Evidence for a possible dietary effect on the isotopic composition of Zn in blood via isotopic analysis of food products by multi-collector ICP-mass spectrometry†
Marta
Costas-Rodríguez
,
Lana
Van Heghe
and
Frank
Vanhaecke
*
Department of Analytical Chemistry, Ghent University, Krijgslaan 281-S12, 9000 Ghent, Belgium. E-mail: frank.vanhaecke@ugent.be
First published on 23rd October 2013
Abstract
In this work, the hypothesis of a possible dietary effect on the isotopic composition of Zn in blood from populations with different feeding habits, i.e. lacto-ovo vegetarians and omnivores, was investigated through isotopic analysis of Zn in common food products by multi-collector ICP – mass spectrometry (MC-ICP-MS). Several certified reference materials (CRMs) were also included in the sample set for comparison purposes. For these CRMs, the isotopic composition of Zn is expressed as δ-values, calculated with respect to both IRMM-3702 and JMC-ZnLyon, as isotopic standards. The range of δ66Zn values observed in food products was approximately 1.9‰. In general, vegetables, cereals and derived products showed an enrichment of the heavier Zn isotopes, whereas a depletion was observed in products of animal origin (meat, fish, egg and semi-skimmed milk), relative to human blood samples. Mussel, however, showed a significant enrichment of the heavier isotopes, which is hypothetically attributed to its accumulation behaviour. Thus, the lower δ66Zn values found in food products of animal origin appear to be reflected in the lower δ66Zn value observed in blood from an omnivorous population compared to that for a vegetarian population.
1. Introduction
Zinc is a vital element for human health as it is present in more than 300 proteins required for normal physiological functions.1 To maintain a steady state in the body, an adequate intake of Zn from the diet is required. Zn absorption takes place in the small intestine mainly through carrier-mediated processes that remain partly unresolved yet.2 The bioavailability of dietary Zn is influenced by the food composition. The chemical form of Zn and the presence of inhibitors or enhancers affect the solubility of Zn in the intestinal lumen. The most significant inhibitor is phytate (inositol hexa- and penta-phosphate), which is the main storage form of phosphorus in bran and seeds.3–6 For diets with a phytate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn molar ratio of 5
Zn molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, such as the lacto-ovo vegetarian diets, the fraction of zinc absorbed can be reduced to values around 35%.7,8 In spite of this, under normal conditions, Zn homeostasis is still maintained as endogenous Zn losses are adjusted to the amount absorbed.3 Proteins from animal products on the other hand enhance the absorption of Zn since they release amino acids that keep Zn in solution and increase the Zn intake.6
15, such as the lacto-ovo vegetarian diets, the fraction of zinc absorbed can be reduced to values around 35%.7,8 In spite of this, under normal conditions, Zn homeostasis is still maintained as endogenous Zn losses are adjusted to the amount absorbed.3 Proteins from animal products on the other hand enhance the absorption of Zn since they release amino acids that keep Zn in solution and increase the Zn intake.6
The partial release of dietary Zn into the lumen and different absorption rates might be associated with isotope fractionation. Zn isotopes are also biologically re-distributed in the organism through processes that to date are still poorly understood.9–11 Since there are no changes in the Zn oxidation state in the organism,2 isotope fractionation seems to be governed by Zn ligand exchange mechanisms, where presumably heavy isotopes tend to be enriched in the compounds with the strongest bonds.12–14 Differences in the isotopic composition of Zn between different organs has been experimentally demonstrated in experiments with animals.10,12,13 The range of δ66Zn variation among the organs of a single sheep raised on a controlled diet was higher than 1‰.10 In humans, an overall variability of about 2‰ has been reported, with δ66Zn values from down to −1‰ in the liver15 and up to +1‰ in bone tissue (relative to the JCM-ZnLyon standard).16
Nowadays, efforts are being made to elucidate the sources of variation in the isotopic composition of Zn in human blood. For apparently healthy individuals (reference population), physiological factors, such as body metabolic rate, age and menstruation, and lifestyle, particularly the diet, are possible sources of variation. Jaouen et al. showed a heavier isotopic composition of blood-Zn in a population with a possibly elevated basal metabolic rate due to cold stress.9 The same authors observed a weak positive correlation of δ66Zn with the age, but the isotopic composition of Zn in blood is not a good age tracer due to the rapid Zn turnover (∼3 months). No significant differences in terms of δ66Zn between premenopausal and postmenopausal women and no significant effect of age on the isotopic composition of Zn have been found in a recent work carried out by our group.17 Ohno et al. showed that seasonal changes did not affect the isotopic composition of Zn in red blood cells,18 while Albarède et al.15 and Van Heghe et al.19 concluded that also gender did not seem to have an effect on δ66Zn in whole blood, serum and red blood cells.
Significant differences in the isotopic composition of Zn in blood from populations with different feeding habits have been reported and the isotopic composition of Zn has been suggested as a potential dietary proxy.9,19 Lacto-ovo vegetarians show higher δ66Zn values than omnivorous individuals.19 Recently, Jaouen et al. observed a δ66Zn enrichment of 0.8‰ in the first step of mammal trophic chains (comparison of plants and bones of herbivores) and a slight depletion of 0.2‰ in the second step (comparison of bones of herbivores and carnivores, respectively).20
To the best of our knowledge, to date there is no literature regarding Zn isotope ratio variations in actual food products consumed by humans. Zn isotope ratio variability has been mainly reported for animals and vegetables grown under strictly controlled conditions10,12,21–23 and even for related food certified reference materials (CRMs) little information on the isotopic composition of Zn is available.24 In the case of vegetables or plants, a widespread variability of δ66Zn was found (about 2.2‰), with δ66Zn values ranging from −0.9‰ in palm leaves25 to 1.3‰ in lentils (relative to the JMC-ZnLyon standard).23 In addition to the inter-species variability, Zn isotope fractionation has also been observed during Zn absorption and translocation by rice, tomato and lettuce grown hydroponically.21,22
The goal of this work was to investigate a possible dietary effect on the isotopic composition of Zn in blood from populations with different feeding habits, i.e. lacto-ovo vegetarians and omnivores, through the isotopic analysis of Zn in food by multi-collector ICP – mass spectrometry (MC-ICP-MS). A variety of common food products and several certified reference materials were analysed. For this purpose, the analytical protocol previously described for blood samples19 was fine-tuned for analysis of food samples.
2. Experimental
2.1 Reagents and standards
Ultrapure water (resistivity >18.2 MΩ cm) obtained from a Milli-Q Element water purification system (Millipore, France) was used throughout. 14 M HNO3 and 12 M HCl, both of pro analysis purity level (ProLabo, Belgium), were further purified by sub-boiling distillation in PFA and quartz equipment, respectively. Ultrapure 9.8 M H2O2 used for sample preparation was acquired from Sigma-Aldrich (Belgium) and used as such.Polypropylene chromatographic columns filled with AG MP-1 strong anion exchange resin (100–200 mesh, chloride form) acquired from Bio-Rad (Belgium) were used for the isolation procedure.
The isotopic reference material IRMM-3702, purchased from the Institute for Reference Materials and Measurements (IRMM, Belgium) was used for standard-sample bracketing and the Johnson Matthey® Zn Lyon solution 3-0749 L (JMC-ZnLyon, Lyon-CNRS laboratory, France) for comparison purposes. In addition, a Zn standard solution (Inorganic Ventures, the Netherlands; lot D2-ZN02061) was used as the in-house isotopic standard for checking the quality of the isotope ratio measurements. This standard is further referred to as A&MS-Zn.
Single-element standard stock solutions (1000 mg L−1) used for mass bias correction (Cu) and for quantification purposes (Zn and some major and minor elements) were acquired from Inorganic Ventures. Standard solutions of appropriate concentration levels were prepared by suitable dilution with 0.42 M sub-boiled HNO3.
All manipulations were carried out in a class-10 clean lab. Teflon Savillex® beakers used for sample handling and storage were cleaned with HNO3 and HCl (pro-analysis) in several steps and subsequently rinsed repeatedly with Milli-Q water before use.
2.2 Samples
Various certified reference materials (CRMs) relevant in the context of “human nutrition” were selected. BCR CRM 063R (skim milk powder), BCR CRM 184 (bovine muscle), BCR CRM 189 (wholemeal flour) and BCR CRM 278R (mussel tissue) were purchased from the Institute for Reference Materials and Measurements (IRMM, Belgium). SRM 1577a (bovine liver), SRM 1570 (spinach powder), SRM 1567a (wheat flour powder), SRM 1568a (rice flour powder) and SRM 1577a (bovine liver) were acquired from the United States National Institute of Standards and Technology (NIST, USA). Also real food samples commonly consumed by lacto-ovo vegetarians and/or omnivores were analyzed. These samples were purchased from a local Belgian supermarket and included meat (chicken, lamb, beef and pork), fish (pangasius, Pangasius hypophthalmus, and salmon, Salmo salar), vegetables (lettuce and brown lentils), cereals (rice and wheat bran), semi-skimmed milk, pasta, egg and dark chocolate.Whole blood samples from lacto-ovo vegetarian and omnivorous individuals were also included in this work. Six samples were from lacto-ovo vegetarians (3 males and 3 females) and twenty-one from omnivores (7 males and 14 females). The blood donors were between 20 and 32 years. The results for 12 of these samples have already been reported in a previous work.18 For the remaining samples, sample pre-treatment and analysis were performed following the same approach. Ethical approval for these research was obtained and patients signed informed consent.
2.3 Sample preparation
Real samples of vegetables and cereals were thoroughly washed with ultrapure water to remove surface contamination. Meat and fish samples were taken from the inner part of the original piece and cut into small portions with a cleaned plastic knife. Subsequently, all samples were dried at 65 °C until constant weight. Finally, they were ground and stored in polypropylene tubes until acid digestion.About 0.1 g of sample or CRM were accurately weighed in a Savillex® PFA vessel and 4 mL of 14 M HNO3 and 1 mL of 9.8 M H2O2 were added. For samples with lower Zn concentrations, 0.5 g or 1 mL of sample were used instead, and hence, 5 mL of HNO3 and 2 mL of H2O2 were used. The mineralization was performed at 110 °C overnight (16 h). The digests thus obtained were subsequently evaporated to dryness and re-dissolved in 5 mL of 8 M HCl + 0.001% H2O2. Blanks were included with each set of digestions. In a next step, the samples were subjected to chemical purification by means of anion exchange chromatography.
Zn was isolated using the protocol described by Van Heghe et al.19 In brief, the Bio-Rad Poly-Prep® columns were filled with 2 mL of AG MP-1 resin. A frit was placed as bed support and a piece of cotton was placed on top of the resin as a stopper. The resin was cleaned with 3 mL of 7 M HNO3 and 10 mL of H2O and conditioned with 10 mL of 8 M HCl + 0.001% H2O2 mixture. The sample was loaded onto the column and 8 mL of 8 M HCl + 0.001% H2O2 was passed through for matrix elution. Subsequently, Cu was eluted with 12 mL of 5 M HCl + 0.001% H2O2 and Fe with 10 mL of 0.6 M HCl. Finally, Zn was eluted with 8 mL of 0.7 M HNO3. The purified Zn fraction was collected and evaporated to dryness at 95 °C to remove residual chlorides. This procedure was performed twice. The final residue was re-dissolved in 0.42 M HNO3.
2.4 Instrumentation and measurements
A Thermo Scientific Neptune MC-ICP-MS instrument was used for Zn isotope ratio measurements. For the introduction of solutions into the ICP ion source, a 100 μL min−1 PFA concentric nebulizer and a double spray chamber, consisting of a cyclonic and a Scott-type sub-unit, were used. Zn isotope ratio measurements were performed by static collection, involving six Faraday collectors connected to 1011 Ω amplifiers. The measurements were carried out at medium mass resolution. The instrument settings and data acquisition parameters used are shown in Table 1. Gain calibration, peak centering and baseline correction were performed before each measurement session.| Instrument settings | |
| RF power (W) | 1300 |
| Guard electrode | Connected |
| Sampler cone | Ni, 1.1 mm aperture diameter |
| Skimmer cone | Ni, H-type, 0.8 mm aperture diameters |
| Lens settings | Optimized for maximum signal intensity |
| Ar flow-rates (L min−1) | Plasma 15; auxiliary 0.6–0.7; nebulizer 0.9–1.0 |
| Sample uptake rate (μL min−1) | 100 |
| Resolution mode | Medium |
| Data acquisition parameters | |
| Acquisition mode | Static; multi-collection |
| Number of blocks | 9 |
| Number of cycles | 5 |
| Integration time (s) | 4 |
| Cup configuration | L3: 63Cu; L2: 64Zn; L1: 65Cu; C: 66Zn; H1: 67Zn; H2: 68Zn |
Zn isotope ratio measurements were carried out following a standard-sample-standard bracketing sequence, i.e. standard (IRMM-3702 or JMC-ZnLyon), sample, standard and so on. Cu was added to all solutions at a final concentration of 500 μg L−1 to serve as an internal standard relied on for mass discrimination correction. An acid blank (0.42 M HNO3 with 500 μg L−1 Cu) and procedural blanks (also containing 500 μg L−1 of the internal standard) were measured at the beginning and in the middle of each measurement session for proper blank correction. The in-house standard A&MS-Zn was included every 5 samples to check the validity of the measurements. The concentration of standards and samples was adjusted to 500 μg L−1 to avoid variations that might affect the extent of instrumental mass bias.
The isotope ratio data obtained were treated off-line after 2s-rejection of outliers. Correction for mass discrimination was performed according to Woodhead.26 The isotopic composition of Zn is expressed in delta notation (δ66Zn, δ67Zn and δ68Zn, ‰), i.e. as the relative difference between the Zn isotope ratio of the sample and that of a standard. These values are expressed relative to both IRMM-3702 and JMC-ZnLyon for comparison purposes.
A Thermo Scientific Element XR sector field ICP-MS instrument (Germany) was used for element quantification purposes. For sample introduction, a 200 μL min−1 nebulizer and a cyclonic spray chamber were used. Table 2 provides the instrument settings and data acquisition parameters used for the elemental assays. Concentrations of Zn and some major and minor elements that can potentially give rise to interferences were determined in the food samples after acid digestion and after Zn isolation. Ga was used as an internal standard to correct for matrix effects and instrument instability.
| Instrument settings | |
| RF power (W) | 1200 |
| Guard electrode | Connected |
| Sampler cone | Ni, 1.1 mm aperture diameter |
| Skimmer cone | Ni, H-type, 0.8 mm aperture diameter |
| Lens settings | Optimized for maximum signal intensity |
| Ar flow-rates (L min−1) | Plasma 15; auxiliary 0.85; nebulizer 1.0–1.1 |
| Sample uptake rate (μL min−1) | 200 |
| Resolution mode | Medium (∼4000) |
| Data acquisition parameters | |
| Acquisition mode | E-scan |
| Dwell time per point (ms) | 10 |
| Points per peak | 20 |
| Number of runs | 5 |
| Number of passes | 5 |
3. Results and discussion
3.1 Assessment of the sample preparation procedure
The sample preparation procedure preceding Zn isotope ratio measurements by MC-ICP-MS in food products is crucial due to the high amount of organic matter and salts originally present in such samples. An efficient decomposition of the organic matrix, by acid digestion, and isolation of the target element to minimize spectral and non-spectral effects, are mandatory. Both procedures have to be performed without isotopic fractionation and to assure this, quantitative recoveries of Zn have to be reached. Common isolation procedures used for Zn isotope ratio measurements by MC-ICP-MS are based on the methodology firstly developed by Maréchal et al.27 and based on the use of AG-MP-1 ion exchange resin. Several adaptations have been implemented to render the method suited for biological samples.9,18,28 As on-column isotope fractionation can occur,29 optimization or fine-tuning of the isolation procedure is necessary for each new sample type.In this work, the isolation procedure previously developed for blood samples19 was evaluated for food products. Zn recoveries were determined for both the acid digests and the purified Zn fractions for all CRMs and real samples via SF-ICP-MS analysis. Samples were analysed in duplicate. The recoveries obtained were quantitative within the experimental uncertainty, ensuring absence of any effect from on-column isotope fractionation. Table 3 shows the Zn concentrations obtained for the CRMs after digestion and isolation, respectively. As can be seen, good agreement was obtained between the experimentally determined and the certified values (t-test; texp < tcrit, p < 0.005).
| CRM | Matrix | Certified valuea (μg g−1) | Found value (μg g−1) |
|---|---|---|---|
| Experimental values are average value ± standard deviation.a Certified values and their uncertainties as reported in the certificate. The uncertainties represent the 95% confidence interval of the mean.b The uncertainties represent the half-width of the 95% confidence interval of the mean value. | |||
| BCR CRM 184 | Bovine muscle | 166 ± 3 | 166 ± 4 |
| NIST SRM 1577a | Bovine liver | 123 ± 0.8 | 130 ± 3 |
| BCR CRM 278 | Mussel tissue | 76 ± 2 | 83 ± 2 |
| NIST SRM 1570 | Spinach | 50 ± 2 | 51 ± 0.2 |
| NIST SRM 1568a | Rice flour | 19.4 ± 0.5 | 21.0 ± 0.5 |
| NIST SRM 1567a | Wheat flour | 10.6 ± 1.0 | 11.8 ± 0.3 |
| BCR CRM 189 | Whole meal flour | 56.5 ± 1.7 | 59.2 ± 0.5 |
| BCR CRM 063R | Skim milk | 49.0 ± 0.6b | 51.5 ± 0.5 |
The efficiency of the anion exchange chromatography to remove the matrix elements was tested as well. The presence of other elements with the target element may alter the extent of instrumental mass discrimination in MC-ICP-MS. As a result, a series of elements (Al, Br, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, Pb, Rb, S, Sr, Ti and V) were determined by SF-ICP-MS in the spinach certified reference material NIST SRM 1570. The elements were selected on the basis of their certified concentrations. Negligible amounts were found in the purified Zn fraction for the majority of them. However, 23% of the original Br content, 1% of that of P and less than 0.1% of those of Ca, Mg, S and K were still seen to end up in the purified Zn fraction. Taken into account that these elements are partially eluted with Zn and that the matrices to be analysed are different in nature (i.e. meat, fish, vegetables, cereals and dairy products among other), these elements were also determined in all samples. The levels of them present in the multi-collector measurement solutions were <3 mg L−1 for K and Ca, <1 mg L−1 for Br, Na, P, S and <0.1 mg L−1 for Mg. To test possible undesirable effects resulting from the presence of these elements, delta values of Zn were determined in the A&MS Zn in-house standard (i) as such and (ii) containing these elements at the maximum levels mentioned. Both solutions were measured in triplicate. The differences between the isotope ratio results for these standards were −0.013‰ for δ66Zn, 0.012‰ for δ67Zn and 0.005‰ for δ68Zn (N = 2). These differences were within experimental uncertainty and thus, the presence of the remaining major elements does not influence the Zn delta values.
The average δ66Zn, δ67Zn and δ68Zn values and their experimental uncertainty (as 2s) for two replicates of the A&MS-Zn in-house standard were −7.03 ± 0.04, −10.54 ± 0.08 and −13.93 ± 0.09‰ (N = 2), respectively. In addition, delta values obtained over a period of 4 years (N = 82) were: −7.06 ± 0.02‰ for δ66Zn, −10.55 ± 0.06‰ for δ67Zn and −13.98 ± 0.07‰ for 68Zn.
Procedural blanks, treated in the same way as the samples, were included in each batch of digestion. The contribution of the procedural blanks was ∼10 ng of Zn, compared to ∼1 μg of Zn in the sample. The differences between the results with and without blank correction were less than 0.05, 0.15 and 0.09‰ for δ66Zn, δ67Zn and δ68Zn, respectively. Blank correction was carried out through before mass bias correction.
3.2 Reference values for Zn isotopic composition
At present, most of the delta Zn values reported in the literature are expressed versus the JMC-ZnLyon standard. However, this material is no longer commercially available30 and thus the use of the new and certified isotopic reference material IRMM-3702 will increase in the future. We therefore prefer expressing the Zn delta values versus IRMM-3702. Table 4 shows the values of δ66Zn, δ67Zn and δ68Zn for the CRMs calculated versus both JMC-ZnLyon and IRMM-3702 to facilitate comparison with values reported in the literature.| CRM | Matrix | Relative to JMC-ZnLyon | Relative to IRMM-3702 | ||||
|---|---|---|---|---|---|---|---|
| δ 66Zn (‰) | δ 67Zn (‰) | δ 68Zn (‰) | δ 66Zn (‰) | δ 67Zn (‰) | δ 68Zna (‰) | ||
| a Uncertainties are expressed as standard deviations. | |||||||
| BCR CRM 184 | Bovine muscle | 0.02 ± 0.02 | 0.02 ± 0.12 | 0.04 ± 0.12 | −0.24 ± 0.02 | −0.37 ± 0.06 | −0.46 ± 0.05 |
| NIST SRM 1577a | Bovine liver | 0.24 ± 0.02 | 0.37 ± 0.07 | 0.47 ± 0.05 | −0.08 ± 0.04 | −0.10 ± 0.07 | −0.13 ± 0.08 |
| BCR CRM 278 | Mussel tissue | 0.73 ± 0.01 | 1.07 ± 0.08 | 1.34 ± 0.05 | 0.45 ± 0.03 | 0.66 ± 0.10 | 0.75 ± 0.11 |
| NIST SRM 1570 | Spinach | 0.69 ± 0.03 | 0.93 ± 0.17 | 1.34 ± 0.09 | 0.42 ± 0.02 | 0.43 ± 0.08 | 0.75 ± 0.04 |
| NIST SRM 1568a | Rice flour | 0.39 ± 0.01 | 0.57 ± 0.05 | 0.72 ± 0.04 | 0.11 ± 0.02 | 0.19 ± 0.07 | 0.12 ± 0.04 |
| NIST SRM 1567a | Wheat flour | 1.17 ± 0.08 | 1.76 ± 0.13 | 2.29 ± 0.06 | 0.94 ± 0.05 | 1.37 ± 0.17 | 1.77 ± 0.14 |
| BCR CRM 189 | Whole meal flour | 0.61 ± 0.05 | 0.90 ± 0.02 | 1.15 ± 0.01 | 0.36 ± 0.03 | 0.50 ± 0.20 | 0.62 ± 0.03 |
| BCR CRM 063R | Skimmed milk | 0.52 ± 0.04 | 0.76 ± 0.09 | 1.00 ± 0.10 | 0.20 ± 0.03 | 0.30 ± 0.14 | 0.37 ± 0.06 |
For some of these CRMs, the Zn isotopic composition has been reported earlier (delta values versus the JMC-ZnLyon standard). For instance, for mussel tissue BCR CRM 278, Maréchal et al. reported a δ66Zn value of 0.82‰,27 while for bovine liver NIST SRM 1577a, Stenberg et al. reported a δ66Zn value of 0.04 ± 0.016 (2s)‰.28 Some differences were observed between the data obtained in this work and these previously reported data. These differences can possibly be explained by the isotopic heterogeneity of the CRMs. In fact, a spread of 0.41‰ in δ66Zn values can be found for the same CRM (BCR CRM 281, rye grass) in the literature.31,32
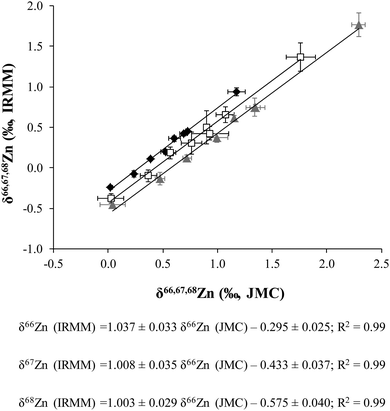
The delta Zn values obtained versus the JMC-ZnLyon and IRMM-3702, respectively, were also plotted against one another (Fig. 1). The corresponding straight line equations are also shown in this figure. The difference in δ66Zn between JMC-ZnLyon (3-0749L) and IRMM-3702 has already been reported in several papers: −0.30 ± 0.04‰ (N = 24);19 −0.29 ± 0.05‰ (N = 5);30 −0.32 ± 0.03‰ (N = 4);33 −0.32 ± 0.16‰ (N = 2)34 and −0.27 ± 0.07‰ (N = 24).35 These values agree very well with the intercept of the δ66Zn regression line (−0.295 ± 0.025‰, Fig. 1).
3.3 Evidence for a possible dietary effect
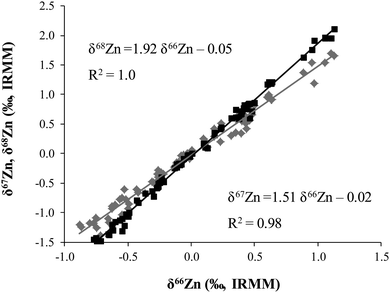
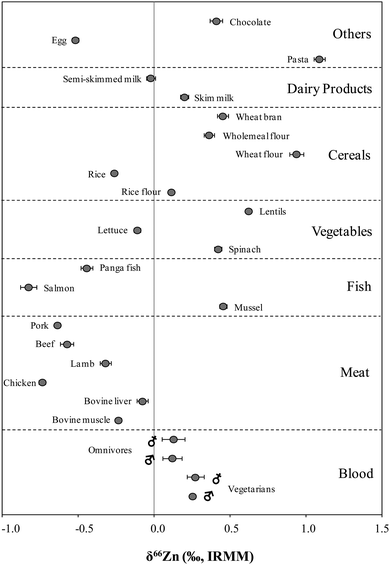
The isotopic composition of Zn was determined in food products in order to evaluate a possible dietary effect on the isotopic composition of Zn in whole blood of populations with different feeding habits, i.e. lacto-ovo vegetarians and omnivores. The δ66Zn values of the samples are reported in the ESI† (Table S1). Results given correspond to the mean value obtained for two different sample digestions and two measurements of each digest. As can be seen in Fig. 2, all sample results fall on the normal mass-dependent fractionation lines, i.e. δ67Zn = 1.5 × δ66Zn and δ68Zn = 2.0 × δ66Zn.Fig. 3 shows the δ66Zn data for the real food products and CRMs. The average of the delta values found in whole blood from lacto-ovo vegetarian and omnivorous individuals were also included in this figure (individual blood data are shown in Table S2, ESI†). The δ66Zn values for the food products range from −0.83 to 1.09‰ (i.e. overall variability 1.92‰). As has been mentioned previously, this widespread range is in agreement with the range of δ66Zn values reported by other authors for biological samples.10,15,16
In general, Zn in products of animal origin, i.e. meat, fish, egg and semi-skimmed milk, is isotopically lighter than that in human blood samples. Only mussel shows a significantly heavier Zn isotopic composition, which is assumed to be a result of the high bioaccumulation capacity of this species. On the other hand, vegetables, cereals, dairy products, pasta and chocolate show an enrichment of the heavier Zn isotopes. The highest δ66Zn values are observed for wheat-based products, i.e. wheat flour and pasta.
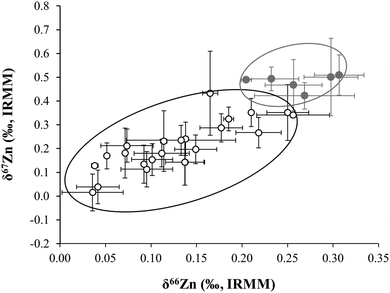
Based on the δ66Zn values determined in blood, it was concluded that the omnivorous population showed a slight, but significant depletion of the heavier Zn isotopes with respect to the lacto-ovo vegetarian population (see Fig. 4). A summary of the average δ66Zn values in food products of animal and plant origin, along with the values in human blood of omnivores and vegetarians, and the isotopic fractionation between diet and blood are shown in Fig. 5. From the results obtained for food products in this work, it can be hypothesized that the lighter isotopic composition in the omnivorous population could be related to the even lighter isotopic composition of Zn in food products of animal origin. In contrast, higher consumption of cereal-based products and vegetables appears to be linked with the relative enrichment of blood in the heavy Zn isotopes seen for the lacto-ovo vegetarian population. Thus, the diet seems to be the predominant source of variation of the Zn isotopic composition in human blood.
4. Conclusions
Analytical methodology based on a conventional acid digestion, combined with anion exchange separation, followed by Zn isotope ratio measurement using an MC-ICP-MS instrument operated at medium mass resolution provides precise and reliable results for Zn isotope ratios in food matrices. The δ66Zn variability observed in food products ranged from −0.83 to 1.09‰. In general, vegetables, cereals and some derived products showed higher δ66Zn values, whereas a depletion of the heavier Zn isotopes was observed in products of animal origin, relative to human blood samples. Only mussel showed a significantly higher δ66Zn value, which is attributed to the high bioaccumulation capability of this species. The lower δ66Zn value observed in blood from omnivorous compared with that from vegetarian individuals appears to be related to the isotopically light isotopic composition of Zn in food products of animal origin. Thus, a dietary effect seems to be reflected in the isotopic composition of Zn in human blood.Acknowledgements
The Flemish Research Foundation (FWO-Vlaanderen) is acknowledged for financial support through Research Project G002111N. Marta Costas-Rodríguez thanks BOF-UGent for her postdoctoral grant. The Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) is acknowledged for financial support through the PhD fellowship of Lana Van Heghe.References
- A. S. Prasad, in Zinc and enzymes, ed. A. S. Prasad, Plenum Press, New York, 1993, pp. 17–53 Search PubMed.
- L. C. Costello, C. C. Fenselau and R. B. Franklin, J. Inorg. Biochem., 2011, 105, 589–599 CAS.
- N. Roohani, R. Hurrell, R. Kelishadi and R. Schulin, J. Res. Med. Sci., 2013, 144–157 CAS.
- V. Kumar, A. K. Sinha, H. P. S. Makkar and K. Becker, Food Chem., 2010, 120, 945–959 CAS.
- J. R. Hunt, Am. J. Clin. Nutr., 2003, 78, 633S–639S CAS.
- B. Lönnerdal, J. Nutr., 2000, 130, 1378S–1383S Search PubMed.
- FAO/WHO, Report of a joint FAO/WHO expert consultation, Human vitamin and mineral requirements, Bangkok, Thailand, 2001.
- J. R. Hunt, L. A. Matthys and L. K. Jhonson, Am. J. Clin. Nutr., 1998, 67, 421–430 CAS.
- K. Jaouen, M. Gilbert, A. Lamboux, P. Telouk, F. Fourel, F. Albarède, A. N. Alekseev, E. Crubézy and V. Balter, Metallomics, 2013, 5, 1016–1024 CAS.
- V. Balter, A. Zazzo, A. P. Moloney, F. Moynier, O. Schmidt, F. J. Monahan and F. Albarède, Rapid Commun. Mass Spectrom., 2010, 24, 605–612 CAS.
- T. Walczyk, in Isotopic Analysis: Fundamentals and Applications Using ICP-MS, ed. F. Vanhaecke and P. Degryse, Wiley-VCH Verlag GmbH & Co. KgaA, Wiengeim, 2012, ch. 16, pp. 435–494 Search PubMed.
- F. Moynier, T. Fujii, A. S. Shaw and M. Le Borgne, Metallomics, 2013, 5, 693–699 CAS.
- V. Balter, A. Lamboux, A. Zazzo, P. Télouk, Y. Leverrier, J. Marvel, A. P. Moloney, F. J. Monahan, O. Schmidt and F. Albarède, Metallomics, 2013, 5, 1470–1482 CAS.
- E. A. Schauble, Rev. Mineral. Geochem., 2004, 55, 65–111 CAS.
- F. Albarède, P. Telouk, A. Lamboux, K. Jaouen and V. Balter, Metallomics, 2011, 3, 926–933 Search PubMed.
- K. Jaouen, V. Balter, E. Herrscher, A. Lamboux, P. Telouk and F. Albarède, Am. J. Phys. Anthropol., 2012, 148, 334–340 Search PubMed.
- L. Van Heghe, O. Deltombe, J. Delanghe, H. Depypere and F. Vanhaecke, J. Anal. At. Spectrom. Search PubMed , submitted.
- T. Ohno, A. Shinoha, M. Chiba and T. Hirata, Anal. Sci., 2005, 21, 425–428 CAS.
- L. Van Heghe, E. Engström, I. Rodushkin, C. Cloquet and F. Vanhaecke, J. Anal. At. Spectrom., 2012, 27, 1327–1334 CAS.
- K. Jaouen, M. L. Pons and V. Balter, Earth Planet. Sci. Lett., 2013, 374, 164–172 CAS.
- T. Arnold, G. J. D. Kirk, M. Wissuwa, M. Frei, F. J. Zhao, T. F. D. Mason and D. Weiss, Plant Cell Environ., 2010, 33, 370–381 CAS.
- D. J. Weiss, T. F. D. Mason, F. J. Zhao, G. J. D. Kirk, B. J. Coles and M. S. A. Horstwood, New Phytol., 2005, 165, 703–710 CAS.
- F. Moynier, S. Pichat, M. L. Pons, D. Fike, V. Balter and F. Albarède, Gems Gemol., 2009, 267, 125–130 CAS.
- C. Cloquet, J. Carignan, M. F. Lehmann and F. Vanhaecke, Anal. Bioanal. Chem., 2008, 390, 451–463 CAS.
- J. Viers, P. Oliva, A. Nonelle, A. Gelabert, J. Sonke, R. Freydier, R. Gainville and B. Dupre, Chem. Geol., 2007, 239, 124–137 CAS.
- J. Woodhead, J. Anal. At. Spectrom., 2002, 17, 1381–1385 CAS.
- C. N. Maréchal, P. Télouk and F. Albarède, Chem. Geol., 1999, 156, 251–273 Search PubMed.
- A. Stenberg, H. Andrén, D. Malinovsky, E. Engström, I. Rodushkin and D. C. Baxter, Anal. Chem., 2004, 76, 3971–3978 CAS.
- C. Maréchal and F. Albarède, Geochim. Cosmochim. Acta, 2002, 66, 1499–1509 Search PubMed.
- K. Moeller, R. Schoenberg, R. B. Pedersen, D. Weiss and S. Dong, Geostand. Geoanal. Res., 2012, 36, 177–199 CAS.
- T. Arnold, M. Schönbächler, M. Rehkämper, S. Dong, F. J. Zhao, G. J. D. Kirk, B. J. Coles and D. Weiss, Anal. Bioanal. Chem., 2010, 398, 3115–3125 CAS.
- D. J. Weiss, N. Rausch, T. F. D. Mason, B. J. Coles, J. J. Wilkinson, L. Ukonmaanaho, T. Arnold and T. Nieminen, Geochim. Cosmochim. Acta, 2007, 71, 942–960 Search PubMed.
- J. C. J. Petit, J. de Jong, L. Chou and N. Mattielli, Geostand. Geoanal. Res., 2008, 32, 149–166 CAS.
- C. Cloquet, J. Carignan and G. Libourel, Environ. Sci. Technol., 2006, 40, 6594–6600 CAS.
- D. M. Borrok, R. Gieré, M. Ren and E. R. Landa, Environ. Sci. Technol., 2010, 44, 9219–9224 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3mt00244f |
| This journal is © The Royal Society of Chemistry 2014 |