Metallothionein polymorphisms in pathological processes
Martina
Raudenska
ab,
Jaromir
Gumulec
ab,
Ondrej
Podlaha
c,
Marketa
Sztalmachova
ad,
Petr
Babula
e,
Tomas
Eckschlager
f,
Vojtech
Adam
bd,
Rene
Kizek
bd and
Michal
Masarik
*ab
aDepartment of Pathological Physiology, Faculty of Medicine, Masaryk University, Kamenice 5, CZ-625 00 Brno, Czech Republic. E-mail: masarik@med.muni.cz; Fax: +420-5-4949-4340; Tel: +420-5-4949-3631
bCentral European Institute of Technology, Brno University of Technology, Technicka 3058/10, CZ-616 00 Brno, Czech Republic
cDepartment of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, and Department of Biostatistics, Harvard School of Public Health, Boston, MA 02115, USA
dDepartment of Chemistry and Biochemistry, Mendel University in Brno/Zemedelska 1, CZ-613 00 Brno, Czech Republic
eDepartment of Natural Drugs, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Palackeho 1-3, CZ-612 42 Brno, Czech Republic
fDepartment of Paediatric Haematology and Oncology, 2nd Faculty of Medicine Charles University and University Hospital Motol, V Uvalu 84, CZ-150 06 Prague 5, Czech Republic
First published on 3rd September 2013
Abstract
Metallothioneins (MTs) are a class of metal-binding proteins characterized by a high cysteine content and low molecular weight. MTs play an important role in metal metabolism and protect cells against the toxic effects of radiation, alkylating agents and oxygen free radicals. The evidence that individual genetic characteristics of MTs play an important role in physiological and pathological processes associated with antioxidant defense and detoxification inspired targeted studies of genetic polymorphisms in a clinical context. In recent years, common MT polymorphisms were identified and associated with, particularly, western lifestyle diseases such as cancer, complications of atherosclerosis, and type 2 diabetes mellitus along with related complications. This review summarizes all evidence regarding MT polymorphisms of major human MTs (MT1, MT2, MT3 and MT4), their relation to pathological processes, and outlines specific applications of MTs as a set of genetic markers for certain pathologies.
1 Introduction
Living organisms constantly need to cope with harmful environmental conditions such as heavy metal load, UV radiation, and oxidative stress. It is well known that the toxicity levels of metals and reactive oxygen species (ROS) vary considerably between, as well as within, species.1 Differential expression and function of metal binding proteins, such as metallothioneins (MTs), might be one of the reasons for this variation.2 In several genome-wide association studies a cluster of MT genes located on chromosome 16 (16q12-22) was found to be an important target in candidate gene finding. Lee et al. associated this gene cluster with obesity3 and Seibold et al. with breast cancer risk.4 Furthermore, promoter methylation and differential gene expression of MT1G, an MT gene paralog, was identified as a novel marker in melanoma in a genome-wide study.5 Notable changes in the expression of a variety of metallothionein genes, with a number of them being clearly upregulated, were identified in the pancreatic beta-cells from type 2 diabetes mellitus (T2DM) patients. These include the metallothioneins 1E, 1G, 1M, 1X and 2A.6 Expression of MTs may be also influenced by different external and internal factors, such as single nucleotide polymorphisms (SNPs).7 The evidence that individual genetic characteristics play an important role in physiological and pathological processes associated with antioxidant defense and detoxification resulted in numerous follow-up studies of genetic polymorphisms in the clinical settings.In this review we discuss the role of MTs and report all relevant single nucleotide polymorphisms (SNPs) in humans, including their locations along the gene sequence. Subsequently, we summarize the evidence regarding the association of MT SNPs with various pathological conditions. To date, no comprehensive review and only one genome-wide association study4 with regard to human MT polymorphisms have been published. Moreover, although numerous studies in the last three decades have described the association of MT SNPs with various pathological conditions, the nomenclature is riddled with inconsistency and inaccuracy. The novelty of this review lies in the synthesis of all studies related to MT polymorphisms, the nomenclature standardization according to the latest recommendations,8 and the exhaustive comparison across multiple databases.
1.1 How SNPs affect protein function
Single nucleotide polymorphisms (SNPs) are the most common source of variation in the human genome.9 Given their location, SNPs can be divided into coding and non-coding region SNPs. A SNP in the coding region may affect the amino acid composition in two different ways:(a) Non-synonymous SNPs (nsSNPs) result in an alteration of the coded amino acid. NsSNPs may lead to missense and nonsense types of mutation. A missense mutation changes one codon into another, thereby causing a change in the resulting amino acid, whereas nonsense mutation results in a misplaced termination codon. NsSNPs usually have a significant effect on the structure or function of the encoded protein. The functional effects caused by nsSNPs can be divided into several categories: SNPs affecting protein structure (protein aggregation, stability, flexibility, functional sites, and protein folding); reaction kinetics and its dependence on the environmental parameters; subcellular protein localization; mRNA stability and protein expression; and finally, interactions with other molecules.10
(b) Due to the redundant nature of the RNA triplet code, many coding region SNPs will not cause an amino acid change of the encoded protein. Such SNPs are called synonymous or silent, but it cannot be said with certainty that they have no effect.11 Synonymous polymorphisms appear to influence the kinetics of translation and protein folding (possibly via translational pausing during the recruitment of rare tRNAs).12 Synonymous polymorphisms also affect the mRNA structure and stability. Furthermore, SNPs in coding as well as in intron regions play a role in alternative splicing.13
SNPs found in promoters and other regulatory regions affect the amount or timing of protein production.
2 Materials and methods
2.1 Identification of relevant studies
MEDLINE (PubMed; 1968 to January 2013), EMBASE (1977 to January 2013), Cochrane Library (1953 to January 2013) and Web of Science (Science citation index expanded 1945 to January 2013) were searched using common keywords related to metallothionein and polymorphisms. The keywords were as follows: “polymorphism”, “SNP”, or “genetic variation” and “metallothionein”. Cited references of found studies were analyzed to find additional articles. Date of publication and language were not a restriction. Database search in the National Center for Biotechnology Information's Gene database (NCBI gene, www.ncbi.nlm.nih.gov/gene) was used to identify all human MT genes. Subsequently, database search was performed in the NCBI Short Genetic Variations database (NCBI dbSNP, www.ncbi.nlm.nih.gov/projects/SNP) and in Boston Children's Hospital's Informatics Program's SNPper database (http://snpper.chip.org) to identify relevant MT polymorphisms and their position within genes.2.2 SNP nomenclature
SNPs localized in 5′ untranslated regions of a gene (5′UTR), in the coding sequence, in introns and in 3′ untranslated regions of a gene (3′UTR) were only taken into account. SNPs localized near gene regions are not included. The data acquisition process is shown in Fig. 1. SNP names are listed in the NCBI rs format. The SNP position within the gene sequence is depicted by SNP nomenclature according to the Human genome variation society' recommendations for the description of DNA sequence variants v2.0 only,8 previous designations, genomic reference sequence, mRNA and protein positions are not listed. According to this nomenclature, nucleotide +1 is the first in the coding sequence (the first nucleotide of the exon). SNPs in the untranslated region at the 5′ end of the gene (5′UTR) are numbered in relation to this first coding nucleotide, for example, substitution of the 12th G to A before the first coding nucleotide, as follows: c.-12G>A. Likewise, SNPs in the coding region (e.g. C to G substitution of the third nucleotide) are designated as follows: c.3G>C. Intron SNPs are designated relative to its 5′ or 3′ end, giving priority to the closer end. For instance, T to G substitution of the second nucleotide in the intron (88 + 2) positioned between coding DNA nucleotides 88 and 89 is as follows: c.88+2T>G. Variants in the 3′ untranslated region of the gene are designated in relation to the termination codon as follows: T to A substitution 70 nucleotides downstream of the termination codon: c.*70T>A. | ||
| Fig. 1 Data acquisition process. Flow diagram for identification of metallothionein (MT) genes, MT single nucleotide polymorphisms (SNPs) and relevant studies. | ||
3 Metallothionein gene family
Metallothioneins (MTs) are a class of metal-binding proteins characterized by high cysteine content (up to 30% of the amino acid residues) and low molecular weight (0.5–15 kDa). Up to seven divalent metal ions can be bound to human MTs and this binding stabilizes the three-dimensional structure of MTs.14 MTs play an important detoxification role in the defense against excessive essential metals; this protective function is related to the ability of MTs to scavenge free radicals.15 Therefore, MTs are highly expressed when exposed to oxidative stress or toxic factors.2a,16Metallothioneins (MTs) can be found in most eukaryotes. Whereas the structure, physiology, and pathophysiology of MTs exhibit differences between individual species, the claims mentioned herein relate only to the human MTs and cannot be generalized to all organisms. There are four main gene subfamilies of MTs expressed in humans: MT1, MT2, MT3, and MT4. In humans, this cluster of genes is located on chromosome 16 (16q12-22).17 MT1 and MT2 consist of nine functional (MT1A, MT1B, MT1E, MT1F, MT1G, MT1H, MT1M (also called MT1K), MT1X, and MT2A) and seven nonfunctional (MT1C, MT1D, MT1I, MT1J, MT1L, PT1P, and MT2B) paralogs.18 Whereas MT1 and MT2 are expressed ubiquitously, MT3 expression appears to be restricted to the brain19 and metallothionein 4 (MT4) was found to be specifically expressed in stratified squamous epithelia.20
The ubiquitously expressed MT1 and MT2 isoforms are inducible in mammalian cells by heavy metals. In contrast, MT3 seems to be not inducible by heavy metals, although its gene contains metal responsive elements (MREs) in its promoter.21 Additionally, the status of the MT4 inducibility is still unclear. The early induction of MTs by metals also makes these proteins a potential biomarker useful to assess the ecotoxicological significance of non-essential (Cd, Pb) and essential, but potentially toxic, (Cu) metals.22 A significant association between the metal levels, MTs expression, and diseases was shown in various tissues2c,23 including breast,24 renal,25 and prostate cancers.26 The overexpression of MT2A is frequently observed in invasive human breast tumors and was linked with more aggressive breast cancers.24b Experimental studies have also shown both enhanced and reduced neuronal protection against a variety of oxidative stress-induced damages in MT-overexpressing and MT-knockout mice, respectively.27 However, mice with targeted deletion of both the MT1 and MT2 genes do not exhibit an altered phenotype under normal laboratory conditions.28 Nevertheless, when exposed to cadmium, these mice showed an increased sensitivity to intoxication and a dramatically reduced myotonic reflex of mesenteric arteries.29 Mice with defective genes for MT were ten times more sensitive to Cd toxicity than wild type strains.23a
MT2A seems to be the most expressed MT in the human body.26d The difference in expression between MT2A and other metallothioneins is attributed to the binding ability of enhancers in the MT2A promoter region.30
Under physiological conditions, MTs primarily bind zinc, but at the same time they have a particularly high affinity for potentially toxic heavy metals. In a study performed by Waalkes and co-workers, the order of binding affinity of MTs was determined as follows, Cd > Pb > Cu > Hg > Zn > Ag > Ni > Co;31 thus MTs are capable of binding Cd and Pb more strongly than Zn.
3.1 Regulation of MT expression
MT expression is controlled mainly at the transcriptional level.23d It could be induced by many different stimuli, such as metal exposure, oxidative stress, glucocorticoids, and changes in pH.16c,d,32 MT expression control elements can be functionally subdivided into two categories: basal and inducible. There are several distinct basal sequences, which include the TATA-box, GC-box, and at least two basal level enhancer (BLE) sequences.33 MT genes also respond to induction by heavy metals, antioxidants, and steroid hormones through the action of metal regulatory elements (MRE), antioxidant responsive elements (AREs), and glucocorticoid responsive elements (GRE) in the 5′-regulatory regions.34The main transcription factor involved in the metal regulation of expression is MRE-binding transcription factor-1 (MTF-1).35 On exposure to various heavy metals and under conditions of oxidative stress MTF-1 is activated and binds to MRE regions with the consensus sequence TGCRCNC and initializes the gene transcription.36 A recent study has shown that antioxidant response element-mediated expression of MTs is preferentially activated by nuclear respiratory factor 1 (Nrf1).37
4 MT1A polymorphisms
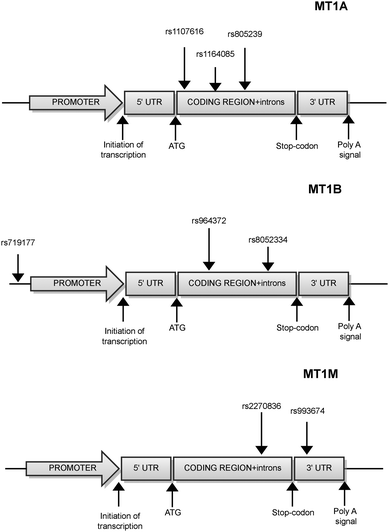
In terms of human MT1A polymorphisms, according to the NCBI database of polymorphisms (http://www.ncbi.nlm.nih.gov/snp/), 41 human SNPs are identified in the MT1A gene region (from the 5′ untranslated region (5′UTR) to the 3′ untranslated region (3′UTR)). According to this database, one is located in the 5′UTR, 6 in the coding sequence, 27 in introns, and 7 in 3′UTR. Of these, three polymorphisms were mentioned in the literature to have a significant impact on physiological and pathophysiological processes (as of January 2013)38 (Fig. 2).4.1 MT1A polymorphism rs11640851
rs11640851 is a c.80C>A single nucleotide polymorphism (80 nucleotides from the beginning of the exon) located in the MT1A coding region. It leads to an amino acid change (Thr27Asn), and can therefore be described as non-synonymous (two different polypeptide sequences can be produced). This polymorphism was associated with longevity in the Italian population and the A allele carriers were predisposed to longevity.38a In contrast, C allele carriers were predisposed to the development of cardiovascular disease and type 2 diabetes (T2DM).38b Concomitantly, older women with the CC genotype showed higher zinc release by MT (detected by a Zinpyr-1 fluorescent probe in the presence of a nitric oxide donor), reduced MT levels and low IL-6 plasma concentrations. MTs induction and zinc metabolism are essential for keeping the inflammatory status under control and for achieving healthy longevity.38b4.2 MT1A polymorphism rs8052394
This is a non-synonymous polymorphism of the MT1A gene, which is located 152 nucleotides from the first codon (ATG) (c.152A>G) and leads to an amino acid change Lys51Arg. Frequency of the rs8052394 G allele was significantly associated with the incidence of the type 2 diabetes mellitus (T2DM).38c Significant differences in the distribution of genotypes and allelic frequencies between T2DM patients and controls were found. Frequency of GA or GG carriers in T2DM groups compared with the control was increased.38c Serum superoxide dismutase (SOD) activity was significantly lower in GG or GA carriers than in AA carriers in diabetic patients.38c SOD catalyzes the conversion of superoxide into oxygen and hydrogen peroxide and represents an important antioxidant defense mechanism against oxidative stress. DM patients overproduce superoxide and their antioxidant system is usually weakened. As a result, damage in multiple organs, such as renal injuries or diabetic cardiomyopathy, occurs.39 Furthermore, the rs8052394 A allele was associated with an increased risk of oral squamous cell carcinoma (OSCC). The AA genotype is associated with alteration of homeostasis of zinc and copper, which may affect molecules containing one of those metals, such as p53. The abnormal expression of MT may also affect its antioxidant role.38d Furthermore Wang et al. found, that rs8052394 GA and GG carriers with increased methylmercury (MeHg) intake had lower levels of mercury in their hair compared to subjects with the AA genotype.38e4.3 MT1A polymorphism rs11076161
This SNP (c. 29-28A>G) is a polymorphism in the first intron of MT1A. Yang et al. found that rs11076161 is significantly related to the occurrence of diabetic neuropathy in the T2DM patients and is explained by the decreased neuronal protection against oxidative stress.38c rs11076161 A allele carriers also showed a protective trend against oral squamous cell carcinoma (OSCC, odds ratio (OR) = 0.53).38d5 MT1B polymorphisms
55 human SNPs were identified in the MT1B gene region according to the NCBI database (as of January 2013). Of these, two are located in the 5′UTR, 15 in the coding sequence, 27 in introns, and 11 in the 3′UTR region. Three of these polymorphisms (rs964372, rs8052394, and rs7191779) have a significant association with diseases (see below)38c,d,40 (Fig. 2).5.1 MT1B polymorphism rs964372
This SNP is located in the MT1B intron 1 (c.28+137C>G). Significant association was observed between this SNP and decreased utilization of fatty acids in T2DM patients.38c These carriers were characterized by hyperlipidemia with increased serum triglycerides and neuropathy. Furthermore, a protective trend was observed between OSCC and rs964372 C allele carriers (OR = 0.49).38d Increased risk of hepatocellular carcinoma was also observed in individuals carrying haplotype AGT of the MT1A rs8052394 A allele, the MT1B rs964372 G allele, and the MT1B rs8052334 T allele (2.25-fold) compared to the most common ACT haplotype.40 In particular, this risk was highlighted in smokers carrying the AGT haplotype (a 6.72-fold increased risk of HCC development). A possible explanation is the different efficiency of individual MT1Bs in coping with oxidative stress induced by smoking.405.2 MT1B polymorphism rs8052334
This SNP is located in the MT1B intron 2 (c.95-68T>C). As indicated above, increased risk of hepatocellular carcinoma was observed in individuals carrying haplotype AGT of the MT1A rs8052394 A allele, the MT1B rs964372 G allele, and the MT1B rs8052334 T allele in smokers.405.3 MT1B polymorphism rs7191779
This SNP is located upstream of the MT1B gene region on chromosome 16 (c.-1975C>G). Similarly, rs7191779 C allele carriers also showed a protective trend against OSCC (OR = 0.36).38d6 MT1M polymorphisms
MT1M, another MT superfamily gene is transcriptionally regulated by both heavy metals and glucocorticoids. The MT1M gene was shown to induce changes of the cell cycle and activate the NF-κB pathway in Hep-G2 cells.41 In total, 29 SNPs were identified, two of which are located in the 5′UTR, 10 in the coding sequence, 12 in introns, and 5 in the 3′UTR region. Two of them were associated with a disease38e (Fig. 2).6.1 MT1M polymorphism rs2270836
rs2270836 is an intron 2 SNP in the MT1M gene (c.95-49G>A). Wang et al. found a significant effect of the minor homozygote genotype AA of MT1M (rs2270836) on urinary Hg levels. Multivariate regression analysis showed that subjects with the AA genotype had lower urinary mercury levels than those with the GG genotype because the binding of heavy metals varies depending on the molecular structures of MTs.38e6.2 MT1M polymorphism rs9936741
This is a 3′UTR single nucleotide polymorphism at the 327th position of the MT1M mRNA sequence (c.*31T>C). As reported by Wang et al., subjects with the TT genotype had lower hair mercury levels than subjects with TC and CC genotypes, respectively, after controlled MeHg intake.38e7 Other functional MT1 polymorphisms
We found a high level of inconsistency between databases with regard to MT1 polymorphisms. Hence, the data from NCBI dbSNP are reported in this review. In case no polymorphisms are indexed in the NCBI dbSNP database, the SNPper database entry is mentioned. To date, there is no evidence of polymorphisms other than those mentioned to be associated with any given pathological condition. However, the number of polymorphisms without known effects was identified for MT1s. There were 13 coding, 4 in 5′UTR, 19 intron and 2 in 3′UTR in the MT1F gene (according to NCBI), 3 coding, 3 in 5′UTR, 10 intron and 14 in 3′UTR in the MT1G gene (according to SNPper, no information in NCBI), 19 coding, 3 in 5′UTR, 29 intron and 8 in 3′UTR in the MT1H gene (according to NCBI), and 6 coding, 3 in 5′UTR, 34 intron and 8 in 3′UTR in the MT1X gene (according to NCBI).8 MT2A polymorphisms
According to NCBI (dbSNP), 24 human SNPs were identified in the MT2A gene region, 4 of them are located in the 5′UTR, 7 in the coding sequence, 9 in introns and 4 in the 3′UTR region (January 2013). Three MT2A polymorphisms with significant impact on the physiological and pathophysiological processes were mentioned in recent publications23c,38a,c,e,42 (Fig. 3).8.1 MT2A polymorphism rs28366003
rs28366003 is an A/G substitution located in the center of the MREa-like consensus sequence TGCACTC (c.-77A>G). This polymorphism was studied in Japanese, Polish, and Turkish populations. The genotype frequencies of AA, AG, and GG were identified in these studies as follows: 82%, 17%, and 0.9% in the Japanese, 88.9%, 10.6%, and 0.5% in the Polish, and 87%, 12.3%, and 0.7% in the Turkish population.42a–c McElroy et al. determined the frequency of the A and G alleles in Caucasian and Afro-American females in the Midwestern United States. The frequency of the G allele was 1.1% in Afro-Americans and 6.4% in Caucasians, which was less frequent than the Japanese and Turkish populations.43 The exact effect of this polymorphism is still not fully understood. Since this SNP position is located in the 5′ regulation region, it is possible that the A/G substitution produces allele-specific MT2A gene expression. Using reporter-gene assay with HEK293 cells, Kita et al. observed that this SNP inhibits the binding of nuclear proteins to the core promoter region of the MT2A gene. As a result, this polymorphism should decrease the induction of gene transcription. It was confirmed that this SNP reduced cadmium-induced transcription of the MT2A gene in the HEK293cells.42aKayaalti et al. in the study of metal levels in kidney autopsies found considerably high accumulation of Cd in individuals having AG and GG genotypes compared with individuals having the AA genotype.38a Furthermore, the G allele carriers also had higher blood levels of cadmium and lead and lower levels of zinc.23c The critical tissue level of cadmium is 200 μg g−1 and exceeding this limit may cause renal dysfunction.44 Absorption of the Cd and Pb is also associated with a decrease in Zn, as a result of the antagonistic relationships between these elements.23b Consequently, rs28366003 SNP could become a promising indicator of increased risk of diseases associated with the exposure to Cd and ROS. Nevertheless, an association between MT2A promoter polymorphisms and sporadic amyotrophic lateral sclerosis (ALS) in a Japanese population was not confirmed although reactive oxygen species (ROS) are assumed to be involved in the pathogenesis of ALS.45
As was shown in another study, maternal blood Cd levels were statistically higher in women with the AG genotype compared to the AA genotype. In contrast, placental Cd levels were significantly higher in mothers with the AA rather than the AG genotype.42d Another study suggested that blood lead levels of the heterozygote genotype (AG) in pregnant women were statistically higher than those of the homozygote genotype (AA) (P < 0.05).46
Since the rs28366003 SNP is located in the center of the MRE consensus sequence, the A/G substitution might alter MT2A gene expression. As a result, the MT protein level could decrease, and differences in cancer development and prognosis might be expected in these allele carriers.
The association between rs28366003 and prostate cancer was shown in a study of the Polish population.42c Compared to homozygous common allele carriers, heterozygous carriers had a significantly increased risk of prostate cancer (OR = 2.30). The association between SNP in MT2A and clinicopathological parameters, such as the prostate-specific antigen (PSA) level, Gleason score (histological grading), tumour stage, and prostate volume, was analyzed. The statistically significant association was only found between rs28366003 and the Gleason score. The AG genotype and the G allele were more frequently found in patients with Gleason score >7 tumors.42c
8.2 MT2A polymorphism rs1610216
Polymorphism rs1610216 is located in the promoter region of the MT2A gene (c.-284C>T). The genotype frequencies of AA, AG, and GG are 90.5%, 0.0%, and 9.5%, respectively, as shown in the healthy Bulgarian population.42k Because of the position in the promoter, this SNP could influence the level of MT expression. Overexpression of MT in various metabolic organs was shown to reduce hyperglycemia-induced oxidative stress, organ specific diabetic complications like diabetic cardiomyopathy,49 nephropathy,50 and DNA damage in diabetic experimental animals, as shown in comparison to MT-knockout mice.51According to Giacconi et al., the AA, rather than the AG, genotype of the rs1610216 polymorphism is associated with chronic inflammation (higher plasma levels of IL-6), hyperglycaemia, enhanced glycosylated hemoglobin (HbA1c), and marked zinc deficiency in atherosclerosis patients. AA patients are at higher risk of developing type 1 DM in association with atherosclerosis (OR = 2.6) and related complications, such as ischaemic cardiomyopathy (OR = 12.6). Nevertheless, no association between this polymorphism and hypertension has been found.42h
Conversely, in the Bulgarian cohort, the G allele (compared to the AA-genotype) was identified as an independent predictor for the development of diabetes without cardiovascular complications (OR = 7.56).42k
8.3 MT2A polymorphism rs10636
This is a 3′ untranslated region MT2A single nucleotide polymorphism (c.*77G>C). The genotype frequencies are 58.3%, 33.3%, and 8.3% for the GG, GC, and CC alleles in the Caucasian population, respectively.42gYang et al. found that rs10636 is significantly related to the occurrence of diabetic neuropathy and hyperlipidemia in the T2DM patients.38c An earlier study compared rs10636 MT2A polymorphisms in 288 patients with atherosclerosis and 218 healthy elderly controls. The GG carriers had a higher risk of atherosclerosis than controls. Furthermore, a significant decrease in intracellular zinc, decreased serum zinc and copper levels, and increased inflammatory cytokines were shown in (C–) carriers. A major incidence of soft carotid plaques was also significantly associated with this allele. This MT2A polymorphism influences inflammatory status, zinc availability, NK cell cytotoxicity, and trace element levels, all of which may promote plaque development.42i
In another research, Gundacker and co-workers42g studied the association between the rs10636 in MT2A and Pb levels. They found a negative association between non-wild type variants and blood lead.42g According to the work of Giacconi et al.,42i the C allele of rs10636 is also associated with decreased NK cell cytotoxicity and increased MCP-1 (monocyte chemotactic protein-1) levels in patients suffering from carotid artery stenosis. Zinc deficiency may be responsible for decreased NK cell activity and enhanced plasma concentrations of MCP-1. MCP-1 was increased in atherosclerotic lesions in humans as well as in animal models.42i Chen et al. identified a weak association of blood cadmium levels and the rs10636 allele variant in females living in the densely polluted conditions.42j Multivariate regression analysis showed that subjects with the rsl0636 CC genotype had lower urinary mercury levels than those with the GG genotype.38e
9 MT3 polymorphisms
MT3 (also called the growth inhibitory factor, GIF, GIFB or GRIF) is expressed primarily in the central nervous system and small amounts of protein can also be traced in the pancreas and intestines.16b MT3 binds Zn and Cd ions more weakly than MT2.52 MT3 functions as a growth inhibitory factor. It plays a major role in the organization and apoptosis of brain cells, and, compared to other MT superfamily members, plays unique biological roles in various neuropathological disorders.19,53Inter alia, MT3 was revealed as a neuron outgrowth inhibitor in Alzheimer's disease.53,54 In total, 32 human SNPs were identified according to the NCBI, 3 of which are located in the 5′UTR region, 4 in the coding sequence, 15 in introns, and 10 in the 3′UTR region. Only one relevant MT3 polymorphism with a significant impact on the physiological and pathophysiological processes was mentioned in recent publications55 (Fig. 3).
9.1 MT3 polymorphism rs45570941
This polymorphism, which is an intron 2 SNP in the MT3 gene (c.97+377G>C), was studied in a Chinese population of autistic children. Significant differences in the frequencies of rs45570941 genotypes and alleles between autistic children and controls were found55b (χ2 = 13.569, P < 0.05 and χ2 = 6.89, P < 0.05, respectively). Heavy metal toxicity was proposed as a hypothetical cause of autism. More specifically, the dysfunction of the MT synthesis and activity may be the major driver of this disorder. Numerous heavy metals, including mercury, lead, and arsenic, were linked with symptoms that resemble the neurological symptoms of autism.55a10 MT4 polymorphisms
The MT4 gene is located about 20 kb upstream from the MT3 gene and is expressed solely in the epithelia of upper parts of the digestive tract, in the footpads, and in the neonatal skin.56 Histologically, MT4 mRNA was detected in the differentiating spinous layer of cornified epithelia, whereas in the basal layer MT1 expression prevailed.56 Furthermore, MT4 showed better Cu binding properties than MT1.57According to NCBI dbSNP, two polymorphisms are located in the coding sequence and 55 in introns. Of these, only one MT4 polymorphism (rs396230) is known to have a significant impact on the physiological and pathophysiological processes58 (Fig. 3).
10.1 MT4 polymorphism rs396230
This is an intron 2 SNP (c.98-137 A>G) in the MT4 gene. Since MT4 is involved in the detoxification of lead, rs396230 genotypes and renal functions were investigated. Serum creatinine, urea, and uric acid levels were measured by Chen et al. as indicators of renal function in a cohort of lead battery-recycling factory workers (65.5% had AA, 15.9% AG, and 18.6% GG genotypes). Workers with the AG genotype had higher serum creatinine and urea levels. Furthermore, after adjusting potential confounders, blood pressure and serum uric acid values showed a positive correlation with AA, AG, and GG genotypes (AA genotype strongest). Workers with the G allele had blood pressures more than 10 mmHg higher than those with the AA genotype.5811 Conclusion
MTs play an important role in the cell transportation and management of metals and have a crucial role in the detoxification processes. MTs expression and their ability to bind metals can be influenced by changes at the DNA level, such as SNPs. In addition to changes in metal levels, some single nucleotide polymorphisms (SNPs) were found to be associated with predisposition to various diseases including cancer, cardiovascular diseases, and faster aging, confirming the key role of MTs in organisms against oxidative stress and toxic metals (Fig. 4).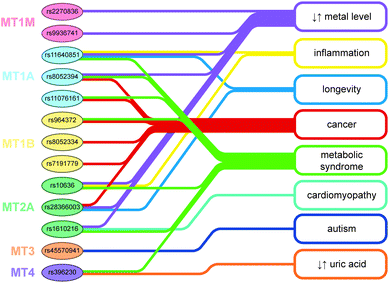 | ||
| Fig. 4 Association of metallothionein polymorphisms with pathological conditions and laboratory changes. See the distinct relation between polymorphisms, metal level change, and disease occurrence. Polymorphism names are in the rs NCBI format. Clinical/laboratory conditions and MT genes are color-coded. Most frequent linkage includes metabolic syndrome-related pathologies (including diabetes and its complications, atherosclerosis, high blood pressure and dyslipoproteinaemia), disbalance of various metal levels, and cancers. For a more detailed view, see Table 1. ↑↓ – alteration of concentration. | ||
| Gene | NCBI rs number | SNP | Associated with | Ref. |
|---|---|---|---|---|
| MT1A | rs11640851 | Non-synonymous in the coding region | Longevity | 38a |
| Cardiovascular disease | 38b | |||
| T2DM | 38b | |||
| MT levels | 38b | |||
| IL-6 plasma concentrations | 38b | |||
| rs8052394 | Non-synonymous in the coding region | T2DM | 38c | |
| Serum superoxide dismutase | 38c | |||
| OSCC | 38d | |||
| Hair mercury levels | 38e | |||
| HCC | 40 | |||
| rs11076161 | In intron | Diabetic neuropathy | 38c | |
| OSCC | 38d | |||
| MT1B | rs964372 | In intron | Hyperlipidemia | 38c |
| Diabetic neuropathy | 38c | |||
| OSCC | 38d | |||
| HCC | 40 | |||
| rs8052334 | In intron | HCC | 40 | |
| rs7191779 | Upstream gene region | OSCC | 38d | |
| MT1M | rs2270836 | In intron | Urinary Hg level | 38e |
| rs9936741 | In 3′UTR | Hair Hg levels | 38e | |
| MT2A | rs28366003 | In the 5′UTR regulatory region | Cd blood levels | 23c |
| Pb blood levels | 23c | |||
| Zn blood levels | 23c | |||
| Autopsy kidney Cd levels | 38a | |||
| Maternal blood Cd levels | 42d | |||
| Placental Cd levels | 42d | |||
| Maternal blood Pb levels | 46 | |||
| Longevity | 59 | |||
| Prostate cancer | 42c | |||
| rs10636 | In 3′UTR | Diabetic neuropathy | 38c | |
| Hyperlipidemia in T2DM | 38c | |||
| Atherosclerosis | 42i | |||
| Intracellular Zn availability | 42i | |||
| Serum Zn and Cu levels | 42i | |||
| Inflammatory cytokines levels | 42i | |||
| Soft carotid plaques incidence | 42i | |||
| Pb blood levels | 42g | |||
| NK cell cytotoxicity | 42i | |||
| Monocyte chemotactic protein-1 | 42i | |||
| Urinary Hg levels | 38e | |||
| Cd blood levels | 42j | |||
| rs1610216 | In the promoter region | Chronic inflammation | 42h | |
| Hyperglycaemia | 42h | |||
| Glycated hemoglobin | 42h | |||
| Zinc deficiency | 42h | |||
| NIDDM | 42h | |||
| Ischaemic cardiomyopathy | 42h | |||
| Development of diabetes | 42k | |||
| MT3 | rs45570941 | In intron | Autism in children | 55b |
| MT4 | rs396230 | In intron | Blood pressure | 58 |
| Serum uric acid | 58 | |||
Although there are numerous association studies investigating the effects of MT polymorphisms in humans, the large level of nomenclature inaccuracy causes an unprecedented degree of confusion in the literature. In addition, the classification of MTs with a clear separation between functional genes and pseudogenes is lacking. Using a systematic approach, we identified in total 388 MT SNPs, of which 18 studies linked 13 MT SNPs in 6 MT paralogs with various pathological conditions. Of these, the most frequent linkage includes metabolic syndrome-related pathologies (including diabetes and its complications, atherosclerosis, high blood pressure and dyslipoproteinaemia) and disbalance of various metal levels, each associated with 7 SNPs. These disorders were followed by cancers, associated with 6 SNPs. The largest numbers of associations have been demonstrated in MT1A and MT2A. These findings emphasize the role of MT in the development and progression of a number of pathological conditions. In addition, the possibility of using MT polymorphisms as risk indicators for a large spectrum of diseases is obvious. However, it is worth noting that because some studies show associations in the laboratory setting only, while others investigate true clinical conditions, there are still missing pieces of the puzzle that would allow a more comprehensive understanding of the issue. Thus, association studies with a more systematic approach are highly desirable.
Conflict of interest
The authors declare they have no competing interests as defined by Metallomics, or other interests that might be perceived to influence the results and discussion reported in this paper.Acknowledgements
Financial support from CEITEC CZ.1.05/1.1.00/02.0068, doc CEITEC.02/2012 (JG), and project for conceptual development of research organization 00064203 is greatly acknowledged.References
- (a) J. Busciglio and B. A. Yankner, Apoptosis and increased generation of reactive oxygen species in Downs-syndrome neurons in-vitro, Nature, 1995, 378, 776–779, DOI:10.1038/378776a0; (b) J. C. A. Marr, H. L. Bergman, J. Lipton and C. Hogstrand, Differences in relative sensitivity of naive and metals-acclimated brown and rainbow-trout exposed to metals representative of the clark-fork river, montana, Can. J. Fish. Aquat. Sci., 1995, 52, 2016–2030, DOI:10.1139/f95-793; (c) S. Silver, Bacterial resistances to toxic metal ions – a review, Gene, 1996, 179, 9–19, DOI:10.1016/s0378-1119(96)00323-x; (d) T. K. S. Janssens, D. Roelofs and N. M. van Straalen, Molecular mechanisms of heavy metal tolerance and evolution in invertebrates, Insect Sci., 2009, 16, 3–18, DOI:10.1111/j.1744-7917.2009.00249.x.
- (a) V. Adam, I. Fabrik, T. Eckschlager, M. Stiborova, L. Trnkova and R. Kizek, Vertebrate metallothioneins as target molecules for analytical techniques, TrAC, Trends Anal. Chem., 2010, 29, 409–418, DOI:10.1016/j.trac.2010.02.004; (b) V. Adam, J. Petrlova, J. Wang, T. Eckschlager, L. Trnkova and R. Kizek, Zeptomole electrochemical detection of metallothioneins, PLoS One, 2010, 5, e11441, DOI:10.1371/journal.pone.0011441; (c) S. Krizkova, I. Fabrik, V. Adam, P. Hrabeta, T. Eckschlager and R. Kizek, Metallothionein – a promising tool for cancer diagnostics, Br. Med. J., 2009, 110, 93–97 CAS.
- B. Y. Lee, D. H. Shin, S. Cho, K. S. Seo and H. Kim, Genome-wide analysis of copy number variations reveals that aging processes influence body fat distribution in Korea Associated Resource (KARE) cohorts, Hum. Genet., 2012, 131, 1795–1804, DOI:10.1007/s00439-012-1203-1.
- P. Seibold, R. Hein, P. Schmezer, P. Hall, J. J. Liu, N. Dahmen, D. Flesch-Janys, O. Popanda and J. Chang-Claude, Polymorphisms in oxidative stress-related genes and postmenopausal breast cancer risk, Int. J. Cancer, 2011, 129, 1467–1476, DOI:10.1002/ijc.25761.
- Y. Koga, M. Pelizzola, E. Cheng, M. Krauthammer, M. Sznol, S. Ariyan, D. Narayan, A. M. Molinaro, R. Halaban and S. M. Weissman, Genome-wide screen of promoter methylation identifies novel markers in melanoma, Genome Res., 2009, 19, 1462–1470, DOI:10.1101/gr.091447.109.
- L. Marselli, J. Thorne, S. Dahiya, D. C. Sgroi, A. Sharma, S. Bonner-Weir, P. Marchetti and G. C. Weir, Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes, PLoS One, 2010, 5, e11499, DOI:10.1371/journal.pone.0011499.
- M. Hlavna, M. Raudenska, K. Hudcova, J. Gumulec, M. Sztalmachova, V. Tanhauserova, P. Babula, V. Adam, T. Eckschlager, R. Kizek and M. Masarik, MicroRNAs and zinc metabolism-related gene expression in prostate cancer cell lines treated with zinc(II) ions, Int. J. Oncol., 2012, 41, 2237–2244, DOI:10.3892/ijo.2012.1655.
- J. T. den Dunnen and S. E. Antonarakis, Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion, Hum. Mutat., 2000, 15, 7–12, DOI:10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N.
- F. S. Collins, L. D. Brooks and A. Chakravarti, A DNA polymorphism discovery resource for research on human genetic variation, Genome Res., 1998, 8, 1229–1231 CAS.
- (a) E. Alexov, Numerical calculations of the pH of maximal protein stability – the effect of the sequence composition and three-dimensional structure, Eur. J. Biochem., 2004, 271, 173–185, DOI:10.1046/j.1432-1033.2003.03917.x; (b) V. Chelliah, L. Chen, T. L. Blundell and S. C. Lovell, Distinguishing structural and functional restraints in evolution in order to identify interaction sites, J. Mol. Biol., 2004, 342, 1487–1504, DOI:10.1016/j.jmb.2004.08.022; (c) D. Gilis and M. Rooman, Predicting protein stability changes upon mutation using database-derived potentials: solvent accessibility determines the importance of local versus non-local interactions along the sequence, J. Mol. Biol., 1997, 272, 276–290, DOI:10.1006/jmbi.1997.1237.
- J. V. Chamary, J. L. Parmley and L. D. Hurst, Hearing silence: non-neutral evolution at synonymous sites in mammals, Nat. Rev. Genet., 2006, 7, 98–108, DOI:10.1038/nrg1770.
- H. Akashi, Synonymous codon usage in Drosophila-melanogaster – natural-selection and translational accuracy, Genetics, 1994, 136, 927–935 CAS.
- (a) Y. Xing and C. Lee, Assessing the application of Ka/Ks ratio test to alternatively spliced exons, Bioinformatics, 2005, 21, 3701–3703, DOI:10.1093/bioinformatics/bit613; (b) A. G. Nackley, S. A. Shabalina, I. E. Tchivileva, K. Satterfield, O. Korchynskyi, S. S. Makarov, W. Maixner and L. Diatchenko, Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure, Science, 2006, 314, 1930–1933, DOI:10.1126/science.1131262.
- (a) C. C. Chang and P. C. Huang, Semi-empirical simulation of Zn/Cd binding site preference in the metal binding domains of mammalian metallothionein, Protein Eng., 1996, 9, 1165–1172, DOI:10.1093/protein/9.12.1165; (b) M. Fedurco and I. Sestakova, Adsorption of Cd,Zn-metallothionein on covered Hg electrodes and its voltammetric determination, Bioelectrochem. Bioenerg., 1996, 40, 223–232, DOI:10.1016/0302-4598(96)01914-9; (c) X. L. Yu, M. Wojciechowski and C. Fenselau, Assessment of metals in reconstituted metallothioneins by electrospray mass-spectrometry, Anal. Chem., 1993, 65, 1355–1359, DOI:10.1021/ac00058a010.
- (a) J. Abel and N. Deruiter, Inhibition of hydroxyl-radical-generated DNA-degradation by metallothionein, Toxicol. Lett., 1989, 47, 191–196, DOI:10.1016/0378-4274(89)90075-1; (b) L. Cai, J. B. Klein and Y. J. Kang, Metallothionein inhibits peroxynitrite-induced DNA and lipoprotein damage, J. Biol. Chem., 2000, 275, 38957–38960, DOI:10.1074/jbc.C000593200; (c) P. J. Thornalley and M. Vasak, Possible role for metallothionein in protection against radiation-induced oxidative stress – kinetics and mechanism of its reaction with superoxide and hydroxyl radicals, Biochim. Biophys. Acta, 1985, 827, 36–44, DOI:10.1016/0167-4838(85)90098-6.
- (a) P. Babula, M. Masarik, V. Adam, T. Eckschlager, M. Stiborova, L. Trnkova, H. Skutkova, I. Provaznik, J. Hubalek and R. Kizek, Mammalian metallothioneins: properties and functions, Metallomics, 2012, 4, 739–750, 10.1039/c2mt20081c; (b) E. Tokuda, S. I. Ono, K. Ishige, A. Naganuma, Y. Ito and T. Suzuki, Metallothionein proteins expression, copper and zinc concentrations, and lipid peroxidation level in a rodent model for amyotrophic lateral sclerosis, Toxicology, 2007, 229, 33–41, DOI:10.1016/j.tox.2006.09.011; (c) J. W. Bauman, J. Liu, Y. P. Liu and C. D. Klaassen, Increase in metallothionein produced by chemicals that induce oxidative stress, Toxicol. Appl. Pharmacol., 1991, 110, 347–354, DOI:10.1016/s0041-008x(05)80017-1; (d) G. K. Andrews, Regulation of metallothionein gene expression by oxidative stress and metal ions, Biochem. Pharmacol., 2000, 59, 95–104, DOI:10.1016/s0006-2952(99)00301-9.
- M. Karin, R. L. Eddy, W. M. Henry, L. L. Haley, M. G. Byers and T. B. Shows, Human metallothionein genes are clustered on chromosome-16, Proc. Natl. Acad. Sci. U. S. A., 1984, 81, 5494–5498, DOI:10.1073/pnas.81.17.5494.
- (a) F. A. Stennard, A. F. Holloway, J. Hamilton and A. K. West, Characterization of 6 additional human metallothionein genes, Biochim. Biophys. Acta, Gene Struct. Expression, 1994, 1218, 357–365, DOI:10.1016/0167-4781(94)90189-9; (b) A. K. West, R. Stallings, C. E. Hildebrand, R. Chiu, M. Karin and R. I. Richards, Human metallothionein genes – structure of the functional locus at 16q13, Genomics, 1990, 8, 513–518, DOI:10.1016/0888-7543(90)90038-v; (c) Z. M. Liu, G. G. Chen, C. K. Y. Shum, A. C. Vlantis, M. G. Cherlan, J. Koropatnick and C. A. van Hasselta, Induction of functional MT1 and MT2 isoforms by calcium in anaplastic thyroid carcinoma cells, FEBS Lett., 2007, 581, 2465–2472, DOI:10.1016/j.febslet.2007.04.049.
- Y. Uchida, F. Gomi, T. Masumizu and Y. Miura, Growth inhibitory factor prevents neurite extension and the death of cortical neurons caused by high oxygen exposure through hydroxyl radical scavenging, J. Biol. Chem., 2002, 277, 32353–32359, DOI:10.1074/jbc.M111263200.
- G. Meloni, K. Zovo, J. Kazantseva, P. Palumaa and M. Vasak, Organization and assembly of metal-thiolate clusters in epithelium-specific metallothionein-4, J. Biol. Chem., 2006, 281, 14588–14595, DOI:10.1074/jbc.M601724200.
- R. Faraonio, P. Moffatt, O. LaRochelle, H. M. Schipper, R. S-Arnaud and C. Seguin, Characterization of cis-acting elements in the promoter of the mouse metallothionein-3 gene – activation of gene expression during neuronal differentiation of P19 embryonal carcinoma cells, Eur. J. Biochem., 2000, 267, 1743–1753, DOI:10.1046/j.1432-1327.2000.01167.x.
- (a) Z. Y. Huang, Q. Zhang, J. Chen, Z. X. Zhuang and X. R. Wang, Bioaccumulation of metals and induction of metallothioneins in selected tissues of common carp (Cyprinus carpio L.) co-exposed to cadmium, mercury and lead, Appl. Organomet. Chem., 2007, 21, 101–107, DOI:10.1002/aoc.1167; (b) M. F. McAleer and R. S. Tuan, Metallothionein overexpression in human trophoblastic cells protects against cadmium-induced apoptosis, In Vitro Mol. Toxicol., 2001, 14, 25–42 CrossRef CAS PubMed.
- (a) Y. P. Liu, J. Liu, S. M. Habeebu, M. P. Waalkes and C. D. Klaassen, Metallothionein-I/II null mice are sensitive to chronic oval cadmium-induced nephrotoxicity, Toxicol. Sci., 2000, 57, 167–176, DOI:10.1093/toxsci/57.1.167; (b) A. U. R. Memon, T. G. Kazi, H. I. Afridi, M. K. Jamali, M. B. Arain, N. Jalbani and N. Syed, Evaluation of zinc status in whole blood and scalp hair of female cancer patients, Clin. Chim. Acta, 2007, 379, 66–70, DOI:10.1016/j.cca.2006.12.009; (c) Z. Kayaalti, V. Aliyev and T. Soylemezogiu, The potential effect of metallothionein 2A-5 A/G single nucleotide polymorphism on blood cadmium, lead, zinc and copper levels, Toxicol. Appl. Pharmacol., 2011, 256, 1–7, DOI:10.1016/j.taap.2011.06.023; (d) T. Eckschlager, V. Adam, J. Hrabeta, K. Figova and R. Kizek, Metallothioneins and cancer, Curr. Protein Pept. Sci., 2009, 10, 360–375, DOI:10.2174/138920309788922243.
- (a) H. Goulding, B. Jasani, H. Pereira, A. Reid, M. Galea, J. A. Bell, C. W. Elston, J. F. Robertson, R. W. Blamey, R. A. Nicholson, K. W. Schmid and I. O. Ellis, Metallothionein expression in human breast-cancer, Br. J. Cancer, 1995, 72, 968–972, DOI:10.1038/bjc.1995.443; (b) H. G. Kim, J. Y. Kim, E. H. Han, Y. P. Hwang, J. H. Choi, B. H. Park and H. G. Jeong, Metallothionein-2A overexpression increases the expression of matrix metalloproteinase-9 and invasion of breast cancer cells, FEBS Lett., 2011, 585, 421–428, DOI:10.1016/j.febslet.2010.12.030.
- J. I. Izawa, M. Moussa, M. G. Cherian, G. Doig and J. L. Chin, Metallothionein expression in renal cancer, Urology, 1998, 52, 767–772, DOI:10.1016/s0090-4295(98)00323-9.
- (a) J. Gumulec, M. Masarik, S. Krizkova, M. Hlavna, P. Babula, R. Hrabec, A. Rovny, M. Masarikova, J. Sochor, V. Adam, T. Eckschlager and R. Kizek, Evaluation of alpha-methylacyl-CoA racemase, metallothionein and prostate specific antigen as prostate cancer prognostic markers, Neoplasma, 2012, 59, 191–200, DOI:10.4149/neo_2012_025; (b) H. Wei, M. M. Desouki, S. Lin, D. Xiao, R. B. Franklin and P. Feng, Differential expression of metallothioneins (MTs) 1, 2, and 3 in response to zinc treatment in human prostate normal and malignant cells and tissues, Mol. Cancer, 2008, 7 DOI:10.1186/1476-4598-7-7; (c) J. Gumulec, M. Masarik, S. Krizkova, V. Adam, J. Hubalek, J. Hrabeta, T. Eckschlager, M. Stiborova and R. Kizek, Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma, Curr. Med. Chem., 2011, 18, 5041–5051, DOI:10.2174/092986711797636126; (d) M. Masarik, J. Gumulec, M. Hlavna, M. Sztalmachova, P. Babula, M. Raudenska, M. Pavkova-Goldbergova, N. Cernei, J. Sochor, O. Zitka, B. Ruttkay-Nedecky, S. Krizkova, V. Adam and R. Kizek, Monitoring of the prostate tumour cells redox state and real-time proliferation by novel biophysical techniques and fluorescent staining, Integr. Biol., 2012, 4, 672–684, 10.1039/c2ib00157h; (e) M. Masarik, J. Gumulec, M. Sztalmachova, M. Hlavna, P. Babula, S. Krizkova, M. Ryvolova, M. Jurajda, J. Sochor, V. Adam and R. Kizek, Isolation of metallothionein from cells derived from aggressive form of high-grade prostate carcinoma using paramagnetic antibody-modified microbeads off-line coupled with electrochemical and electrophoretic analysis, Electrophoresis, 2011, 32, 3576–3588, DOI:10.1002/elps.201100301; (f) S. Krizkova, M. Ryvolova, J. Hrabeta, V. Adam, M. Stiborova, T. Eckschlager and R. Kizek, Metallothioneins and zinc in cancer diagnosis and therapy, Drug Metab. Rev., 2012, 44, 287–301, DOI:10.3109/03602532.2012.725414; (g) V. Pekarik, J. Gumulec, M. Masarik, R. Kizek and V. Adam, Prostate cancer, miRNAs, metallothioneins and resistance to cytostatic drugs, Curr. Med. Chem., 2013, 20, 534–544, DOI:10.2174/092986713804910102; (h) J. K. Choi, U. S. Yu, O. J. Yoo and S. Kim, Differential coexpression analysis using microarray data and its application to human cancer, Bioinformatics, 2005, 21, 4348–4355, DOI:10.1093/bioinformatics/bti722.
- (a) S. Suemori, M. Shimazawa, K. Kawase, M. Satoh, H. Nagase, T. Yamamoto and H. Hara, Metallothionein, an endogenous antioxidant, protects against retinal neuron damage in mice, Invest. Ophthalmol. Visual Sci., 2006, 47, 3975–3982, DOI:10.1167/iovs.06-0275; (b) K. Wakida, M. Shimazawa, I. Hozumi, M. Satoh, H. Nagase, T. Inuzuka and H. Hara, Neuroprotective effect of erythropoietin, and role of metallothionein-1 and -2, in permanent focal cerebral ischemia, Neuroscience, 2007, 148, 105–114, DOI:10.1016/j.neuroscience.2007.04.063; (c) M. Penkowa, S. Florit, M. Giralt, A. Quintana, A. Molinero, J. Carrasco and J. Hidalgo, Metallothionein reduces central nervous system inflammation, neuro degeneration, and cell death following kainic acid-induced epileptic seizures, J. Neurosci. Res., 2005, 79, 522–534, DOI:10.1002/jnr.20387.
- A. E. Michalska and K. H. A. Choo, Targeting and germ-line transmission of a null mutation at the metallothionein I-loci and II-loci in mouse, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 8088–8092, DOI:10.1073/pnas.90.17.8088.
- (a) B. A. Masters, E. J. Kelly, C. J. Quaife, R. L. Brinster and R. D. Palmiter, Targeted disruption of metallothionein-I and metallothionein-II genes increases sensitivity to cadmium, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 584–588, DOI:10.1073/pnas.91.2.584; (b) L. L. Pearce, R. E. Gandley, W. P. Han, K. Wasserloos, M. Stitt, A. J. Kanai, M. K. McLaughlin, B. R. Pitt and E. S. Levitan, Role of metallothionein in nitric oxide signaling as revealed by a green fluorescent fusion protein, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 477–482, DOI:10.1073/pnas.97.1.477; (c) J. Carrasco, M. Penkowa, H. Hadberg, A. Molinero and J. Hidalgo, Enhanced seizures and hippocampal neurodegeneration following kainic acid-induced seizures in metallothionein-I plus II-deficient mice, Eur. J. Neurosci., 2000, 12, 2311–2322, DOI:10.1046/j.1460-9568.2000.00128.x.
- (a) Y. Y. Yang, P. D. Robbins and J. S. Lazo, Differential transactivation of human metallothionein-IIa in cisplatin-resistant and -sensitive cells, Oncol. Res., 1998, 10, 85–98 CAS; (b) M. Karin, A. Haslinger, A. Heguy, T. Dietlin and T. Cooke, Metal-responsive elements act as positive modulators of human metallothionein-IIA enhancer activity, Mol. Cell. Biol., 1987, 7, 606–613 CrossRef CAS PubMed.
- M. P. Waalkes, M. J. Harvey and C. D. Klaassen, Relative in vitro affinity of hepatic metallothionein for metals, Toxicol. Lett., 1984, 20, 33–39, DOI:10.1016/0378-4274(84)90179-6.
- (a) R. D. Palmiter, Regulation of metallothionein genes by heavy-metals appears to be mediated by a zinc-sensitive inhibitor that interacts with a constitutively active transcription factor, MTF-1, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 1219–1223, DOI:10.1073/pnas.91.4.1219; (b) L. J. Hager and R. D. Palmiter, Transcriptional regulation of mouse-liver metallothionein-I gene by glucocorticoids, Nature, 1981, 291, 340–342, DOI:10.1038/291340a0.
- W. Lee, A. Haslinger, M. Karin and R. Tjian, Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40, Nature, 1987, 325, 368–372, DOI:10.1038/325368a0.
- A. Haslinger and M. Karin, Upstream promoter element of the human metallothionein-IIA gene can act like an enhancer element, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 8572–8576, DOI:10.1073/pnas.82.24.8572.
- F. Otsuka, A. Iwamatsu, K. Suzuki, M. Ohsawa, D. H. Hamer and S. Koizumi, Purification and characterization of a protein that binds to metal-responsive elements of the human metallothionein II(A) gene, J. Biol. Chem., 1994, 269, 23700–23707 CAS.
- (a) M. Karin, A. Haslinger, H. Holtgreve, R. I. Richards, P. Krauter, H. M. Westphal and M. Beato, Characterization of DNA-sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIa gene, Nature, 1984, 308, 513–519, DOI:10.1038/308513a0; (b) K. Suzuki and S. Koizumi, Individual metal responsive elements of the human metallothionein-IIA gene independently mediate responses to various heavy metal signals, Ind. Health, 2000, 38, 87–90, DOI:10.2486/indhealth.38.87.
- M. Ohtsuji, F. Katsuoka, A. Kobayashi, H. Aburatani, J. D. Hayes and M. Yamamoto, Nrf1 and Nrf2 Play distinct roles in activation of antioxidant response element-dependent genes, J. Biol. Chem., 2008, 283, 33554–33562, DOI:10.1074/jbc.M804597200.
- (a) Z. Kayaalti, G. Mergen and T. Soylemezoglu, Effect of metallothionein core promoter region polymorphism on cadmium, zinc and copper levels in autopsy kidney tissues from a Turkish population, Toxicol. Appl. Pharmacol., 2010, 245, 252–255, DOI:10.1016/j.taap.2010.03.007; (b) C. Cipriano, M. Malavolta, L. Costarelli, R. Giacconi, E. Muti, N. Gasparini, M. Cardelli, D. Monti, E. Mariani and E. Mocchegiani, Polymorphisms in MT1a gene coding region are associated with longevity in Italian central female population, Biogerontology, 2006, 7, 357–365, DOI:10.1007/s10522-006-9050-x; (c) L. Yang, H. Y. Li, T. Yu, H. J. Zhao, M. G. Cherian, L. Cai and Y. Liu, Polymorphisms in metallothionein-1 and -2 genes associated with the risk of type 2 diabetes mellitus and its complications, Am. J. Physiol.: Endocrinol. Metab., 2008, 294, E987–E992, DOI:10.1152/ajpendo.90234.2008; (d) A. I. Zavras, A. J. Yoon, M. K. Chen, C. W. Lin and S. F. Yang, Metallothionein-1 genotypes in the risk of oral squamous cell carcinoma, Ann. Surg. Oncol., 2011, 18, 1478–1483, DOI:10.1245/s10434-010-1431-3; (e) Y. Wang, J. M. Goodrich, B. Gillespie, R. Werner, N. Basu and A. Franzblau, An investigation of modifying effects of metallothionein single-nucleotide polymorphisms on the association between mercury exposure and biomarker levels, Environ. Health Perspect., 2012, 120, 530–534, DOI:10.1289/ehp.1104079.
- (a) F. R. DeRubertis, P. A. Craven and M. F. Melhem, Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: effects of tempol, Metab., Clin. Exp., 2007, 56, 1256–1264, DOI:10.1016/j.metabol.2007.04.024; (b) T. Nishikawa, D. Edelstein, X. L. Du, S. Yamagishi, T. Matsumura, Y. Kaneda, M. A. Yorek, D. Beebe, P. J. Oates, H. P. Hammes, I. Giardino and M. Brownlee, Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage, Nature, 2000, 404, 787–790, DOI:10.1038/35008121; (c) V. Elangovan, E. Shohami, I. Gati and R. Kohen, Increased hepatic lipid soluble antioxidant capacity as compared to other organs of streptozotocin-induced diabetic rats: a cyclic voltammetry study, Free Radical Res., 2000, 32, 125–134, DOI:10.1080/10715760000300131.
- R. H. Wong, C. H. Huang, C. B. Yeh, H. S. Lee, M. H. Chien and S. F. Yang, Effects of metallothionein-1 genetic polymorphism and cigarette smoking on the development of hepatocellular carcinoma, Ann. Surg. Oncol., 2012, 20, 2088–2095, DOI:10.1245/s10434-012-2456-6.
- L. Sun, X. Zhang, Y. Kong and L. Yu, Effect of MT1M gene on the cell cycle and signaling pathway of Hep-G2, Int. J. Androl., 2004, 10, 932–934 CAS.
- (a) K. Kita, N. Miura, M. Yoshida, K. Yamazaki, T. Ohkubo, Y. Imai and A. Naganuma, Potential effect on cellular response to cadmium of a single-nucleotide A → G polymorphism in the promoter of the human gene for metallothionein IIA, Hum. Genet., 2006, 120, 553–560, DOI:10.1007/s00439-006-0238-6; (b) Z. Kayaalti and T. Soylemezoglu, The polymorphism of core promoter region on metallothionein 2A-metal binding protein in Turkish population, Mol. Biol. Rep., 2010, 37, 185–190, DOI:10.1007/s11033-009-9586-3; (c) E. Forma, A. Krzeslak, J. Wilkosz, P. Jozwiak, A. Szymczyk, W. Rozanski and M. Brys, Metallothionein 2A genetic polymorphisms and risk of prostate cancer in a Polish population, Cancer Genet., 2012, 205, 432–435, DOI:10.1016/j.cancergen.2012.05.005; (d) D. Tekin, Z. Kayaalti, V. Aliyev and T. Soylemezoglu, The effects of metallothionein 2A polymorphism on placental cadmium accumulation: is metallothionein a modifiying factor in transfer of micronutrients to the fetus?, J. Appl. Toxicol., 2012, 32, 270–275, DOI:10.1002/jat.1661; (e) E. Mocchegiani, R. Giacconi, C. Cipriano, M. Muzzioli, N. Gasparini, R. Moresi, R. Stecconi, H. Suzuki, E. Cavalieri and E. Mariani, MtmRNA gene expression, via IL-6 and glucocorticoids, as potential genetic marker of immunosenescence: lessons from very old mice and humans, Exp. Gerontol., 2002, 37, 349–357, DOI:10.1016/s0531-5565(01)00202-9; (f) M. G. Cherian, A. Jayasurya and B. H. Bay, Metallothioneins in human tumors and potential roles in carcinogenesis, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2003, 533, 201–209, DOI:10.1016/j.mrfmmm.2003.07.013; (g) C. Gundacker, K. J. Wittmann, M. Kukuckova, G. Komarnicki, I. Hikkel and M. Gencik, Genetic background of lead and mercury metabolism in a group of medical students in Austria, Environ. Res., 2009, 109, 786–796, DOI:10.1016/j.envres.2009.05.003; (h) R. Giacconi, C. Cipriano, E. Muti, L. Costarelli, C. Maurizio, V. Saba, N. Gasparini, M. Malavolta and E. Mocchegiani, Novel-209A/G MT2A polymorphism in old patients with type 2 diabetes and atherosclerosis: relationship with inflammation (IL-6) and zinc, Biogerontology, 2005, 6, 407–413, DOI:10.1007/s10522-005-4907-y; (i) R. Giacconi, E. Muti, M. Malavolta, C. Cipriano, L. Costarelli, G. Bernardini, N. Gasparini, E. Mariani, V. Saba, G. Boccoli and E. Mocchegiani, The +838 C/G MT2A polymorphism, metals, and the inflammatory/immune response in carotid artery stenosis in elderly people, Mol. Med., 2007, 13, 388–395, DOI:10.2119/2007-00045.Giacconi; (j) X. Chen, L. J. Lei, L. T. Tian, G. Y. Zhu and T. Y. Jin, Bone mineral density and polymorphisms in metallothionein 1A and 2A in a Chinese population exposed to cadmium, Sci. Total Environ., 2012, 423, 12–17, DOI:10.1016/j.scitotenv.2012.02.020; (k) R. Kozarova, A. Postadzhiyan, B. Finkov and M. Apostolova, Association of copy number variations and single nucleotide polymorphisms in metallothionein genes with pathogenesis of diabetes and coronary artery disease, Atheroscler. Suppl., 2011, 12, 107, DOI:10.1016/S1567-5688(11)70504-9.
- J. A. McElroy, E. C. Bryda, S. D. McKay, R. D. Schnabel and J. F. Taylor, Genetic variation at a metallothionein 2A promoter single-nucleotide polymorphism in white and black females in midwestern United States, J. Toxicol. Environ. Health, Part A, 2010, 73, 1283–1287, DOI:10.1080/15287394.2010.485067.
- N. Miura, Individual susceptibility to cadmium toxicity and metallothionein gene polymorphisms: with references to current status of occupational cadmium exposure, Ind. Health, 2009, 47, 487–494 CrossRef CAS PubMed.
- Y. Hayashi, T. Hashizume, K. Wakida, M. Satoh, Y. Uchida, K. Watabe, Z. Matsuyama, A. Kimura, T. Inuzuka and I. Hozumi, Association between metallothionein genes polymorphisms and sporadic amyotrophic lateral sclerosis in a Japanese population, Amyotrophic Lateral Scler. Other Mot. Neuron Disord., 2006, 7, 22–26, DOI:10.1080/14660820600618766.
- Z. Kayaalti, D. Tekin and T. Soylemezoglu, Role of metallothionein 2A polymorphism on lead metabolism: are pregnant women with a heterozygote genotype for metallothionein 2A polymorphism and their newborns at risk of having higher blood lead levels?, Toxicol. Lett., 2011, 205, S106, DOI:10.1016/j.toxlet.2011.05.383.
- (a) K. Shibuya, N. Nishimura, J. S. Suzuki, C. Tohyama, A. Naganuma and M. Satoh, Role of metallothionein as a protective factor against radiation carcinogenesis, J. Toxicol. Sci., 2008, 33, 651–655, DOI:10.2131/jts.33.651; (b) M. P. Waalkes, J. Liu, K. S. Kasprzak and B. A. Diwan, Minimal influence of metallothionein over-expression on nickel carcinogenesis in mice, Toxicol. Lett., 2004, 153, 357–364, DOI:10.1016/j.toxlet.2004.06.003; (c) J. Zeng, B. L. Vallee and J. H. R. Kagi, Zinc transfer from transcription factor-IIIA fingers to thionein clusters, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 9984–9988, DOI:10.1073/pnas.88.22.9984; (d) R. Shimoda, W. E. Achanzar, W. Qu, T. Nagamine, H. Takagi, M. Mori and M. P. Waalkes, Metallothionein is a potential negative regulator of apoptosis, Toxicol. Sci., 2003, 73, 294–300, DOI:10.1093/toxsci/kfg095; (e) B. Werynska, B. Pula, B. Muszczynska-Bernhard, A. Gomulkiewicz, A. Jethon, M. Podhorska-Okolow, R. Jankowska and P. Dziegiel, Expression of metallothionein-III in patients with non-small cell lung cancer, Anticancer Res., 2013, 33, 965–974 Search PubMed.
- (a) A. M. L. Janssen, W. van Duijn, F. Kubben, G. Griffioen, C. Lamers, J. van Krieken, C. J. H. van de Velde and H. W. Verspaget, Prognostic significance of metallothionein in human gastrointestinal cancer, Clin. Cancer Res., 2002, 8, 1889–1896 CAS; (b) M. Moussa, D. Kloth, G. Peers, M. G. Cherian, J. V. Frei and J. L. Chin, Metallothionein expression in prostatic carcinoma: correlation with Gleason grade, pathologic stage, DNA content and serum level of prostate-specific antigen, Clin. Invest. Med., 1997, 20, 371–380 CAS.
- Q. R. Liang, E. C. Carlson, R. V. Donthi, P. M. Kralik, X. Shen and P. N. Epstein, Overexpression of metallothionein reduces diabetic cardiomyopathy, Diabetes, 2002, 51, 174–181 CrossRef CAS PubMed.
- S. R. Zheng, E. C. Carlson, L. Yang, P. M. Kralik, Y. Huang and P. N. Epstein, Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy, J. Am. Soc. Nephrol., 2008, 19, 2077–2085, DOI:10.1681/asn.2007080967.
- M. D. Apostolova, K. H. A. Choo, A. E. Michalska and C. Tohyama, Analysis of the possible protective role of metallothionein in streptozotocin-induced diabetes using metallothionein-null mice, J. Trace Elem. Med. Biol., 1997, 11, 1–7 CAS.
- P. Palumaa, I. Tammiste, K. Kruusel, L. Kangur, H. Jornvall and R. Sillard, Metal binding of metallothionein-3 versus metallothionein-2: lower affinity and higher plasticity, Biochim. Biophys. Acta, Proteins Proteomics, 2005, 1747, 205–211, DOI:10.1016/j.bbapap.2004.11.007.
- J. Hidalgo, M. Aschner, P. Zatta and M. Vasak, Roles of the metallothionein family of proteins in the central nervous system, Brain Res. Bull., 2001, 55, 133–145, DOI:10.1016/s0361-9230(01)00452-x.
- (a) A. K. Sewell, L. T. Jensen, J. C. Erickson, R. D. Palmiter and D. R. Winge, Bioactivity of metallothionein-3 correlates with its novel beta-domain sequence rather than metal-binding properties, Biochemistry, 1995, 34, 4740–4747, DOI:10.1021/bi00014a031; (b) Y. Manso, J. Carrasco, G. Comes, G. Meloni, P. A. Adlard, A. I. Bush, M. Vasak and J. Hidalgo, Characterization of the role of metallothionein-3 in an animal model of Alzheimer's disease, Cell. Mol. Life Sci., 2012, 69, 3683–3700, DOI:10.1007/s00018-012-1047-9; (c) M. C. Amoureux, D. Van Gool, M. T. Herrero, R. Dom, F. C. Colpaert and P. J. Pauwels, Regulation of metallothionein-III (GIF) mRNA in the brain of patients with Alzheimer disease is not impaired, Mol. Chem. Neuropathol., 1997, 32, 101–121, DOI:10.1007/bf02815170.
- (a) D. A. Drum, Are toxic biometals destroying your children's future?, Biometals, 2009, 22, 697–700, DOI:10.1007/s10534-009-9212-9; (b) H. Fusheng, X. Feiyan, X. Han and Z. Fang, Study on the relationship between gene polymorphism of metallothionein 3 and childhood autism, Chin. J. Clin., 2011, 5, 2559–2562 Search PubMed.
- C. J. Quaife, S. D. Findley, J. C. Erickson, G. J. Froelick, E. J. Kelly, B. P. Zambrowicz and R. D. Palmiter, Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia, Biochemistry, 1994, 33, 7250–7259, DOI:10.1021/bi00189a029.
- L. Tio, L. Villarreal, S. Atrian and M. Capdevila, Functional differentiation in the mammalian metallothionein gene family – metal binding features of mouse MT4 and comparison with its paralog MT1, J. Biol. Chem., 2004, 279, 24403–24413, DOI:10.1074/jbc.M401346200.
- H. I. Chen, Y. W. Chiu, Y. K. Hsu, W. F. Li, Y. C. Chen and H. Y. Chuang, The association of metallothionein-4 gene polymorphism and renal function in long-term lead-exposed workers, Biol. Trace Elem. Res., 2010, 137, 55–62, DOI:10.1007/s12011-009-8564-x.
- Z. Kayaalti, L. Sahiner, M. E. Durakoglugil and T. Soylemezoglu, Distributions of interleukin-6 (IL-6) promoter and metallothionein 2A (MT2A) core promoter region gene polymorphisms and their associations with aging in Turkish population, Arch. Gerontol. Geriatr., 2011, 53, 354–358, DOI:10.1016/j.archger.2011.01.001.
| This journal is © The Royal Society of Chemistry 2014 |