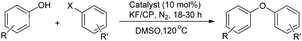
KF/Clinoptilolite, an effective solid base in Ullmann ether synthesis catalyzed by CuO nanoparticles
Mohammad A.
Khalilzadeh
*a,
Hoda
Keipour
b,
Abolfazl
Hosseini
b and
Daryoush
Zareyee
a
aDepartment of Chemistry, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran. E-mail: m.khalilzadeh@hotmail.com; Fax: +98 1232211647; Tel: +98 9111130400
bDepartment of Chemistry, Science and Research Branch, Islamic Azad University, Mazandaran, Iran
First published on 11th October 2013
Abstract
Employing KF/Clinoptilolite as an efficient base the cross-coupling reactions of various phenols with aryl iodides could be successfully carried out in the presence of copper oxide nanoparticles. The C–O coupling products were obtained in moderate to good yields (62–87%) for a variety of substrates.
The synthesis of diaryl ethers has been an important objective in organic synthesis since they are important intermediates in the synthesis of biologically and pharmaceutically active molecules.1 The classical Ullmann method for carbon–heteroatom bond formation is performed using stoichiometric amounts of copper reagents at high temperature, which leads to environmental and waste disposal problems at a larger scale.2 To overcome these drawbacks, considerable attention has been recently focused on development of catalytic systems for this purpose.3 During the past few years, metal-catalyzed methods for the preparation of diaryl ethers, in particular, with aryl halide derivatives facilitated by transition metal catalysts4 have been reported. Recent approaches in the use of copper salt nanoparticles have been proved to be an effective alternative for the classical Ullmann reaction under ligand-free conditions. The high surface area and reactive morphology of the CuO and copper salt nanoparticles made them successful catalysts in C–N, C–O, and C–S cross-coupling reactions.5 Besides the importance of catalysts used in cross-coupling reactions, the results established the significant role of the base in the outcome of the reaction. Among the bases used, solid bases6 have become popular due to their environmentally-friendly nature and unique properties. In contrast, homogeneous base catalysts inescapably involve in the emission of a large amount of wastewater and the separation difficulty of the catalysts from products owing to the emulsification and the saponification caused by aqueous quenching. In the past decades, however, there has been increasing interest in looking for new applications of clays, natural, quite common and very cheap materials with superior performances over the currently used materials.7 Among the salts with potentially basic properties potassium fluoride is more popular because of its cheapness and availability. A lot of supports have been introduced for increasing the basicity of potassium fluoride such as KF/ZnO,8 KF/Ca–Mg–Al,9 KF/CaO–Fe3O4,10 KF/celite,11 KF/NaY zeolite,12 KF/montmorillonite,13 KF/CaF2,14 KF/natural phosphate15 and KF/Ca–Al hydrotalcite16 mainly applied for biodiesel production. Although, a majority of the abovementioned solid bases are effective in organic transformations, they suffer from some drawbacks such as low basicity, use of expensive solid supports and tedious preparation steps.
Even though solid supported bases have found wide applications in organic transformation, there are only a few reports on the Ullmann-type coupling reactions with solid bases.
Clinoptilolite (CP) is a naturally occurring zeolite mineral, with an open aluminosilicate framework structure, and a high internal surface area with broad applications in chemistry and other industries.17
Clinoptilolite has high cation exchange capacity especially for potassium cations.18 Thus the use of this property by impregnation of potassium fluoride on CP causes a more free fluoride anion that is able to act as an effective base and it is obtained by simple mixing in water and evaporation without the need for any preparation or preactivation.
Prompted by this finding, we have recently developed a simple, cheap and effective solid base for C–O cross-coupling reaction with potassium fluoride impregnated on Clinoptilolite.19 In continuation of our interest in exploring coupling methodology, we report herein our results obtained with a nanosized CuO catalyst for an efficient synthesis of diaryl ethers in the presence of KF/CP as the base.
The reactions are successful at 120 °C in DMSO in the presence of KF/CP under an inert atmosphere. The reaction of phenol with iodobenzene was first studied as model substrates in the presence of CuO nanoparticles and KF/CP as the base (Table 1).
| Entry | Catalyst | Solvent | Base | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: phenol (1.5 mmol), catalyst (10 mol%), iodobenzene (1 mmol), base (0.33 g, 2 equiv.) and solvent (5 mL) at 120 °C for 12 h. b Isolated yield. c Yield in parentheses refers to the isolated yield after 20 h. d 2.5 equiv. of KF/CP was used. | ||||
| 1 | CuSO4 | DMSO | KF/CP | 28 |
| 2 | Cu(OAc)2 | DMSO | KF/CP | 30 |
| 3 | CuO | DMSO | KF/CP | 22 |
| 4 | CuBr | DMSO | KF/CP | 42 |
| 5 | CuCl | DMSO | KF/CP | 38 |
| 6 | CuO NP | Toluene | KF/CP | 12 |
| 7 | CuO NP | Dioxane | KF/CP | 18 |
| 8 | CuO NP | DMSO | KF/CP | 78 (86)c |
| 9 | CuO NP | DMSO | KF/CP | 80d |
| 10 | CuO NP | DMSO | K3PO4/CP | 16 |
To our pleasure, diphenylether was obtained in 78% yield when the reaction was carried out at 120 °C in the presence of 10 mol% of copper oxide nanoparticles and 2 equiv. of KF/CP in DMSO under nitrogen (Table 1, entry 8).
When the amount of base (KF/CP) was increased to 2.5 equiv. the yield was inappreciably enhanced to 80% (Table 1, entry 9) while the reaction time had more effect and the yield was increased to 86% after 20 hours (Table 1, entry 8).
Other copper salts such as CuSO4, Cu(OAc)2, CuBr, CuCl and CuO showed lower catalytic activity than CuO nanoparticles for this reaction. Among the solvents used, DMSO afforded the highest conversion. In addition, the reaction was more effective with KF/CP as a base rather than K3PO4/CP.
To study the scope of the procedure, the reaction of other phenols was studied next. The simplest case reaction of phenol with iodobenzene afforded the corresponding diaryl ether in high yield (Table 2, entry 1). Substituted halophenols underwent reaction with iodobenzene in 68–78% yield (Table 2, entries 2–4). Similar reactivity was observed with alkyl and methoxy substituted phenols (Table 2, entries 5–11). When 1-naphthol was used for the reaction, the corresponding naphthyl ether was obtained in moderate yield (Table 2, entry 12). Phenols having electron-donating groups showed greater reactivity in comparison to those with moderate electron-withdrawing halophenols.
| Entry | Aryl iodide | Phenol | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: phenol (1.5 mmol), catalyst (10 mol%), iodobenzene (1 mmol), base (0.33 g, 2 equiv.) and solvent (5 mL) at 120 °C under N2. b Isolated yield. | ||||
| 1 |
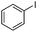
|
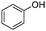
|
20 | 86 |
| 2 |
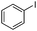
|
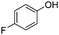
|
24 | 78 |
| 3 |
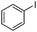
|
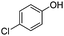
|
24 | 75 |
| 4 |
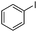
|
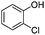
|
24 | 68 |
| 5 |
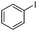
|
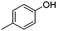
|
18 | 85 |
| 6 |
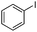
|
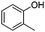
|
18 | 70 |
| 7 |
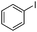
|
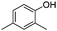
|
18 | 70 |
| 8 |
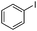
|
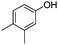
|
18 | 83 |
| 9 |
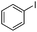
|
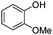
|
21 | 74 |
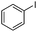
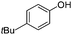
| 10 |
|
|
18 | 84 |
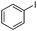
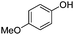
| 11 |
|
|
22 | 87 |
| 12 |
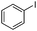
|
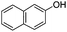
|
20 | 67 |
| 13 |
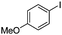
|
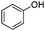
|
20 | 62 |
| 14 |
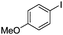
|
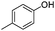
|
18 | 65 |
| 15 |
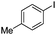
|
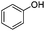
|
18 | 80 |
| 16 |
|
|
30 | 46 |
| 17 |
|
|
30 | 42 |
The coupling reaction of sterically hindered phenols was slightly disfavoured. Hence, when employing ortho substituted phenols, the reaction occurred with a lower yield than with less hindered para substituted phenols (Table 2, entries 3–10).
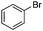
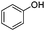
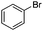
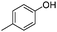
The reaction of phenols with substituted iodobenzenes was also studied. 1-Iodo-4-methoxybenzene and 1-iodo-4-methylbenzene provide the corresponding cross-coupled products in reasonable yields (Table 2, entries 13–15). Finally, it was found that the protocol is also active in activating bromobenzene and afforded the corresponding ethers albeit with low yields compared to iodobenzene (Table 2, entries 16 and 17).
Based on previous work20 a plausible mechanism for the CuO NP catalyzed reductive Ullmann reaction of aryl halides is shown in Scheme 1. As shown in Scheme 1, the catalytic cycle may be initiated by adsorption of aryl halides on the surface of the CuO nanoparticles through an oxidative addition. In the next step a proton was abstracted by a negatively charged fluoride moiety from phenol to generate a phenoxide anion stabilized at the potassium surface. After that the reaction may undergo anion substitution, followed by a reductive elimination process on the surface of the CuO NPs.
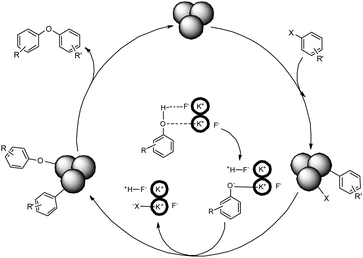 | ||
| Scheme 1 Proposed mechanism for CuO nanoparticle catalyzed O-arylation of phenols with aryl halides. | ||
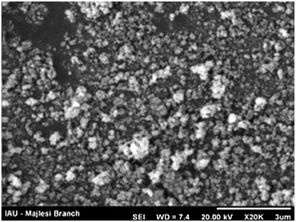
SEM analysis
Copper nanoparticles were prepared according to a previously reported procedure.5b The nanoparticles were characterized by SEM analysis, and it gives a fairly well dispersed mixture of small particles (<100 nm) (Fig. 1).In summary, a simple protocol for the O-arylation of phenols has been established through an effective new solid base. The strategy described herein includes many characteristics that are often considered as useful synthetic methods: solid phase reaction using available nanoparticles is operationally simple and scalable, employs starting materials that are commercially available and cheap, generates an innocuous byproduct, provides product diversity, and is compatible with a number of useful functional groups. Moreover, this methodology provides an efficient route to a wide variety of substituted phenolic compounds and it also enabled phenyl bromides to perform the reaction with moderate yields. In addition, an environmentally friendly solid base can be used instead of a strong base such as Cs2CO3.
Experimental section
Materials
The raw material was an Iranian commercial Clinoptilolite (Afrandtooska Company) obtained from deposits in the region of Semnan. The catalyst was prepared according to the literature procedure.5b,19 All starting materials were purchased from Aldrich or Fluka and were used without further purification. A silica gel (60 mesh) was used for product purification. Melting points were determined using an Electrothermal IA 9100 Digital Melting Point apparatus. 1H and 13C NMR spectra were recorded in [d6] DMSO using a Bruker DRX-300 Avance spectrometer operating at 300.13 and 75.47 MHz, respectively.Typical procedure
A mixture of phenol (1.5 mmol), the appropriate aryl halide (1 mmol), CuO nanoparticles (10 mol%) and 35% w/w potassium fluoride/Clinoptilolite (0.33 g) in dry DMSO (5 mL) was heated at 120 °C. The mixture was stirred under a nitrogen atmosphere until the reaction was complete (by TLC or GC monitoring). After completion, the reaction mixture was cooled to room temperature and filtered off to separate the solids (which was washed with 15 mL EtOAc) and the combined solution was placed in a separatory funnel and washed twice with water. The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo. Products were purified by column chromatography on a silica gel eluting with ethyl acetate–hexane mixtures.Notes and references
- (a) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054–3131 CrossRef CAS PubMed; (b) P. Cristau, J.-P. Vors and J. Zhu, Tetrahedron, 2003, 59, 7859–7870 CrossRef CAS PubMed; (c) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS PubMed.
- (a) F. Ullmann, Ber. Dtsch. Chem. Ges., 1904, 37, 853–857 CrossRef; (b) J. Lindley, Tetrahedron, 1984, 40, 1433–1456 CrossRef CAS.
- F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954–6971 CrossRef CAS PubMed.
- (a) G. Mann and J. F. Hartwig, Tetrahedron Lett., 1997, 38, 8005–8008 CrossRef CAS; (b) G. Mann, C. Incarvito, A. L. Rheingold and J. F. Hartwig, J. Am. Chem. Soc., 1999, 121, 3224–3225 CrossRef CAS; (c) Q. Shelby, N. Kataoka, G. Mann and J. F. Hartwig, J. Am. Chem. Soc., 2000, 122, 10718–10719 CrossRef CAS; (d) K. E. Torraca, X. Huang, C. A. Parrish and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 10770–10771 CrossRef CAS; (e) M. Wolter, G. Nordmann, G. E. Job and S. L. Buchwald, Org. Lett., 2002, 4, 973–976 CrossRef CAS PubMed; (f) R. A. Altman, A. Shafir, A. Choi, P. A. Lichtor and S. L. Buchwald, J. Org. Chem., 2008, 73, 284–286 CrossRef CAS PubMed; (g) X. Lv and W. Bao, J. Org. Chem., 2007, 72, 3863–3867 CrossRef CAS PubMed; (h) D. Ma and Q. Cai, Org. Lett., 2003, 5, 3799–3802 CrossRef CAS PubMed; (i) H.-J. Cristau, P. P. Cellier, S. Hamada, J. F. Spindler and M. Taillefer, Org. Lett., 2004, 6, 913–916 CrossRef CAS PubMed; (j) A. Ouali, J.-F. Spindler, H.-J. Cristau and M. Taillefer, Adv. Synth. Catal., 2006, 348, 499–505 CrossRef CAS; (k) P. J. Fagan, E. Hauptman, R. Shapiro and A. Casalnuovo, J. Am. Chem. Soc., 2000, 122, 5043–5051 CrossRef CAS; (l) S. Benyahya, F. Monnier, M. Taillefer, M. Wong Chi Man, C. Bied and F. Ouazzani, Adv. Synth. Catal., 2008, 350, 2205–2208 CrossRef CAS; (m) O. Bistri, A. Correa and C. Bolm, Angew. Chem., Int. Ed., 2008, 47, 586–588 CrossRef CAS PubMed; (n) H. J. Kim, M. Kim and S. Chang, Org. Lett., 2011, 13, 2368–2371 CrossRef CAS PubMed.
- (a) L. Rout, T. K. Sen and T. Punniyamurthy, Angew. Chem., Int. Ed., 2007, 46, 5583–5586 CrossRef CAS PubMed; (b) C. K. Chen, Y. W. Chen, C. H. Lin, H. P. Lin and C. F. Lee, Chem. Commun., 2010, 46, 282–284 RSC; (c) J. Zhang, Z. Zhang, Y. Wang, X. Zheng and Z. Wang, Eur. J. Org. Chem., 2008, 5112–5116 CrossRef CAS; (d) V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 951–953 CrossRef CAS PubMed; (e) B. Sreedhar, R. Arundhathi, P. L. Reddy and M. L. Kantam, J. Org. Chem., 2009, 74, 7951–7954 CrossRef CAS PubMed; (f) L. Rout, S. Jammi and T. Punniyamurthy, Org. Lett., 2007, 9, 3397–3399 CrossRef CAS PubMed; (g) V. P. Reddy, A. V. Kumar and K. R. Rao, J. Org. Chem., 2010, 75, 8720–8723 CrossRef CAS PubMed; (h) Y. Isomura, T. Narushima, H. Narushima, T. Yonezawa and Y. Obora, Chem. Commun., 2012, 48, 3784–3786 RSC; (i) R. Zhang, J. Liu, S. Wang, J. Niu, C. Xia and W. Sun, ChemCatChem, 2011, 3, 146–149 CrossRef CAS.
- (a) H. Hattori, Appl. Catal., A, 2001, 222, 247–259 CrossRef CAS; (b) R. A. Sheldon and R. S. Downing, Appl. Catal., A, 1999, 189, 163–183 CrossRef CAS; (c) J. H. Clark, D. G. Cork and M. S. Robertson, Chem. Lett., 1983, 1145–1148 CrossRef CAS; (d) K. Ebitani, K. Motokura, K. Mori, T. Mizugaki and K. Kaneda, J. Org. Chem., 2006, 71, 5440–5447 CrossRef CAS PubMed; (e) R. Ballini and P. Marziali, J. Org. Chem., 1996, 61, 3209–3211 CrossRef CAS; (f) B. Veldurthy, J. M. Clacens and F. Figueras, Eur. J. Org. Chem., 2005, 1972–1976 CrossRef CAS; (g) H. A. Prescott, Z.-J. Li, E. Kamnitz, J. Deutsch and H. Lieske, J. Mater. Chem., 2005, 15, 4616–4628 RSC; (h) R. Ballini, G. Bosica, D. Livi, A. Palmieri, R. Maggi and G. Sartori, Tetrahedron Lett., 2003, 44, 2271–2273 CrossRef CAS; (i) B. C. Ranu and B. Subhash, Org. Lett., 2005, 7, 3049–3052 CrossRef CAS PubMed; (j) J. M. Clacens, D. Genuit, L. Delmotte, A. Garcia-Ruiz, G. Bergeret, R. Montiel, J. Lopez and F. Figueras, J. Catal., 2004, 221, 483–490 CrossRef CAS PubMed; (k) M. Zahouily, B. Bahlaouane, M. Aadil, A. Rayadh and S. Sebti, Org. Process Res. Dev., 2004, 8, 275–278 CrossRef CAS; (l) M. Zahouily, Y. Abrouki, A. Rayadh, S. Sebti, H. Dhimane and M. David, Tetrahedron Lett., 2003, 44, 2463–2465 CrossRef CAS.
- R. Song, D. Tong, J. Tang and C. Hu, Energy Fuels, 2011, 25, 2679–2686 CrossRef CAS.
- W. L. Xie and X. M. Huang, Catal. Lett., 2006, 107, 53–59 CrossRef CAS.
- L. J. Gao, G. Y. Teng, J. H. Lv and G. M. Xiao, Energy Fuels, 2010, 24, 646–651 CrossRef CAS.
- S. Hu, Y. Guan, Y. Wang and H. Han, Appl. Energy, 2011, 88, 2685–2690 CrossRef CAS PubMed.
- T. Ando and J. Yamawaki, Chem. Lett., 1979, 45–46 CrossRef CAS.
- J. H. Zhu, Y. Chun, Y. Qin and Q.-H. Xu, Microporous Mesoporous Mater., 1998, 24, 19–28 CrossRef CAS.
- F. M. Asseid, C. V. A. Duke and J. M. Miller, Can. J. Chem., 1990, 68, 1420–1424 CrossRef CAS.
- J. Ichihara, T. Matsuo, T. Harafusa and T. Ando, J. Chem. Soc., Chem. Commun., 1986, 793–794 RSC.
- M. Zahouily, B. Bahlaouane, M. Aadil, A. Rayadh and S. Sebti, Org. Process Res. Dev., 2004, 8, 275–278 CrossRef CAS.
- L. Gao, G. Teng, G. Xiao and R. Wei, Biomass Bioenergy, 2010, 34, 1283–1288 CrossRef CAS PubMed.
- J. V. Smith, Chem. Rev., 1988, 88, 149–182 CrossRef CAS.
- L. L. Ames, Am. Mineral., 1960, 45, 689–700 CAS.
- M. A. Khalilzadeh, A. Hosseini and A. Pilevar, Eur. J. Org. Chem., 2011, 1587–1592 CrossRef CAS.
- (a) V. Pace, F. Martinez, M. Fernandez, J. V. Sinisterra and A. R. Alcantara, Org. Lett., 2007, 9, 2661–2664 CrossRef CAS PubMed; (b) B. Veldurthy, J. M. Clacens and F. Figueras, Adv. Synth. Catal., 2005, 347, 767–771 CrossRef CAS; (c) S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra and P. Saha, J. Org. Chem., 2009, 74, 1971–1976 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 |