Appl. Phys. Lett., 1993, 63, 3294 CrossRef CAS; (b) A. Miller, A. Mackinnon and D. Weaire, J. Phys. C: Solid State Phys., 1981, 36, 119 CAS.
- M. D. Regulacio, C. Ye, S. H. Lim, Y. Zheng, Q. H. Xu and M. Y. Han, CrystEngComm, 2013, 15, 5214 RSC.
- (a) M. D. Regulacio, C. Ye, S. H. Lim, M. Bosman, E. Y. Ye, S. Y. Chen, Q. H. Xu and M. Y. Han, Chem.–Eur. J., 2012, 18, 3127 CrossRef CAS PubMed; (b) A. Singh, H. Geaney, F. Laffir and K. M. Ryan, J. Am. Chem. Soc., 2012, 134, 2910 CrossRef CAS PubMed.
- I. Osipishi, N. I. Butsko, B. I. Gasii and I. D. Zhezhnic, Semiconductors, 1972, 6, 974 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nj00928a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |