First sub-kilogram-scale synthesis of high quality ultrathin tellurium nanowires†
Kai
Wang‡
,
Yuan
Yang‡
,
Hai-Wei
Liang‡
,
Jian-Wei
Liu
and
Shu-Hong
Yu
*
Division of Nanomaterials and Chemistry, Hefei National Laboratory for Physical Sciences at Microscale, Collaborative Innovation Center of Suzhou Nano Science and Technology, Department of Chemistry, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: shyu@ustc.edu.cn; Fax: +86 0551-63603040
First published on 28th January 2014
Abstract
Ultrathin semiconductor nanowires as ideal nanoscale building blocks have gained much research attention. However, the macroscopic-scale synthesis of high quality nanowires remains a challenge in the field of nanowire science and technology. Here, we demonstrate the first sub-kilogram-scale synthesis of ultrathin tellurium nanowires (TeNWs) by a one-pot scale-up hydrothermal process. As much as 150 g of uniform TeNWs with diameters of 7–9 nm and lengths of several micrometers can be synthesized. The ultra-large-scale synthesized TeNWs also can be used as a versatile template to prepare a family of high quality metal telluride nanowires in large quantities because of their high chemical reactivity and excellent dispersibility in various solvents. This research sheds light on the possibility of ultra-large-scale synthesis of high quality nanowires in the future, which is of crucial significance from the viewpoint of practical applications of functional inorganic nanowires.
Currently, research on nanomaterials has attracted more and more attention, not only because of their unique morphological structures and their mystique—invisible to the naked eye—but also for their excellent performance in catalytic, biomedical, optical, magnetism, electronics and energy applications etc.1,2 However, in order to allow nanomaterials to be truly applied in daily production and life, it is necessary to realize their large-scale production, which also is a big problem for nanotechnology at present. So far, after much hard work, a variety of nanomaterials can be up-scale synthesized. For example, Hyeon and coworkers have reported many results on the large-scale synthesis of magnetite nanoparticles. One of the most significant achievements was that 40 g of monodisperse magnetite nanocrystals could be synthesized successfully in a single reaction,3 and they have also reported that TaO nanoparticles,4 Cu2O nanocubes,5 CdSe nanosheets6 and Bi nanocrystals7 could be prepared in a large scale. Research on the large-scale synthesis of quantum dots (QDs), which are a type of important nanomaterials with outstanding optical properties, has also made great progress in recent years, such as InP/ZnS QDs,8 silica-hybridized CdTe QDs9 and CdS QDs.10 What's more, the gram-scale synthesis of Fe2O3 nanorings,11 SiO2 nanorattles,12 Bi2S3 nanodots13 and Bi2S3 necklace nanowires14 have been reported.
1D nanomaterials have attracted a lot of attention because of their basic scientific research value, unique performance and functionality.15 As an important chemical element, elemental tellurium and its corresponding tellurides also attracted a lot of interest for their 1D nanostructures. So far, a variety of tellurium nanowires (TeNWs) with different lengths and diameters have been synthesized through different methods.16–18 However, the synthesis of 1D telluride nanostructures mainly relies on template-assisted chemical transformations. Because of the limitation of the synthesis methods and the experimental conditions, both the uniformity and the quality of the nanowires will be very difficult to guarantee if the synthesis scale is enlarged. Nevertheless, to obtain plentiful high-quality nanowires by using the templating strategy, the large-scale synthesis of high-quality and uniform template materials is highly desirable.19 To the best of our knowledge, the sub-kilogram-scale syntheses of high quality ultrathin tellurium nanowires have not been reported to date.
Over the last several years, our group has also carried out intensive research on template-directed synthesis by using highly reactive TeNWs as templates. Firstly, the high-quality ultrathin tellurium nanowires with a diameter of only several nanometers and a high aspect ratio were first synthesized to act as an excellent template,20 and then the uniform telluride nanowires, 1D noble metal nanomaterials and carbonaceous nanofibers could be prepared through template-directed synthesis, such as CdTe, PbTe,21 Bi2Te3,22 Ag2Te, Cu2Te,23 and Pt or Pd24 nanowires. Additionally, the as-prepared carbonaceous nanofibers could also be used as the template to synthesize other multifunctional nanofibers.25 All of the above proved that the ultrathin TeNWs are emerging as important nanomaterials and as an excellent template to access a rich family of ultrathin nanowires. However, the only fly in the ointment is that the limited scale synthesis of these nanomaterials is strongly dependent on the output of the precursor templates. Thus, ultra-large-scale synthesis of these high quality nanowires is highly desirable.
Herein, we demonstrate the first sub-kilogram-scale synthesis of high quality ultrathin tellurium nanowires (TeNWs) by a reproducible and facile chemical solution approach. As much as 150 g of uniform TeNWs with diameters of 7–9 nm and lengths of several micrometers can be simply synthesized through an one-pot scale-up hydrothermal process, which implies the possibility to prepare a family of metal telluride nanowires in large quantities in consideration of their highly chemical reactivity and excellent dispersibility in various solvents.
As is known to all, a popular method to synthesize 1D telluride nanomaterials involves using the TeNWs as templates.19,20 Therefore, it is highly important to achieve the large-scale synthesis of TeNWs for the mass-production of high-quality telluride materials. In the previous report,21 the concentrations of the reactants were very low and the volume of the reaction vessel was only 50 mL, which greatly influenced the yield of the TeNWs. To increase the output of the TeNWs, it is necessary to increase the concentration of the reactants and the volume of the reaction container. So after improving the synthesis method on the basis of the above theory, we succeeded in carrying out the mass-production of ultrathin TeNWs.
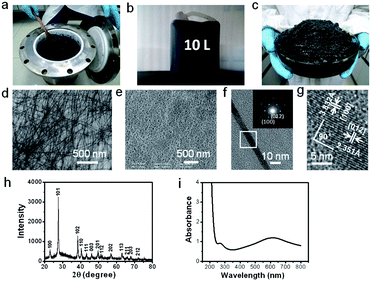
Fig. 1 shows the up-scale synthesized ultrathin TeNWs which were prepared in 12 L of double distilled water with 1.36 M of Na2TeO3 at 180 °C in the 16 L Teflon-lined stainless steel autoclave in one pot. Firstly, the X-ray diffraction (XRD) pattern exhibited in Fig. 1h testified that the up-scale as-synthesized products were pure hexagonal-phase Te, as there were no other peaks in existence, and all the diffraction peaks were in good agreement with the standard literature of hexagonal-phase Te (JCPDS 36-1452). Because the concentrations of the reactants that we used in the synthetic experiment were very high, the products were very concentrated and looked like mud (Fig. 1a). The photograph of the products which were held in a 10 L plastic drum container without any treatment (Fig. 1b) and the photograph of the products in a petri plate which were extracted by acetone (Fig. 1c) illustrate that the output of the TeNWs was significant. Additionally, about 6.5 g TeNW powder can be obtained by adding an appropriate amount of acetone into 500 mL solution and washing with acetone several times, before drying in vacuum at 60 °C (ESI, Fig. S1a†). It was demonstrated that our products could be purified very simply, as shown in the photographs of the partial sample which were extracted by acetone in a beaker (ESI, Fig. S1b and S1c†). The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images illustrate that the products were all wire-like 1D nanostructures, and the nanowires had diameters of 7–9 nm and lengths of tens of micrometers (Fig. 1d and e). Additionally, the TEM image of a single Te nanowire was also observed, and the selected area was detected by high-resolution TEM (HRTEM) (Fig. 1f and g). As marked in Fig. 1h, the lattice spacings were measured as 3.82 Å and 2.35 Å, corresponding to the (100) and (012) planes of hexagonal Te, respectively. The corresponding selected area electron diffraction (SAED) pattern (the inset in Fig. 1f) could further prove the above results; meanwhile the results also indicated that the TeNWs possessed good single crystallinity. Lastly, it can be seen from Fig. 1i that the UV-vis absorption spectrum of the TeNWs with deep blue color had two broad typical absorption peaks located at 278 nm and 586 nm, respectively, which are consistent with the results reported previously. All of the above results clearly demonstrate that the up-scale synthesis of ultrathin TeNWs can be realized, which have great potential for serving as templates for the synthesis of other 1D nanomaterials with uniform morphology and high quality. The BET and pore size of the as-prepared TeNWs have also been detected (ESI, Fig. S2†). Nitrogen adsorption isotherm analysis reveals a Brunauer–Emmett–Teller (BET) surface area of 1.1489 m2 g−1 for the TeNWs, which is a very low value. There are no obvious pores in the as-prepared TeNWs, which is consistent with the SEM and TEM observations. Additionally, the reaction instrument is shown in Fig. S2 (ESI†). Fig. S3a† shows the photograph of the 16 L Teflon-lined stainless steel autoclave and Fig. S3b† exhibits the diagrammatic drawing of the autoclave. The shell material of the autoclave is 316 stainless steel and the shell thickness is about 23 mm; the inner diameter of the Teflon lining is 142 mm, the height is 250 mm and the wall thickness is 15 mm. Moreover, the part in the red circle is the air pressure protection valve, and the maximum pressure that it can support is 12 MPa.
The detailed process of the up-scale synthesis method will now be discussed. Firstly, the changes of the concentrations of the reactants would directly influence the yield of the products. Fig. S4† shows TEM images of the as-synthesized samples prepared in 32 mL double distilled water with the addition of different concentrations of Na2TeO3 in a 50 mL Teflon-lined stainless steel autoclave (ESI†). When increasing the molar quantity of Na2TeO3 to 1.35 mmol, 2.26 mmol, 3.16 mmol and 4.52 mmol respectively, it can be seen from Fig. S4a–d† that all of the samples exhibited wire-like nanostructures with lengths of tens of micrometers and diameters of 7–9 nanometers, with the same high quality and uniformity as the products that were reported previously (ESI†). However, when increasing the molar quantity of Na2TeO3 to 6.78 mmol and 9.04 mmol, the products were still nanowires, but the lengths and diameters of the nanowires were only several micrometers and 12–15 nanometers, respectively (ESI, Fig. S4e and f†). This result suggests that the increasing of the concentration of reactant should be controlled in a certain range. Otherwise, it would affect the diameters and the uniformity of the TeNWs. As the concentration of the reactant can not be increased without limitation, the capacity of the Teflon vessel determines the output of as-synthesized TeNWs in one pot.
Simultaneously, the influence of the amount of PVP on the TeNWs was studied in order to find a suitable amount of PVP to form the uniform nanowires. Fig. S5† shows TEM images of the samples which were synthesized using the same experimental conditions as above, except for the amount of PVP in a 50 mL Teflon-lined stainless steel autoclave (ESI†). It can clearly be seen that when reducing the amount of PVP to 0.7 g, 0.5 g and 0.3 g, the wire-like nanostructures of the samples were not uniform, which was specifically manifested by the different diameters of each nanowire (ESI, Fig. S5a–c†). However, when increasing the amount of PVP to 1.5 g, compared to the typical sample, there was no difference in morphology and uniformity (ESI, Fig. S5d†). Thus, we can conclude that the amount of PVP has a significant influence on the quality of the nanostructures of the samples, and it should be kept in a specific range to obtain high quality nanowires.
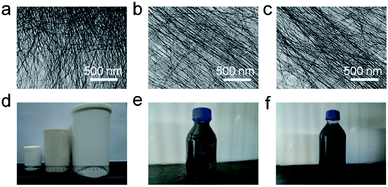
After choosing the suitable concentration of the reactant, the volume of the reaction vessel was gradually enlarged to ensure more product was obtained in the one-pot reaction. It is generally known that the reactor usually used for hydrothermal reactions is composed of a Teflon vessel and a stainless steel autoclave. As the volume of the reactor becomes larger, the thickness of the Teflon vessel and stainless steel autoclave must be thicker to ensure safety, especially when it is used at high temperature. However, increasing the thickness of the Teflon vessel and stainless steel autoclave will greatly influence the heat transfer process. If we do not consider these factors, the reaction will not occur at all. However, when the increase of the scope of the reactor volume is not very big and the airflow in the oven is strong enough, the influence of the wall thickness can be neglected. Therefore, as long as the proportion of the concentration of the raw precursors is expanded, we can successfully synthesize the TeNWs with a high-yield in Teflon vessels with volumes of 100 mL, 500 mL and 1600 mL. The TEM images of the as-prepared TeNWs showed that the particles were uniform nanowires with diameters of 7–9 nm and lengths of several micrometers (Fig. 2a–c). The photograph in Fig. 2d shows three Teflon vessels with different volumes. The thicknesses of the vessels are not much different from each other. The photographs shown in Fig. 2e and f indicate that as much as 400 mL and 1200 mL of the nanowire dispersion with deep blue color could be prepared in one pot in the Teflon-lined stainless steel autoclaves with volumes of 500 mL and 1600 mL, respectively.
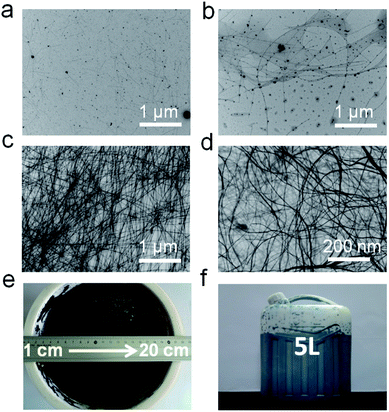
As mentioned above, when increasing the volume of the reactor to 5 L, the thickness of the Teflon vessel and stainless steel autoclave would markedly change, while the heat transfer would also change greatly which would be predicted to influence the process of the reaction. This effect would slow up the reaction, so time-dependent experiments were performed to find the best synthesis conditions. The TEM images shown in Fig. 3 exhibit the morphology evolution of the samples that were synthesized in 4 L double distilled water with 0.452 mol Na2TeO3 and 100 g PVP at 180 °C for different reaction times. When the reaction was set at 2 h, only nanoparticles and nanorods were formed in the solution (Fig. 2a). Meanwhile, when the reaction time was prolonged to 3 h, some nanowires with low aspect ratios appeared in the solution (Fig. 3b), which indicates that the reaction time for the synthesis of TeNWs in a 5 L reactor was not enough. Then, many more nanowires with fewer nanoparticles were obtained (Fig. 3c) when the reaction was further prolonged to 5 h. Finally, the sample which was produced with a reaction time of 6 h featured all wire-like nanostructures with diameters of 7–9 nm and lengths of several micrometers without any nanoparticles (Fig. 3d). Herein, the best reaction time for the up-scale synthesis of ultrathin TeNWs in a 5 L reactor should be set at 6 h. Similarly, the reaction time for the up-scale synthesis of ultrathin TeNWs in a 16 L reactor should be prolonged to 10 h. Fig. 3e and f show photographs of the as-synthesized TeNW magma held in the 16 L Teflon vessel and a 10 L plastic drum container, respectively. It can be summarized that with the increase of the reactor volume, because of the influence of the heat transfer, the reaction time should change correspondingly, which was required to be regulated carefully.
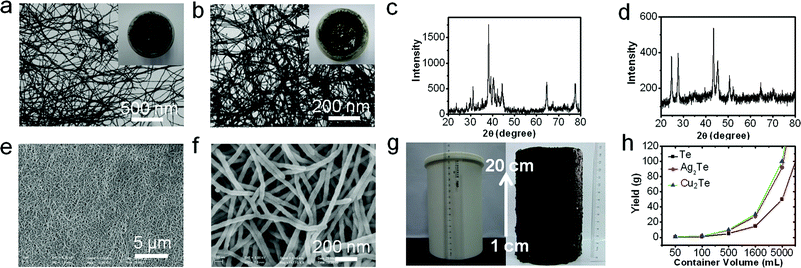
Recently, Ag2Te nanowires have been used as templates to synthesize single crystalline CdTe, PbTe, ZnTe and Pt2Te nanowires through chemical transformation by Jeong and coworkers.26 Similarly, 1D Bi2Te3–Te heterojunctions and Bi2Te3 nanotubes have been prepared through the solvothermal method with the assistance of TeNWs.27 In order to confirm the high chemical activity of the up-scale synthesized TeNWs and their ability for large-scale template-directed synthesis, Ag2Te, Cu2Te nanowires and carbonaceous nanofibers (CNFs) have been successfully prepared.26,28 The XRD patterns proved that the obtained Ag2Te and Cu2Te samples had good crystallinity and were of pure phase (Fig. 4b and d). In addition, their diffraction peaks were consistent with hexagonal-phase Ag2Te (JCPDS 34-0124) and Cu2Te (JCPDS 10-0421) reported in the literature, respectively. The TEM images show that the as-synthesized Ag2Te and Cu2Te samples both had one-dimensional wire-like nanostructures with diameters of 15–17 nm, respectively (Fig. 4a and c). The insets in Fig. 4a and c are the photographs of the as-prepared highly concentrated Ag2Te and Cu2Te nanowires. Apart from the Ag2Te and Cu2Te nanowires demonstrated in the present study, we believe that other telluride nanowires, such as CdTe, PbTe and Bi2Te3, can also be prepared by using the up-scale prepared TeNWs as templates. Additionally, we also tried to fabricate a three dimensional CNF gel with the assistance of the up-scale synthesized TeNWs, which is an important functional nanomaterial.28 From the different magnifications of the SEM images in Fig. 4e and f, it can be seen that the CNF gel sample presents a well-defined net-like structure which consists of one dimensional nanowires. Fig. 4g shows a photograph of the 800 mL Teflon-lined stainless steel autoclave which was used as the reaction vessel, and a photograph of the as-prepared monolithic wet gel. According to the above results, it can be concluded that the up-scale synthesized TeNWs have the same chemical activity as the previous reported TeNWs.
Thus, by improving the experimental conditions, we can successfully obtain high quality ultrathin TeNWs with a high output in one-pot. The product not only has the same morphology and physical properties, but also has the same chemical reactivity compared to the previously reported TeNWs. However, the high chemical reactivity causes the problem that the TeNWs can be oxidized very easily in air, so it is hard to calculate the yield by weighing directly. To calculate the actual yield, we first extracted them from the mother liquor by adding acetone. Then through detecting the residual TeO32− in the mixed solution by ICP, we could calculate the approximate quantity of TeNWs that could be practically synthesized in a one-pot reaction. Because of the influence of the heat transfer, the production efficiency would decrease with the increase of the volume of the reaction containers (ESI, Fig. S6†). This means that 150 g pure TeNWs could be prepared in one pot by using the 16 L autoclave. If there is a bigger container, many more high quality TeNWs can be prepared in a one-pot reaction. It also can be concluded that if there is a large enough container allowing us to use 150 g TeNWs as raw materials for the synthesis of Cu2Te and Ag2Te, we can obtain as much as 276 g Cu2Te and 300 g Ag2Te, respectively. The relationship between the quantity of the different products and the volume of the reaction containers can be demonstrated as a simple linear dependence, as shown in Fig. 4h. In particular, if the same amount of TeNWs is used to synthesize CNFs, theoretically about 45 kg CNFs can be produced in a one-pot reaction. To the best of our knowledge, such abundant high-quality uniform ultrathin nanowires synthesized by a one-pot reaction have not been reported before. The reproducible synthesis method demonstrated here will open a new avenue to access a large family of metal telluride nanowires, polymer and carbon nanofibers, as well as their hybrids.
Conclusions
In conclusion, we have demonstrated the first sub-kilogram-scale synthesis of high quality ultrathin tellurium nanowires by a scale-up hydrothermal process. The success of the scale-up synthesis of high quality TeNWs is of great significance in the field of nanowire nanotechnology, not only because it can provide a highly reactive template to scale-up the synthesis of other 1D nanostructures, but it can also guarantee the stability, homogeneity and reproducibility of the nanowires in the synthetic process, making it possible for practical applications in technical fields. The successful access to high quality nanowires in large quantities with the consideration of their bulk scale assembling behavior29 will make it possible for a broad range of practical applications of these functional nanowires in the future, such as printable electronics and nanowire painting for memory and logic devices.30 On the basis of this, it is possible to design and fabricate new macroscopic-scale composite nanowire films or nanowire monoliths for tailoring their functionalities. Further work is definitely needed to further optimize the reaction conditions and explore the possibility for the industrial production of this type of technically important nanowires.Acknowledgements
We acknowledge the funding support from the Ministry of Science and Technology of China (Grants 2012BAD32B05-4, 2010CB934700, 2013CB933900, 2014CB931800), the National Natural Science Foundation of China (Grants 91022032, 91227103, 21061160492), and the Chinese Academy of Sciences (Grant KJZD-EW-M01-1).Notes and references
- K. J. Klabunds, Nanoscale Materials in Chemistry, Wiley-Interscience 2001 Search PubMed.
- G. Schmid, Nanoparticles: from theory to application, Wiley-VCH, Weinheim, 2004 Search PubMed.
- J. Park, K. An, Y. Hwang, J. G. Park, H. J. Noh, J. Y. Kim, J. H. Park, N. M. Hwang and T. Hyeon, Adv. Mater., 2004, 3, 891–895 CAS.
- M. H. Oh, N. Lee, H. Kim, S. P. Park, Y. Piao, J. Lee, S. W. Jun, W. K. Moon, S. H. Choi and T. Hyeon, J. Am. Chem. Soc., 2011, 133, 5508 CrossRef CAS PubMed.
- J. C. Park, J. Kim, H. Kwon and H. Song, Adv. Mater., 2009, 21, 803 CrossRef CAS.
- J. S. Son, X. D. Wen, J. Joo, J. Chae, S. I. Baek, K. Park, J. H. Kim, K. An, J. H. Yu, S. G. Kwon, S. H. Choi, Z. W. Wang, Y. W. Kim, Y. Kuk, R. Hoffmann and T. Hyeon, Angew. Chem., Int. Ed., 2009, 48, 6861 CrossRef CAS PubMed.
- J. S. Son, K. Park, M. K. Han, C. Kang, S. G. Park, J. H. Kim, W. Kim, S. J. Kim and T. Hyeon, Angew. Chem., Int. Ed., 2011, 50, 1363 CrossRef CAS PubMed.
- K. Kim, S. Jeong, J. Y. Woo and C. S. Han, Nanotechnology, 2012, 23, 065602 CrossRef PubMed.
- L. Zhou, C. Gao, X. Z. Hu and W. J. Xu, ACS Appl. Mater. Interfaces, 2010, 2, 1211 CAS.
- X. M. Liu, Y. Jiang, X. Z. Lan, S. Y. Li, D. Wu, T. T. Han, H. H. Zhong and Z. P. Zhang, J. Colloid Interface Sci., 2011, 354, 15 CrossRef CAS PubMed.
- C. J. Jia, L. D. Sun, F. Luo, X. D. Han, L. J. Heyderman, Z. G. Yan, C. H. Yan, K. Zheng, Z. Zhang, M. Takano, N. Hayashi, M. Eltschka, M. Klaui, U. Rudiger, T. Kasama, L. Cervera-Gontard, R. E. Dunin-Borkowski, G. Tzvetkov and J. Raabe, J. Am. Chem. Soc., 2008, 130, 16968 CrossRef CAS PubMed.
- D. Chen, L. Li, F. Tang and S. Qi, Adv. Mater., 2009, 21, 3804 CrossRef CAS.
- K. Ai, Y. Liu, J. Liu, Q. Yuan, Y. He and L. Lu, Adv. Mater., 2011, 23, 4886 CrossRef CAS PubMed.
- L. Cademartiri, R. Malakooti, P. G. O'Brien, A. Migliori, S. Petrov, N. P. Kherani and G. A. Ozin, Angew. Chem., Int. Ed., 2008, 47, 3814 CrossRef CAS PubMed.
- Y. N. Xia, P. D. Yang, Y. G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and Y. Q. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS.
- Q. Y. Lu, F. Gao and S. Komarneni, Adv. Mater., 2004, 16, 1629 CrossRef CAS.
- Y. J. Zhu, W. W. Wang, R. J. Qi and X. L. Hu, Angew. Chem., Int. Ed., 2004, 43, 1410 CrossRef CAS PubMed.
- Z. Y. Tang, Y. Wang, K. Sun and N. A. Kotov, Adv. Mater., 2005, 17, 358 CrossRef CAS.
- G. D. Moon, S. Ko, Y. Min, J. Zeng, Y. N. Xia and U. Jeong, Nano Today, 2011, 6, 186 CrossRef CAS PubMed.
- H. S. Qian, S. H. Yu, J. Y. Gong, L. B. Luo and L. F. Fei, Langmuir, 2006, 22, 3830 CrossRef CAS PubMed.
- H. W. Liang, S. Liu, Q. S. Wu and S. H. Yu, Inorg. Chem., 2009, 48, 4927 CrossRef CAS PubMed.
- K. Wang, H. W. Liang, W. T. Yao and S. H. Yu, J. Mater. Chem., 2011, 21, 15057 RSC.
- J. W. Liu, J. Xu, H. W. Liang, K. Wang and S. H. Yu, Angew. Chem., Int. Ed., 2012, 51, 7420 CrossRef CAS PubMed.
- G. Q. Zhang, W. Wang and X. G. Li, Adv. Mater., 2008, 20, 3654 CrossRef CAS.
- H. W. Liang, W. J. Zhang, Y. N. Ma, X. Cao, Q. F. Guan, W. P. Xu and S. H. Yu, ACS Nano, 2011, 5, 8148 CrossRef CAS PubMed.
- G. D. Moon, S. Ko, Y. N. Xia and U. Jeong, ACS Nano, 2010, 4, 2307 CrossRef CAS PubMed.
- H. W. Liang, S. Liu, J. Y. Gong, S. B. Wang, L. Wang and S. H. Yu, Adv. Mater., 2009, 21, 1850 CrossRef CAS.
- H. W. Liang, Q. F. Guan, L. F. Chen, Z. Zhu, W. J. Zhang and S. H. Yu, Angew. Chem., Int. Ed., 2012, 51, 5101 CrossRef CAS PubMed.
- J. W. Liu, H. W. Liang and S. H. Yu, Chem. Rev., 2012, 112, 4770 CrossRef CAS PubMed.
- (a) H. W. Liang, J. W. Liu, H. S. Qian and S. H. Yu, Acc. Chem. Res., 2013, 46, 1450 CrossRef CAS PubMed; (b) H. W. Liang, S. Liu and S. H. Yu, Adv. Mater., 2010, 22, 3925 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4mh00004h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |