Side-chain fullerene polyesters: a new class of high refractive index polymers†
Honghong
Yan
a,
Shuang
Chen
a,
Min
Lu
a,
Xiang
Zhu
a,
Yanqing
Li
b,
Dezhen
Wu
c,
Yingfeng
Tu
*a and
Xiulin
Zhu
a
aJiangsu Key Laboratory of Advanced Functional Polymer Design and Application, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China. E-mail: tuyingfeng@suda.edu.cn; Fax: +86-512-65882130; Tel: +86-512-65882130
bJiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, Suzhou 215123, P. R. China
cState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China
First published on 17th January 2014
Abstract
By introducing flexible spacers between pendant fullerenes and the backbone, side-chain fullerene polyesters with high fullerene content and good solubility were synthesized by condensation polymerization. These polyesters are a new class of soluble high refractive index polymers (HRIPs) with the highest value of 1.79 at the sodium D line (589 nm).
Fullerene-based polymers have attracted great interest as they incorporate the good processability of polymeric materials with fullerene's special electronic and optical properties.1–12 Because of the multiple additions of free radicals and nucleophiles onto fullerenes, polymerization of fullerene monomers by free radical, anionic or metal-catalyzed polymerization usually produces star, branched or crosslinked polymers with uncontrolled structures.13–28 Though a clear structure is important for understanding the structure–property relationships of fullerene polymers, due to the synthetic problems stated above, there are only a few reports about the synthesis of fullerene polymer with clear structure, which mainly used the condensation polymerization method.7–12 This method can produce fullerene polymers with well-defined structure, yet the poor solubility problem of the obtained polymers limits their applications due to the high stiffness of the polymer backbones from the repulsion between fullerenes.7–9 Recently, Nierengarten et al. reported the synthesis of fullerene polyesters with good solubility by introducing long alkyl chains.10–12 However, the application of these fullerene polymers in all-polymer solar cells failed due to the low fullerene content in the polymers (∼30%).11 How to synthesize well-defined fullerene polymers with high fullerene content (for outstanding fullerene properties) and good solubility in organic solvents (for good processability) is still a big challenge for polymer chemists.
We report here the design and synthesis of fullerene polyesters P1–P3 with clear structure as shown in Scheme 1. By utilizing liquid crystalline polymers' Finkelmann's decoupling effect method,29 we introduce flexible long spacer groups between the pendent fullerene and polymer backbone to minimize the fullerene's influence on chain stiffness, and obtain polyesters with high fullerene content (>50 wt%) and good solubility. The fullerenoacetic acid was used to synthesize monomer 7, since methanofullerene derivatives are stable and widely used in the opto-electronics field.30–32 Condensation polymerization of this monomer with three different diacyl chlorides at mild temperature afforded three polyesters P1–P3. The detailed synthesis and characterization of the monomer and polymers are described in ESI (Fig. S1–S7†). These polyesters have good solubility in organic solvents (chloroform, dichloromethane, anisole, etc.) at room temperature. In particular, the high solubility of 20 mg mL−1 in chloroform and the good film-forming properties make them useful for applications.
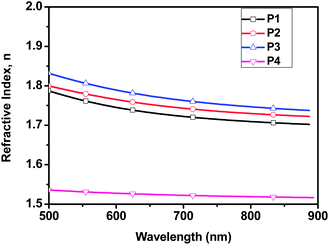
Fig. 1 is the 1H NMR spectra of monomer 7 and polyester P2. The diminished peak k (δ = 4.67 ppm) and the emerging peak k′ (δ = 5.05 ppm) clearly indicate the formation of ester bonds in the polymer. By the end-group estimation, the number-average molecular weights of the polymers were calculated by 1H NMR. Detailed calculation can be found in ESI.† The molecular weights calculated from NMR for polyesters are listed in Table 1.
 | ||
| Fig. 1 1H NMR spectra of monomer 7 (upper part) and fullerene polyester P2 (lower part) in d-chloroform. s: solvent peak. | ||
| Sample | M n (g mol−1) | M n (g mol−1) | PDIb | T d (°C) |
|---|---|---|---|---|
| a Determined by 1H NMR in d-CHCl3. b Determined by GPC using CHCl3 as eluent and PS as standard. c Determined by GPC using THF as eluent and PS as standard. d Temperature at 5% weight loss by a heating rate of 10 °C min−1 under nitrogen. | ||||
| P1 | 9720 | 3900 | 1.29 | 361 |
| P2 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
3200 | 1.35 | 346 |
| P3 | 7440 | 3100 | 1.20 | 360 |
| P4 | 6840 | 7700c | 1.47c | 378 |
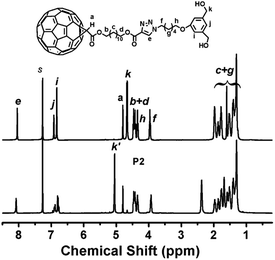
Analysis of the polyesters by gel permeation chromatography (GPC) (Fig. 2) revealed a single peak of the polymers, suggesting there should be no detectable side reactions. The measured molecular weight of the monomer 7 (Mn = 670 g mol−1) using polystyrene (PS) as standard is much lower than the theoretical value (1293 g mol−1). This is due to the difference in hydrodynamic volume of the monomer compared to linear polystyrene standards, and the interaction of fullerenes with the phenyl groups of PS stationary phase, which leads to the increase of elution time with the corresponding decrease of the molecular weights.11,33–36 Nevertheless, the apparent number-average molecular weights obtained by GPC for P1–P3 are between 3000 g mol−1 and 4000 g mol−1, suggesting the successful synthesis of the polymers. The polydispersity index (Mw/Mn) values (1.2–1.4) are smaller than traditional condensation polymerization. This comes from the postpolymerization treatment by precipitating the obtained material into tetrahydrofuran (THF), where monomer and oligomers are washed out.
 | ||
| Fig. 2 GPC analysis of monomer 7 and fullerene polyesters P1–P3. Eluent: chloroform; flow rate: 1.0 mL min−1. Concentration normalized to 1.0 mg mL−1. | ||
The fullerene polymers were further characterized by UV-Vis spectroscopy (see Fig. S4 and S5†), thermogravimetric analysis (TGA, Fig. S6†) and Fourier transform Infrared (FTIR) spectroscopy (Fig. S7†). The sharp peaks at 428 nm and small peaks at 690 nm in Fig. S4† are characteristic features in the spectra of methano[60]fullerenes.37,38 The similar absorption spectra and peaks in solution and in film form indicate the chemical structure of the fullerene polymers is the same in solution and in film. In Fig. S6,† all polymers exhibit high thermal stability with the 5% weight loss temperatures (Td) higher than 340 °C. It should be noted that even at 600 °C, there is more than 60% weight remaining for all the polymers, indicating the high fullerene content of the polyesters. The fullerene polymers' structure is further proved by the FTIR spectra (Fig. S7†), where the peaks at 526 cm−1, 574 cm−1, 1183 cm−1, and 1427 cm−1 are the characteristic peaks of C60 corresponding to its intramolecular vibration modes.39
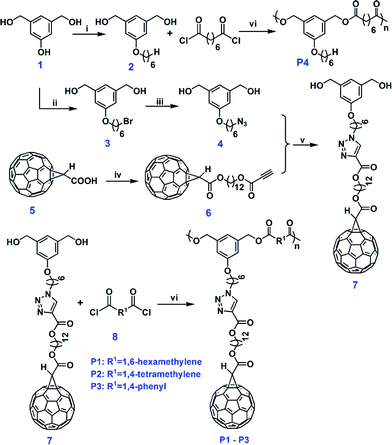
Thin films of P1–P3 polymers were prepared by spin coating the corresponding chloroform solution onto silicon wafers. The refractive indices as a function of wavelength for the polymer films were determined using a variable angle spectroscopic ellipsometer, and the results are presented in Fig. 3. Detailed fitting method and data can be found in ESI (Fig. S8 and S9†). All the fullerene polymers exhibit refractive indices higher than 1.70 in the measured wavelength range from 500 to 900 nm, with the value decreasing slightly with increasing wavelength. As we know, these are the first reported high refractive index polymers (HRIPs) by incorporating fullerenes in the polymers.40–43 The refractive index at the sodium D line (589 nm) for P3 (n = 1.79) is so high that it exceeds most of the polymers which normally exhibit values between 1.30 and 1.70.40 This value is comparable to the refractive indices of well-known HRIPs (∼1.80), which mainly consist of two types of material: conjugated polymers and polymer/inorganic material hybrids.41,44–48 Due to the stability and processability problems for polymer hybrid systems, conjugated polymers have attracted much interest recently. In these polymers, the high refractive indices are due to the highly polarizable π-conjugated functionalities. Since [60]fullerene is a buckyball-like molecule surrounded by conjugated π-electrons and has a high refractive index, we believe this is the origin of the high refractive index for our fullerene polyesters.
To see if the high refractive indices of these polyesters are from the incorporation of side-chain fullerenes, controlled experiments were carried where a similar polyester without pendent fullerenes (P4, Scheme 1) was synthesized. This polymer has good solubility in common organic solvents, with a refractive index around 1.52 at the measured wavelength (Fig. 3), similar to typical polyesters. This result clearly indicates that the pendent fullerenes are the key for the high index of refraction.
To explain the high refractive indices of the fullerene polymers, the Lorentz–Lorenz equation (1) is introduced, which is commonly used to predict a polymer's refractive index:40,49
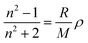 | (1) |
The calculated refractive indices agree well with the measured refractive indices, indicating the good reliability of our measured data. According to eqn (1), the high refractive index of these fullerene polymers can be attributed to the high molar refraction of fullerene and the high density of the polymers. On the other hand, the refractive index of the polymers has the trend of P3 > P2 > P1 at the same measured wavelength, despite the fact that the fullerene content in the polymers is P2 > P3 > P1. This is due to the highest density of P3 (1.430 g cm−3) among the fullerene polyesters (1.418 g cm−3 for P2, and 1.404 g cm−3 for P1).
Since HRIPs have many applications in advanced optical fields to improve the performance of optoelectronic devices,41–47 our results provide a new method to design HRIPs by the incorporation of fullerenes. On the other hand, since fullerene derivatives are widely used in organic solar cells as acceptor materials,50,51 the potential application of our fullerene polymers in these field is under current investigation.
In summary, we have successfully synthesized a series of side-chain high fullerene content polyesters by condensation polymerization. The polyesters have high refractive indices (above 1.70) due to the incorporation of pendent fullerenes. Among them, polymer P3 has the highest refractive index (1.79 at 589 nm) due to its high density, and is among the highest values reported for HRIPs with good solubility and without metal elements. With the semiconducting behaviour from the fullerene moieties, these polymers can be viewed as molecular wires with the insulating polyester backbone as the core and semiconducting fullerenes outside, and have potential applications in optoelectronics devices.
Acknowledgements
The financial support from the National Natural Science Foundation of China (Grant no. 21074079, 21274099), Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant no. 20103201120004), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions is gratefully acknowledged.Notes and references
- Fullerenes, ed. A. Hirsch and M. Brettreich, Willey-VCH, Weinheim, 2005 Search PubMed.
- Fullerenes: Principles and Applications, ed. F. Langa and J. F. Nierengarten, Royal Society of Chemistry, Cambridge, 2007 Search PubMed.
- Fullerene Polymers: Synthesis, Properties and Applications, ed. N. Martín and F. Giacalone, Wiley-VCH, Weinheim, 2009 Search PubMed.
- Y. Kojima, T. Matsuoka, H. Takahashi and T. Kurauchi, Macromolecules, 1995, 28, 8868–8869 CrossRef CAS.
- M. Heuken, H. Komber, T. Erdmann, V. Senkovskyy, A. Kiriy and B. Voit, Macromolecules, 2012, 45, 4101–4114 CrossRef CAS.
- S. Miyanishi, Y. Zhang, K. Hashimoto and K. Tajima, Macromolecules, 2012, 45, 6424–6437 CrossRef CAS.
- S. Shi, K. C. Khemani, Q. C. Li and F. Wudl, J. Am. Chem. Soc., 1992, 114, 10656–10657 CrossRef CAS.
- K. L. Wooley, C. J. Hawker, J. M. J. Fréchet, F. Wudl, G. Srdanov, S. Shi, C. Li and M. Kao, J. Am. Chem. Soc., 1993, 115, 9836–9837 CrossRef CAS.
- M. Berrada, Y. Hashimoto and S. Miyata, Chem. Mater., 1994, 6, 2023–2025 CrossRef CAS.
- Y. Rio, G. Accorsi, H. Nierengarten, J.-L. Rehspringer, B. Hönerlage, G. Kopitkovas, A. Chugreev, A. V. Dorsselaer, N. Armaroli and J.-F. Nierengarten, New J. Chem., 2002, 26, 1146–1154 RSC.
- M. G. Nava, S. Setayesh, A. Rameau, P. Masson and J.-F. Nierengarten, New J. Chem., 2002, 26, 1584–1589 RSC.
- U. Hahn, E. Maisonharte, C. Amatore and J.-F. Nierengarten, Angew. Chem., Int. Ed., 2007, 46, 951–954 CrossRef CAS PubMed.
- F. Wudl, Acc. Chem. Res., 1992, 25, 157–161 CrossRef CAS.
- X. Zhang, A. Romero and C. S. Foote, J. Am. Chem. Soc., 1993, 115, 11024–11025 CrossRef CAS.
- S. R. Wilson, N. Kaprinidis, Y. Wu and D. I. Schuster, J. Am. Chem. Soc., 1993, 115, 8495–8496 CrossRef CAS.
- J. Wang, G. Javahery, S. Petrie and D. K. Bohme, J. Am. Chem. Soc., 1992, 114, 9665–9666 CrossRef CAS.
- V. Baranov, J. Wang, G. Javahery, S. Petrie, A. C. Hopkinson and D. K. Bohme, J. Am. Chem. Soc., 1997, 119, 2040–2049 CrossRef CAS.
- T. Zhang, K. Xi, X. Yu, M. Gu, S. Guo, B. Gu and H. Wang, Polymer, 2003, 44, 2647–2654 CrossRef CAS.
- Y. Huang, H. Peng, J. W. Y. Lam, Z. Xu, F. S. M. Leung, J. W. Mays and B. Z. Tang, Polymer, 2004, 45, 4811–4817 CrossRef CAS PubMed.
- A. M. Ramos, M. T. Rispens, J. C. Hummelen and R. A. J. Janssen, Synth. Met., 2001, 119, 171–172 CrossRef.
- T. Nishimura, K. Takatani, S. Sakurai, K. Maeda and E. Yashima, Angew. Chem., Int. Ed., 2002, 41, 3602–3604 CrossRef CAS.
- F. Lu, S. Xiao, Y. Li, H. Liu, H. Li, J. Zhuang, Y. Liu, N. Wang, X. He, X. Li, L. Gan and D. Zhu, Macromolecules, 2004, 37, 7444–7450 CrossRef CAS.
- Z. T. Ball, K. Sivula and J. M. J. Fréchet, Macromolecules, 2006, 39, 70–72 CrossRef CAS.
- S. Ohsawa, K. Maeda and E. Yashima, Macromolecules, 2007, 40, 9244–9251 CrossRef CAS.
- J. Kim, M. H. Yun, J. Lee, J. Y. Kim, F. Wudl and C. Yang, Chem. Commun., 2011, 47, 3078–3080 RSC.
- B. Z. Tang, H. Xu, J. W. Y. Lam, P. P. S. Lee, K. Xu, Q. Sun and K. K. L. Cheuk, Chem. Mater., 2000, 12, 1446–1455 CrossRef CAS.
- M. Li, X. Li, P. Xu and S. Yang, Macromol. Chem. Phys., 2010, 211, 443–452 CrossRef CAS.
- S.-J. Wang and W.-Y. Yeh, Organometallics, 2012, 31, 6491–6495 CrossRef CAS.
- H. Finkelmann, H. Ringsdorf and J. H. Wendorff, Makromol. Chem., 1978, 179, 273–276 CrossRef CAS.
- N. S. Sariciftci, D. Braun, C. Zhang, V. I. Srdanor, A. J. Heeger, G. Stucky and F. Wudl, Appl. Phys. Lett., 1993, 62, 585–587 CrossRef CAS PubMed.
- B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed.
- A. Kraus and K. Müllen, Macromolecules, 1999, 32, 4214–4219 CrossRef CAS.
- W.-B. Zhang, Y. Tu, R. Ranjan, R. M. V. Horn, S. Leng, J. Wang, M. J. Polce, C. Wesdemiotis, R. P. Quirk, G. R. Newkome and S. Z. D. Cheng, Macromolecules, 2008, 41, 515–517 CrossRef CAS.
- R. Charvet, S. Acharya, J. P. Hill, M. Akada, M. Liao, S. Seki, Y. Honsho, A. Saeki and K. Ariga, J. Am. Chem. Soc., 2009, 131, 18030–18031 CrossRef CAS PubMed.
- M. Heuken, H. Komber and B. Voit, Macromol. Chem. Phys., 2012, 213, 97–107 CrossRef CAS.
- J. C. Hummelen, B. W. Knight, F. LePeq and F. Wudl, J. Org. Chem., 1995, 60, 532–538 CrossRef CAS.
- B. Ma, C. E. Bunker, R. Gudura, X.-F. Zhang and Y.-P. Sun, J. Phys. Chem. A, 1997, 101, 5626–5632 CrossRef CAS.
- W. Krätschmer, L. D. Lamb, K. Fostiropoulos and D. R. Huffman, Nature, 1990, 347, 354–358 CrossRef.
- J. C. Seferis, in Polymer Handbook, ed. J. Brandrup, E. H. Immergut, E. A. Grulke, A. Abe and D. R. Bloch, John Wiley & Sons, New York, 4th edn, 1999 Search PubMed.
- J.-G. Liu and M. Ueda, J. Mater. Chem., 2009, 19, 8907–8919 RSC.
- H. Kudo, M. Yamamoto, T. Nishikubo and O. Moriya, Macromolecules, 2006, 39, 1759–1765 CrossRef CAS.
- S. A. Sydlik, Z. Chen and T. M. Swager, Macromolecules, 2011, 44, 976–983 CrossRef CAS.
- F. Fitrilawati, M. O. Tjia, S. Pfeiffer, H.-H. Hörhold, A. Deutesfeld, H. Eichner and C. Bubeck, Opt. Mater., 2003, 21, 511–519 CrossRef CAS.
- C. M. Ramsdale and N. C. Greenham, Adv. Mater., 2002, 14, 212–215 CrossRef CAS.
- T. Erb, S. Raleva, U. Zhokhavets, G. Gobsch, B. Stühn, M. Spode and O. Ambacher, Thin Solid Films, 2004, 450, 97–100 CrossRef CAS PubMed.
- M. Campoy-Quiles, G. Heliotis, R. Xia, M. Ariu, M. Pintani, P. Etchegoin and D. D. C. Bradley, Adv. Funct. Mater., 2005, 15, 925–933 CrossRef CAS.
- M. Russo, M. Campoy-Quiles, P. Lacharmoise, T. A. M. Ferenczi, M. Garriga, W. R. Caseri and N. Stingelin, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 65–74 CrossRef CAS.
- W. Groh and A. Zimmermann, Macromolecules, 1991, 24, 6660–6663 CrossRef CAS.
- Z. Li, H. C. Wong, Z. Huang, H. Zhong, C. H. Tan, W. C. Tsoi, J. S. Kim, J. R. Durrant and J. T. Cabral, Nat. Commun., 2013, 4, 2227 Search PubMed.
- B. Watts, W. J. Belcher, L. Thomsen, H. Ade and P. C. Dastoor, Macromolecules, 2009, 42, 8392–8397 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis, characterization of fullerene monomers and polymers by MALDI-TOF, 1H NMR, UV-Vis, TGA, and theoretical refractive index calculations. See DOI: 10.1039/c3mh00105a |
| This journal is © The Royal Society of Chemistry 2014 |