Photoresponsive metal–organic materials: exploiting the azobenzene switch
Rahul Dev
Mukhopadhyay
ab,
Vakayil K.
Praveen
a and
Ayyappanpillai
Ajayaghosh
*ab
aPhotosciences and Photonics Group, Chemical Sciences and Technology Division, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR NIIST), Trivandrum-695019, India. E-mail: ajayaghosh@niist.res.in
bAcademy of Scientific and Innovative Research (AcSIR), CSIR, CSIR-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Trivandrum-695019, India
First published on 27th August 2014
Abstract
Photoinduced cis–trans isomerisation of azobenzene has been an inspiration to chemists to design smart materials that respond to light. Lately, this chemistry has also made a mark in the emerging area of metal–organic materials. If one can regulate the properties of porous hybrid materials with light, their scope of applications can be expanded. The field is shown to be promising and gradually progressing from nano to mesospace. However, one needs more in-depth research to deliver materials that are truly smart and practically viable.
Introduction
Photoinduced cis–trans isomerisation is nature inspired chemistry as it is well-understood with respect to the phenomenon of vision in nature. Azobenzene photochemistry may be the most widely studied cis–trans isomerisation in an artificial molecule. The large change in the size, shape and polarity of the molecule associated with trans to cis photoisomerisation, has been extensively used to design a variety of smart materials when integrated into polymers, elastomers, liquid crystals, etc.1–3 Incorporation of azobenzene in various self-assembled soft materials like supramolecular and polymeric gels has facilitated the modulation of their reversible functional properties.4,5 While efficient photoisomerisation of azobenzene has been shown to facilitate drastic changes in properties, partial isomerisation can also be of advantage for the controlled property modulation as demonstrated with the Ostwald type ripening of organic nanodots to 1-D supramolecular rods and chopping of gel fibers.6,7 Another interesting report pertaining to partial isomerisation of an azobenzene moiety is the thermally assisted photonic inversion of supramolecular handedness in π-systems.8 The above systems deal with covalent linkages (strength, 150–1000 kJ mol−1), or non-covalent interactions such as the hydrophobic effect (strength, 12–15 kJ mol−1) and/or H-bonding (strength, 10–20 kJ mol−1). While designing porous photoresponsive metal–organic materials (MOMs), we should consider the fact that the metal–ligand bond strength can vary from moderate to very high values (strength, 40–120 kJ mol−1).9,10 These MOMs comprise of both zero dimensional structures like metal–organic polyhedra (MOP), polygons, nanoballs and nanocontainers or as polymeric networks often mentioned as infinite coordination polymers (ICPs) or porous coordination polymers (PCPs) or porous coordination networks (PCNs) and most frequently as metal–organic frameworks (MOFs).11 Any attempt to manipulate the size, shape and related properties of the above structures with azobenzene photochemistry is not that easy due to their rigid framework. However, in recent times, scientists could utilise azobenzene photoisomerisation to control their porosity thereby triggering the encapsulation and release of guest molecules. We discuss some of the exciting developments with photoresponsive MOMs, the basic design principles, the difficulties encountered in creating such photoresponsive smart materials, stressing the need for further improvements.Early developments with zero-dimensional structures
One of the earliest reports of a photoresponsive discrete metallosupramolecular cyclic nanostructure can be dated back to 2002, when Lees and co-workers synthesized a series of Pd–Re as well as Pd tetranuclear squares bridged by azobenzene linkages.12 Irradiation of these squares with UV light lead to the formation of corresponding dinuclear squares that subsequently reconverted to tetranuclear squares on thermal treatment. The Pt–Re squares did not show this transformation ability, instead a phototriggered disassembly of these metallosquares was observed.Later in 2007, Fujita and co-workers reported a ‘crystalline molecular flask’ composed of 24 pyridine based bent bridging ligands and 12 metal ion centres.13 It is known that the molecular environment experienced by a reactant in the bulk solvent is different from that of discrete metallo-supramolecular cages in solution where distinct microenvironments are created inside the hollow cavities of nanoporous MOMs. However in this case, the ligand was designed in such a way that the azobenzene functionality was attached to the concave side of the bridging ligand such that they project towards the core of the spherical complex making it hydrophobic in nature (Fig. 1). In CH3CN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent, a hydrophobic probe, 1-pyrenecarboxaldehyde was incorporated within the pores of the cage. Upon UV light irradiation, the interior of the cage became less hydrophobic as cis azobenzene is more polar than the trans isomer. As a result, the interaction between the cage and the guest was sufficiently weakened which drove the guest out from the host cavity. Thus, the photonic switching of hydrophobicity in a 0-D MOM in solution with simultaneous guest encapsulation and release was successfully demonstrated in this report.
1) solvent, a hydrophobic probe, 1-pyrenecarboxaldehyde was incorporated within the pores of the cage. Upon UV light irradiation, the interior of the cage became less hydrophobic as cis azobenzene is more polar than the trans isomer. As a result, the interaction between the cage and the guest was sufficiently weakened which drove the guest out from the host cavity. Thus, the photonic switching of hydrophobicity in a 0-D MOM in solution with simultaneous guest encapsulation and release was successfully demonstrated in this report.
 | ||
| Fig. 1 A crystalline molecular flask with an azobenzene appendage that can encapsulate a hydrophobic guest. Reproduced from ref. 13 by permission. Copyright 2007 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
Solving the ‘azobenzene riddle’ in polymeric MOMs
The early success with the 0-D MOMs encouraged researchers to use MOM nanocontainers with extended polymeric structures. These are robust structures that can withstand a range of chemical and physical processes. The guest moieties can be introduced post-synthetically inside the host and this process does not require co-crystallization of host–guest complexes from solution. Although a number of azobenzene based MOMs were reported recently, their isomerisation as direct linkers in the rigid MOM matrix was found to be difficult. Stock and co-workers, understood this problem and were able to show that a covalently linked azobenzene moiety dangling in the MOF pore walls retains its inherent isomerisation ability.14 Soon after this, the same group extended this chemistry to a variety of frameworks and also via post-synthetic modification, whereby successful photoisomerisation was carried out in the synthesized MOFs.15–17 Subsequently, Zhou and co-workers utilised this chemistry to reversibly alter CO2 uptake via photochemical and thermal treatment, see Fig. 2.18 A structural envelope generated from powder X-ray diffraction data shows that the electron density around the Zn4O clusters increased in the UV irradiated materials (cis state) than in the heat treated (trans state) ones, explaining the reason why the CO2 molecules faced a difficulty in approaching the metal clusters, the main sites of CO2 adsorption. The interpenetrated nature of MOFs can produce steric restrictions limiting such photoswitching applications as in this case, where a delay in change of gas adsorption was observed.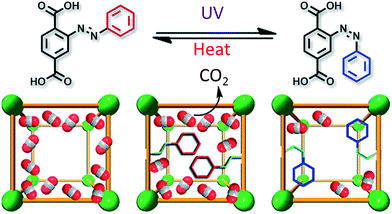 | ||
| Fig. 2 Reversible alteration of CO2 uptake in a MOF with an azobenzene appendage. Reproduced from ref. 18 by permission. Copyright 2012 American Chemical Society. | ||
Yaghi and co-workers therefore, tried to solve this problem with a MOF-74 based structure, having an azobenzene appendage.19 In this case, the magnesium clusters and organic ligands stack in a linear fashion, leading to the formation of large, non-interpenetrated 1-D hexagonal pores, see Fig. 3. Spectroscopic studies revealed that an encapsulated luminescent dye was not released under ambient conditions, but the release of the dye from the pores was triggered only upon irradiation of the MOF at the isosbestic point to induce a wagging motion of the azobenzene appendages via a dual way cis–trans isomerisation.
 | ||
| Fig. 3 A non-interpenetrated MOF with an azobenzene appendage that can encapsulate a luminescent dye as a guest. Reproduced from ref. 19 by permission. Copyright 2013 The Royal Society of Chemistry. | ||
In another strategy, Kitagawa and co-workers reported the isomerisation of azobenzene as a guest within the pores of a flexible PCP.20 The isomerisation yield was found to be higher than that in the previous reports, probably due to the less steric constraints around the azobenzene guests, see Fig. 4. The photoisomerisation of azobenzene triggered a remarkable structural transformation of the host PCP framework. The PCP with azobenzene in the trans state did not adsorb N2 due to the close contact between the host framework and the encapsulated azobenzene molecules, which caused pore blockage. Upon UV irradiation, the cis azobenzene molecules caused the expansion of the framework to a tetragonal form with an 8.3 fold increase in N2 adsorption. In this work, light induced photoisomerisation of azobenzene was utilised to trigger guest (N2) encapsulation rather than release via light induced host to guest structural transformation. Such isomerisation also depends on the close packing of guest molecules within the MOF pores.21
 | ||
| Fig. 4 Structural changes of an azobenzene guest in a soft PCP host, allow varying gas adsorption behaviour. Reproduced from ref. 20 by permission. Copyright 2012 American Chemical Society. | ||
A MOF prepared by Hill et. al., exhibits low energy photoresponsive behaviour, where a dynamic bending of the azo core was observed, although on a local scale, see Fig. 5.22 During adsorption experiments, this photoresponsive behaviour was exploited to trigger the uptake and release of CO2 in real time. On exposure to UV light, 64% of the adsorbed CO2 was instantaneously released and this was achieved using broadband radiation, much like concentrated solar sources.
 | ||
| Fig. 5 Dynamic photoswitching in MOF containing azobenzene-4,4′-dicarboxylate (AzDC) and trans-1,2-bis(4-pyridyl)ethylene (4,4′-BPE) ligands utilised for low energy CO2 uptake and release. Reproduced from ref. 22 by permission. Copyright 2013 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
Recent developments
The next bigger challenge is perhaps to control such stimuli responsive behaviour at the mesoscopic dimensions to construct devices out of functional materials for real applications.23 Unearthing unprecedented properties from MOMs by exploiting chemistry at the mesoscale is therefore no exception.24,25 In this context, photoswitching and cargo release in a dual reservoir and a valve layered surface mounted metal–organic framework (SURMOF) is a promising study, see Fig. 6a.26 Quartz crystal microbalance experiments proved that the release of the guest molecule could be optically triggered. Synthesis of such intelligent two component systems is possible by allowing the growth of a second layer of photoresponsive MOF over a SURMOF via liquid phase epitaxy. MOFs having such interfaces between two distinct mesoscale crystal boundaries can be of great significance in the near future. | ||
| Fig. 6 Photoisomerisation at the mesoscale. (a) Schematic representation of light mediated cargo capture, storage and release in a two component SURMOF. Reproduced from ref. 26 by permission. Copyright 2014 American Chemical Society. (b) Photo controlled solubility variation and cargo release in a MOP. Reproduced from ref. 27 by permission. Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA. | ||
Recently, control on the solubility and guest inclusion properties of a MOP (0 dimensional MOM) by light has been reported, (Fig. 6b).27 A MOP with azobenzene appendages pointing outside the polyhedral nanospace favours π–π interactions with an adjacent MOP leading to aggregation and formation of an insoluble precipitate. The mesoscopic pockets created in between the MOPs are suitable for inclusion of comparatively large guest molecules like methylene blue. On irradiation with UV light, the trans azobenzene appendages isomerise to the cis state, thereby disfavouring the π–π interactions, leading to the breaking down of the aggregates and increase in solubility. In addition to this, cis azobenzene, due to its higher dipole moment facilitates the solubility of the MOP in polar solvents. This disaggregation process is accompanied by simultaneous release of the trapped methylene blue molecules. Both the guest capture as well as precipitation can be reversely triggered using blue light as the opposite optical stimulus and the overall process can be repeated for several cycles.
Outlook and future perspectives
Azobenzene photochemistry has been quite successful for the design of polymers, elastomers and liquid crystal based smart materials. It has also been shown that the properties of metal nanoparticles and inorganic semiconductors can be modulated by appended azobenzene photoisomerization.28–30 However, with MOMs, an attempt to use azobenzene photochemistry to develop smart porous materials with control of the pore size for gas adsorption and release is just at a nascent stage. Therefore, the question is what next? How to explore the wonderful photochemistry of azobenzene to develop smart MOMs? In MOM chemistry what is required is improved gas storage capacity and controlled release, which can be activated with light when needed. The question is whether molecules that undergo photoisomerisation can function as a valve to simultaneously control the gas intake and release in a MOM? Significant knowledge is currently available and therefore there is a great challenge ahead of the scientific community to come up with more exciting results in the near future by taking advantage of the knowledge available, to deliver novel photoresponsive materials that were never dreamt of.Acknowledgements
We thank the Council of Scientific and Industrial Research (CSIR), Govt. of India for financial support. A.A. is grateful to the Department of Atomic Energy, Govt. of India for an Outstanding Researcher award. R.D.M. is thankful to CSIR for a research fellowship.References
- Smart Light-Responsive Materials: Azobenzene-Containing Polymers and Liquid Crystals, ed. Y. Zhao and T. Ikeda, John Wiley & Sons, Inc., Hoboken, New Jersey, 2009 Search PubMed.
- S. Yagai and A. Kitamura, Chem. Soc. Rev., 2008, 37, 1520 RSC.
- M.-M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348 CrossRef CAS PubMed.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed.
- A. Harada, Y. Takashima and M. Nakahata, Acc. Chem. Res., 2014, 47, 2128 CrossRef CAS PubMed.
- S. Mahesh, A. Gopal, R. Thirumalai and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 7227 CrossRef CAS PubMed.
- R. Rajaganesh, A. Gopal, T. M. Das and A. Ajayaghosh, Org. Lett., 2012, 14, 748 CrossRef CAS PubMed.
- A. Gopal, M. Hifsudheen, S. Furumi, M. Takeuchi and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 10505 CrossRef CAS PubMed.
- V. A. Friese and D. G. Kurth, Curr. Opin. Colloid Interface Sci., 2009, 14, 81 CrossRef CAS PubMed.
- D. J. Tranchemontagne, J. L. Mendoza-Cortés, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257 RSC.
- J. J. Perry IV, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 RSC.
- S.-S. Sun, J. A. Anspach and A. J. Lees, Inorg. Chem., 2002, 41, 1862 CrossRef CAS PubMed.
- T. Murase, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2007, 46, 5133 CrossRef CAS PubMed.
- A. Modrow, D. Zarqarani, R. Herges and N. Stock, Dalton Trans., 2011, 40, 4217 RSC.
- S. Bernt, M. Feyand, A. Modrow, J. Wack, J. Senker and N. Stock, Eur. J. Inorg. Chem., 2011, 5378 CrossRef CAS PubMed.
- A. Modrow, D. Zarqarani, R. Herges and N. Stock, Dalton Trans., 2012, 41, 8690 RSC.
- A. Modrow, M. Feyand, D. Zarqarani, R. Herges and N. Stock, Z. Anorg. Allg. Chem., 2012, 2138 CrossRef CAS PubMed.
- J. Park, D. Yuan, K. T. Pham, J.-R. Li, A. Yakovenko and H.-C. Zhou, J. Am. Chem. Soc., 2012, 134, 99 CrossRef CAS PubMed.
- J. W. Brown, B. L. Henderson, M. D. Kiesz, A. C. Whalley, W. Morris, S. Grunder, H. Deng, H. Furukawa, J. I. Zink, J. F. Stoddart and O. M. Yaghi, Chem. Sci., 2013, 4, 2858 RSC.
- N. Yanai, T. Uemura, M. Inoue, R. Matsuda, T. Fukushima, M. Tsujimoto, S. Isoda and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 4501 CrossRef CAS PubMed.
- D. Hermann, H. Emerich, R. Lepski, D. Schaniel and U. Ruschewitz, Inorg. Chem., 2013, 52, 2744 CrossRef CAS PubMed.
- R. Lyndon, K. Konstas, B. P. Ladewig, P. D. Southon, C. J. Kepert and M. R. Hill, Angew. Chem., Int. Ed., 2013, 52, 3695 CrossRef CAS PubMed.
- R. F. Service, Science, 2012, 335, 1167 CrossRef CAS PubMed.
- J. Reboul, S. Furukawa, N. Horike, M. Tsotsalas, K. Hirai, H. Uehara, M. Kondo, N. Louvain, O. Sakata and S. Kitagawa, Nat. Mater., 2012, 11, 717 CrossRef CAS PubMed.
- Y. Sakata, S. Furukawa, M. Kondo, K. Hirai, N. Horike, Y. Takashima, H. Uehara, N. Louvain, M. Meilikhov, T. Tsuruoka, S. Isoda, W. Kosaka, O. Sakata and S. Kitagawa, Science, 2013, 339, 193 CrossRef CAS PubMed.
- L. Heinke, M. Cakici, M. Dommaschk, S. Grosjean, R. Herges, S. Bräse and C. Wöll, ACS Nano, 2014, 8, 1463 CrossRef CAS PubMed.
- J. Park, L.-B. Sun, Y.-P. Chen, Z. Perry and H.-C. Zhou, Angew. Chem., Int. Ed., 2014, 53, 5842 CrossRef CAS PubMed.
- R. Klajn, P. J. Wesson, K. J. M. Bishop and B. A. Grzybowski, Angew. Chem., Int. Ed., 2009, 48, 7035 CrossRef CAS PubMed.
- O. Nachtigall, C. Kördel, L. H. Urner and R. Haag, Angew. Chem., Int. Ed., 2014, 53, 9669 CrossRef CAS PubMed.
- A. Ikegami, M. Suda, T. Watanabe and Y. Einaga, Angew. Chem., Int. Ed., 2010, 49, 372 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |



