Mutagenesis modulates the uptake efficiency, cell-selectivity, and functional enzyme delivery of a protein transduction domain†
Sandra M.
DePorter‡
a,
Irene
Lui‡
b,
Virginia J.
Bruce‡
a,
Melissa A.
Gray
a,
Monica
Lopez-Islas
b and
Brian R.
McNaughton
*ab
aDepartment of Chemistry, Colorado State University, Fort Collins, Colorado 80523, USA. E-mail: brian.mcnaughton@colostate.edu
bDepartment of Biochemistry & Molecular Biology, Colorado State University, Fort Collins, Colorado 80523, USA
First published on 30th October 2013
Abstract
Alanine scanning mutagenesis of a recently reported prostate cancer cell-selective Protein Transduction Domain (PTD) was used to assess the specific contribution each residue plays in cell uptake efficiency and cell-selectivity. These studies resulted in the identification of two key residues. Extensive mutagenesis at these key residues generated multiple mutants with significantly improved uptake efficiency and cell-selectivity profiles for targeted cells. The best mutant exhibits ∼19-fold better uptake efficiency and ∼4-fold improved cell-selectivity for a human prostate cancer cell line. In addition, while the native PTD sequence was capable of delivering functional fluorescent protein to the interior of a prostate cancer cells, only modest functional enzyme delivery was achieved. In contrast, the most potent mutant was able to deliver large quantities of a functional enzyme to the interior of human prostate cancer cells. Taken together, the research described herein has significantly improved the efficiency, cell-selectivity, and functional utility of a prostate cancer PTD.
Introduction
Proteins are of great value as research tools, therapeutics, and imaging reagents.1 The size and complexity of proteins can often endow these reagents with the ability to potently and selectively recognize disease-relevant macromolecules that confound drug discovery efforts limited to small molecules (<800 Da). In addition, a number of protein enzymes such as luciferase and horseradish peroxidase are commonly used for cellular imaging and basic research applications.2,3 In many ways, these reagents are ideally suited for imaging applications. For example, by virtue of their enzymatic activity, appreciable signal can be generated from a relatively small number of functional proteins. Despite clear advantages of protein-based approaches to therapy and cell imaging, the inability of most proteins to penetrate the lipid bilayer membrane of mammalian cells largely limits access of these reagents to cellular components displayed on the cell surface. The potential utility of functional proteins and enzymes with the ability to access the interior of mammalian cells has compelled researchers to address barriers to the intracellular delivery of these agents in mammalian cells. Technologies including electroporation,3 microinjection,4 liposomes,5 lipid-linked compounds,6 nanoparticles,7 fusions to receptor ligands,8 arginine grafting,9 supercharged proteins,10–13 and protein transduction domains (PTDs)14–16 have previously been reported.In many ways, PTDs are ideally suited as protein delivery reagents. The proteinogenic amino acid composition and relatively small size (typically 7–20 L-amino acids) of these reagents can allow researchers to express and purify soluble protein-PTD fusions with relative ease. A number of PTDs such as HIV-1 transactivator of transcription (Tat) peptide,17 the Drosophila Antennapedia-derived penetratin peptide,18 and polyarginine19,20 have been used for intracellular protein delivery. However, the relatively modest uptake efficiency exhibited by some common PTDs, and a general lack of selectivity for diseased cells over healthy cells limits the full potential of these reagents. The development of new PTDs capable of both potent and cell-selective delivery of functional proteins, such as enzymes, to diseased cells would potentially expand the utility of protein-based approaches to cellular perturbation and imaging.
PC-3 cells are one of the most commonly used human prostate cancer cell lines in basic and translational research, and are useful in investigating the biochemical changes in advanced prostate cancer cells and in assessing their response to chemotherapeutic agents.21 Reagents that selectively recognize PC-3 cells are of potential value, since these cells do not express appreciable levels of prostate-specific membrane antigen (PSMA) – the most commonly used marker for prostate cancer cell detection and targeted delivery.22 Therefore, PC-3 cells, or prostate cancer cells with a similar phenotype, would likely evade targeted imaging or drug delivery strategies centered on PSMA recognition.
We recently reported a new PTD referred to as Ypep (Fig. 1A), which selectively recognizes and penetrates PC-3 cells in a manner that is controlled by multivalency effects.23 Green Fluorescent Protein (GFP) bearing Ypep at both the N- and C-termini potently and selectively penetrate PC-3 cells. However, GFP bearing a single N-terminal copy of Ypep was taken up by PC-3 cells with much lower efficiency and selectivity. The need for multivalent display for efficient and selective uptake is inherently limiting, since many proteins are not amenable to fusion at both termini. Perhaps most importantly, while Ypep delivers appreciable levels of GFP to the interior of PC-3 cells, we observed only modest intracellular delivery of a functional enzyme. Taken together, these initial findings place limitations on the functional utility of monomeric Ypep for functional intracellular delivery to prostate cancer cells.
In order to better understand the requirements for uptake of Ypep by PC-3 cells, as well as optimize the potency and cell-selectivity of this new PTD, we prepared a significant number of Ypep mutants and assayed these variants for the ability to deliver functional fluorescent protein or enzyme to PC-3 cells. We show that the best mutant delivers ∼19-fold more fused protein to PC-3 cells than off-target non-cancer human embryonic kidney cells (HEK-293), with ∼4-fold higher cell-selectivity, compared to Ypep. In addition, the most potent and cell-selective mutant we identified delivers large quantities of functional enzyme to human prostate cancer cells. Similar to Ypep, uptake of the most potent and cell-selective mutant proceeds via energy-dependent endocytosis, suggesting that improvement in potency and cell-selectivity is not due to internalization via a different mechanism.
Results and discussion
Alanine scanning illuminates the specific contribution each residue plays in Ypep uptake by PC-3 cells
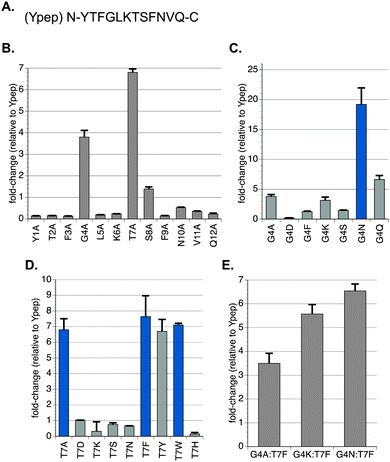
To assess the specific contribution each residue in Ypep plays in cell uptake efficiency, we made a library of Ypep alanine mutants and expressed these peptides as N-terminal fusions to GFP. PC-3 cells were treated with 5 μM of each Ypep-GFP mutant, a concentration previously shown to be sufficient for appreciable Ypep-GFP uptake.23 Cells were then exhaustively washed using conditions that we,23 and others,10–12,24 have previously shown to remove cell surface-bound protein. The amount of internalized GFP was measured by flow cytometry. As seen in Fig. 1B, most mutations resulted in significantly lower GFP delivery. However, Ypep-Gly4Ala and Ypep-Thr7Ala delivered ∼3.8- and ∼6.8-fold more GFP to PC-3 cells compared to native Ypep, respectively (Fig. 1B). A number of commonly used PTDs such as Tat, polyarginine and penetratin are polycationic, and rely on high-theoretical net charge for cell uptake.24 In contrast, Ypep has a theoretical net change of +1. Interestingly, mutating the single positively charged residue (Lys6) to alanine decreased GFP uptake ∼4-fold (Fig. 1B). Based on these initial findings, we prepared a focused library of mutants with molecularly diverse residues at position 4 or 7.Optimizing cellular uptake of a prostate cancer cell-selective protein transduction domain
Ypep mutants containing either negatively charged (aspartic acid), positively charged (lysine), aromatic (phenylalanine), hydrogen bond donating (serine), or amide (asparagine) functional groups at positions 4 or 7 were expressed as N-terminal fusions to GFP. As seen in Fig. 1C, the Gly4Asp mutant exhibited significantly lower uptake, and Gly4Phe and Gly4Ser mutants achieved only slightly higher uptake than Ypep-GFP. However, Gly4Lys and Gly4Asn mutants were significantly improved. Gly4Lys or Gly4Asn mutants delivered ∼3.2- and ∼19.2-fold more GFP to PC-3 cells, compared to native Ypep-GFP. Interestingly, small structural changes at position 4 significantly lowered uptake. While the Gly4Gln mutant was ∼6.6-fold improved over Ypep, it was ∼2.8-fold less efficient than the Gly4Asn mutant. While the cell surface receptor of Ypep and Ypep mutants is currently the object of investigation, the fact that addition of a methylene unit significantly lowers uptake supports a model wherein a well-defined interaction between Ypep-Gly4Asn and a cell surface receptor is required for efficient uptake, and not a less refined interaction that simply requires sequence-defined functional group display on the PTD.We next performed identical experiments to optimize residue 7. The Thr7Asp mutant exhibited essentially identical uptake efficiency as native Ypep. However, Thr7Lys, Thr7Ser, and Thr7Asn mutants all showed significantly lower transduction efficiencies. In contrast, the Thr7Phe mutant was significantly improved, and was able to deliver ∼7.6-fold more GFP to PC-3 cells, compared to native Ypep (Fig. 1D). Based on this finding, we measured uptake efficiencies for Ypep variants containing all possible proteinogenic aromatic residues at position 7 (Fig. 1D). While both Thr7Tyr and Thr7Trp mutants significantly outperformed native Ypep, delivering ∼6.8- and ∼7.1-fold more GFP, respectively, neither outperformed the Thr7Phe mutant. In contrast, the Thr7His mutant showed significantly lower cell uptake compared to Thr7Tyr and Thr7Trp mutants, as well as native Ypep. Taken together, the reduced transduction we observed for the Thr7His and Thr7Lys mutants suggest that residues with positive charge, or partial positive charge, may not be tolerated at this position.
The effect of double beneficial mutations on the uptake efficiency of PTD-linked green fluorescent protein
Combined synergistic effects can play important roles in many biological processes and macromolecule – substrate interactions.25 To assess if combinations of beneficial mutants are synergistic, we prepared three Ypep double mutants that contain combinations of the most beneficial single mutations at residues 4 and 7. Ypep double mutants that contain alanine, lysine, or asparagine at position 4, and phenylalanine at position 7 were expressed as N-terminal fusions to GFP and added to PC-3 cells as previously described. The Gly4Ala:Thr7Phe, Gly4Lys:Thr7Phe, and Gly4Asn:Thr7Phe double mutants were found to be ∼3.5-, ∼5.6-, and ∼6.5-fold more efficient at GFP transduction than Ypep-GFP, respectively (Fig. 1E). Interestingly, however, none of these double mutants exhibited higher uptake compared to the single mutant Ypep variants from which they were derived.Ypep mutants are not cytotoxic, Gly4Asn Ypep outperforms Tat and penetratin protein transduction domains, and is internalized via energy-dependent endocytosis
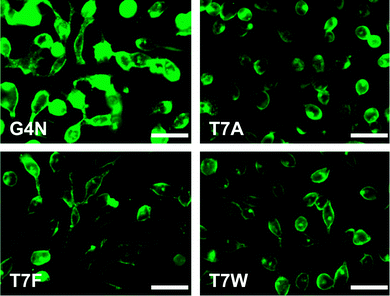
Based on these data, the Gly4Asn, Thr7Phe, Thr7Trp, and Thr7Ala mutants (bars are colored blue in Fig. 1C and D) were most improved over Ypep, with increased transduction efficiencies of 19.2-, 7.6-, 7.1-, and 6.8-fold, respectively. To assess the cytotoxicity of Ypep variants under conditions required for appreciable uptake, we performed 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT) on PC-3 cells after treatment with 5 μM Ypep(mutant)-GFP. These assays revealed no apparent cytotoxicity to PC-3 cells for any of the Ypep mutants (ESI,† Fig. S4). GFP uptake was confirmed for these four mutants by live-cell fluorescence microscopy. While only a very small amount of internalized GFP was observed in PC-3 cells following treatment with 5 μM Ypep-GFP (ESI,† Fig. S5), large amounts of internalized GFP was observed in cells following treatment with the same concentration of the four most active mutant Ypep-GFP fusions (Fig. 2). Consistent with our flow cytometry data, the Gly4Asn mutant delivered the highest amount of GFP to the cell interior. To compare the uptake efficiencies of these reagents to commonly used PTDs, we treated PC-3 cells with solutions containing either Tat-GFP, penetratin-GFP, or the four best Ypep variants identified as a result of mutagenesis studies. As shown in Fig. 3, following treatment with 1 μM PTD-GFP fusion, and washing, all Ypep mutants delivered significantly more GFP to the interior of PC-3 cells, compared to Tat-GFP fusions. Most notably, uptake efficiency in PC-3 cells treated with 1 μM Ypep(Gly4Asn)-GFP was ∼1.5-fold and 23-fold higher than cells treated with either penetratin-GFP or Tat-GFP fusions, respectively. Interestingly, we were unable to express appreciable amounts of soluble (Arg)9-GFP, suggesting potential limitations to polyarginine-based approaches to protein transduction – at least in some cases.Previously, we performed extensive studies to elucidate the mechanism of Ypep uptake. We found that while Ypep is taken up at 37 °C, it is not appreciably internalized when PC-3 cells are incubated with a Ypep solution at 4 °C. This finding suggests that Ypep internalization proceeds via an energy-dependent endocytotic pathway. In order to determine if Ypep(Gly4Asn) uptake is consistent with the parent peptide, or if internalization proceeds via an alternative pathway, we incubated PC-3 cells with 1 μM Ypep(Gly4Asn)-GFP at either 37 °C or 4 °C, washed cells to remove surface-bound material, and measured GFP internalization by microscopy and flow cytometry. Interestingly, similar to our findings for Ypep-GFP uptake, high levels of cell fluorescence is observed following treatment at 37 °C; however, no appreciable fluorescence is observed when cells are treated at 4 °C (ESI,† Fig. S7). Taken together, these findings suggest that like the parent peptide, the Ypep(Gly4Asn) mutant also relies on energy-dependent endocytosis for internalization.
Mutations beneficial to uptake efficiency also increase the cell-selectivity of protein delivery
While the above mutational studies on Ypep resulted in numerous variants with improved transduction efficiency, the effect of these beneficial mutations on cell-selectivity was unclear. In order to assess the impact of these mutations on cell-selectivity, we compared PTD-GFP fusion uptake in PC-3 (target) and off-target non-cancer human embryonic kidney cells (HEK-293). Cells were treated with 0.1–1 μM PTD-GFP fusion, washed as previously described to remove cell surface-bound material, and internalized GFP was measured by flow cytometry. As shown in Fig. 4, a majority of the most efficient Ypep mutants also exhibited increased selectivity for PC-3 human prostate cancer cells. Consistent with our previous findings, Ypep delivered ∼1.6-, ∼1.8-, ∼1.7-, or 2.8-fold more GFP to PC-3 cells compared to HEK-293 cells, following treatment with 0.1, 0.25, 0.5, or 1 μM solutions, respectively. While the Thr7Phe mutant exhibited similar selectivity for PC-3 cells (∼1.6, ∼2.0, ∼2.6, and ∼2.8-fold following 0.1–1 μM treatment), the Gly4Asn, Thr7Trp, and Thr7Ala mutants were significantly more selective for PC-3 cells. For example, Gly4Asn, Thr7Trp, and Thr7Ala Ypep mutants were ∼5.3, ∼5.8, and ∼5.0-fold more selective for PC-3 prostate cancer cells compared to HEK-293 cells. Taken together, these studies demonstrate a significant improvement in both the transduction efficiency and PC-3 cell-selectivity of multiple Ypep mutants found as a result of these studies.Gly4Asn Ypep mutant delivers appreciable levels of a functional enzyme to PC-3 cells
Perhaps the ultimate test of a PTD is intracellular delivery of a functional enzyme. Luciferase is a class of enzymes that oxidize photon-emitting substrate, resulting in bioluminescence. These enzymes enjoy extensive use as reporters and cell imaging reagents because of their high sensitivity, broad dynamic range, and operational simplicity.26 NanoLuc luciferase (nLuc) is a recently reported variant of the small luciferase subunit from the deep sea shrimp Oplophorus gracilirostris.27 As a simple test for functional intracellular enzyme delivery, we measured luciferase activity in PC-3 cells following treatment with 1 μM nLuc, Ypep-nLuc, or Ypep(Gly4Asn)-nLuc. Consistent with the overwhelming majority of proteins, appreciable amounts of nLuc do not penetrate mammalian cells. Cells treated with nLuc, washed to remove surface-bound protein, and treated with furimazine, exhibited very little luminescence (Fig. 5). Similar to our previous findings, relatively modest functional enzyme delivery was achieved via Ypep-dependent delivery. Cells treated with Ypep-nLuc, then washed as described above, were ∼6.1-fold more luminescent than cells treated with nLuc alone (Fig. 5). In contrast, cells similarly treated with Ypep(Gly4Asn)-nLuc were ∼41.6-fold more luminescent that cells treated with nLuc. These findings suggest that relatively large amounts of enzymatically active Ypep(Gly4Asn)-nLuc were delivered to the interior of PC-3 cells (Fig. 5). Importantly, cells are not lysed at any point during the luciferase assay. Therefore, luminescence generated during these experiments is the action of active nLuc enzyme within the cell interior.Experimental
Mammalian cell culture
Human prostate adenocarcinoma cells (PC-3) cells were cultured in F12K with 10% Fetal Bovine Serum (FBS) and HEK293T cells cultured in high glucose Dulbecco's modified Eagle medium (DMEM) with 10% Fetal Bovine Serum (FBS). All cells were incubated at 37 °C with 5% CO2 environment. All cells were obtained from ATCC.Cloning
All plasmids were constructed on a pETDuet-1 backbone. All peptides and GGS linkers on the N-terminus and C-terminus of sfGFP were assembled from a set of overlapping oligonucleotides. The peptides were then amplified with the sfGFP or nLuc proteins and the plasmids were ligated into NcoI and KpnI restriction enzyme cleavage sites in the pETDuet-1 plasmid.Protein purification
BL21 E. coli was grown in 500 mL LB cultures at 37 °C to OD600 = ∼0.6 and induced with 1 mM IPTG at 30 °C overnight. Cells were then collected by centrifugation and stored at −20 °C. Frozen pellets were thawed and 20 mL B-PER was added to lyse cells. The lysate was cleared by centrifugation (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 30 min) and the supernatant was mixed with 1 mL of Ni-NTA agarose resin for 1 hour. The resin was collected by centrifugation (4950 rpm, 10 min). The resin was washed with 50 mL of phosphate buffered saline (PBS) with 300 mM NaCl and 20 mM imidazole. The protein was then eluted with 5 mL PBS containing 300 mM NaCl and 500 mM imidazole. The proteins were dialyzed against PBS and analyzed for purity by SDS-PAGE staining with Coomassie Blue. The proteins were then quantified using a modified Lowry protein assay kit. nLuc proteins were purified in the same way, except washed with Tris buffers (25 mM Tris-HCl, 100 mM NaCl, pH = 8.0) instead of phosphate buffers.
000 rpm, 30 min) and the supernatant was mixed with 1 mL of Ni-NTA agarose resin for 1 hour. The resin was collected by centrifugation (4950 rpm, 10 min). The resin was washed with 50 mL of phosphate buffered saline (PBS) with 300 mM NaCl and 20 mM imidazole. The protein was then eluted with 5 mL PBS containing 300 mM NaCl and 500 mM imidazole. The proteins were dialyzed against PBS and analyzed for purity by SDS-PAGE staining with Coomassie Blue. The proteins were then quantified using a modified Lowry protein assay kit. nLuc proteins were purified in the same way, except washed with Tris buffers (25 mM Tris-HCl, 100 mM NaCl, pH = 8.0) instead of phosphate buffers.
Flow cytometry analysis
Mammalian cells were grown to 90% confluency in a 12-well plate. Cells were then washed once with PBS and 500 μL of diluted protein in PBS was added. The cells were incubated with the protein solution for 3 hours at 37 °C, 5% CO2 environment. After the incubation period, cells were then washed once with PBS and two times with PBS-HS (heparin sulfate 20 U mL−1) for 10 minutes at 37 °C/5% CO2. The cells were then removed from dish with 0.5 mL of 0.25% trypsin and collected by centrifugation. The cells were then resuspended in PBS-HS and taken for flow cytometry analysis.Live cell fluorescence microscopy
Mammalian cells were grown to 90% confluency in a 12-well plate. Cells were then washed once with PBS and 500 μL of 5 μM protein in PBS was added. The cells were incubated with the protein solution for 3 hours at 37 °C, 5% CO2 environment. After the incubation period, cells were then washed once with PBS and three times with PBS-HS (heparin sulfate 20 U mL−1) for 10 minutes at 37 °C/5% CO2. The cells were then imaged on the EVOS FL fluorescence microscope. For 4 °C experiments, the PC-3 cells were incubated at 4 °C for 30 minutes prior to the addition of the diluted protein. The incubation period was carried out at 4 °C and washed as described above.MTT assay
PC-3 cells were grown to 90% confluency in a 12-well plate. Cells were then washed once with PBS and incubated with the protein in PBS for 3 hours at 37 °C/5% CO2. The solution was removed and the cells were washed twice with PBS-HS (heparin sulfate 20 U mL−1). The cells were then incubated with 0.5 mL medium with 25 μL of MTT reagent for 4.5 hours. After the incubation, 250 μL detergent was added to the cells and they were incubated for an addition 30 minutes. MTT assay readings were taken with a Synergy Mx microplate reader at 570 nm.NanoGlo luciferase assay
PC-3 cells were grown to ∼80% confluency in a 24-well plate (clear bottom, black well). The nLuc proteins were diluted in TBS (25 mM Tris-HCl, 150 mM NaCl, pH = 7.0) and added to the PC-3 cells. Cells were incubated with each solution for 3 h at 37 °C under 5% CO2 environment. The cells were then washed with TBS, TBS-0.1% tween-20, and TBS-HS (heparin sulfate 20 U mL−1). This washing procedure was repeated a total of two times. Then, the cells were incubated with 200 μL TBS and 200 μL Nano-Glo Luciferase Assay Reagent for 10 minutes. Luminescence was measured on a Synergy Mx microplate reader.Conclusions
In summary, we recently reported Ypep, a prostate cancer cell-selective PTD, with uptake efficiency and cell-selectivity profiles that are dependent on multivalency effects. When a single copy of Ypep is fused to GFP, modest uptake efficiency and cell selectivity is observed. Fusion of a single Ypep to the N-terminus of nLuc does not deliver appreciable functional protein to PC-3 cells. Mutational studies have revealed a number of Ypep variants with significantly improved protein transduction efficiency and selectivity for PC-3 human prostate cancer cells. Amazingly, a single mutation to Ypep (Gly4Asn) resulted in a variant with ∼19.2-fold higher transduction efficiency and ∼4-fold better selectivity for PC-3 cells over off-target HEK-293 cells. In contrast to Ypep, Ypep(Gly4Asn) delivered appreciable levels of nanoluciferase (nLuc) to the interior of PC-3 cells. Taken together, the findings described in this paper significantly improve the functional utility of Ypep-dependent delivery of exogenous proteins to the interior of PC-3 prostate cancer cells. Our data suggest that the Ypep mutants described here are well suited to serve as reagents for PC-3 cell-selective delivery of imaging and enzymatic proteins for basic research and biomedical applications.Acknowledgements
This work was supported in part by institutional funds provided by Colorado State University and the Department of Defense (CDMRP Prostate Cancer New Innovator Award).Notes and references
- P. J. Carter, Exp. Cell Res., 2011, 317, 1261–1269 CrossRef CAS PubMed.
- X. Li, Y. Nakajima, K. Niwa, V. R. Viviani and Y. Ohmiya, Protein Sci., 2010, 19, 26–33 CAS.
- M. Yan, J. Du, Z. Gu, M. Liang, Y. Hu, W. Zhang, S. Priceman, L. Wu, Z. H. Zhou, Z. Liu, T. Segura, Y. Tang and Y. Lu, Nat. Nanotechnol., 2010, 5, 48–53 CrossRef CAS PubMed.
- D. Bar-Sagi and J. R. Feramisco, Cell, 1985, 42, 841–848 CrossRef CAS.
- G. Gregoriadis, Trends Biotechnol., 1995, 13, 527–537 CrossRef CAS.
- O. Zelphati, Y. Wang, S. Kitada, J. C. Reed, P. L. Felgner and J. Corbeil, J. Biol. Chem., 2001, 276, 35103–35110 CrossRef CAS PubMed.
- R. H. Utama, Y. Guo, P. B. Zetterlund and M. H. Stenzel, Chem. Commun., 2012, 48, 11103–11105 RSC.
- C. A. Gabel and S. A. Foster, J. Cell Biol., 1986, 103, 1817–1827 CrossRef CAS.
- S. M. Fuchs and R. T. Raines, ACS Chem. Biol., 2007, 2, 167–170 CrossRef CAS PubMed.
- J. J. Cronican, K. T. Beier, T. N. Davis, J. C. Tseng, W. Li, D. B. Thompson, A. F. Shih, E. M. May, C. L. Cepko, A. L. Kung, Q. Zhou and D. R. Liu, Chem. Biol., 2011, 18, 833–838 CrossRef CAS PubMed.
- J. J. Cronican, D. B. Thompson, K. T. Beier, B. R. McNaughton, C. L. Cepko and D. R. Liu, ACS Chem. Biol., 2010, 5, 747–752 CrossRef CAS PubMed.
- B. R. McNaughton, J. J. Cronican, D. B. Thompson and D. R. Liu, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 6111–6116 CrossRef CAS PubMed.
- D. B. Thompson, J. J. Cronican and D. R. Liu, Methods Enzymol., 2012, 503, 293–319 CAS.
- S. Deshayes, M. C. Morris, G. Divita and F. Heitz, Cell. Mol. Life Sci., 2005, 62, 1839–1849 CrossRef CAS PubMed.
- M. Mae and U. Langel, Curr. Opin. Pharmacol., 2006, 6, 509–514 CrossRef PubMed.
- M. C. Morris, S. Deshayes, F. Heitz and G. Divita, Biol. Cell., 2008, 100, 201–217 CrossRef CAS PubMed.
- M. Green and P. M. Loewenstein, Cell, 1988, 55, 1179–1188 CrossRef CAS.
- D. Derossi, A. H. Joliot, G. Chassaing and A. Prochiantz, J. Biol. Chem., 1994, 269, 10444–10450 CAS.
- E. A. Dubikovskaya, S. H. Thorne, T. H. Pillow, C. H. Contag and P. A. Wender, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12128–12133 CrossRef CAS PubMed.
- S. M. Fuchs and R. T. Raines, Protein Sci., 2005, 14, 1538–1544 CrossRef CAS PubMed.
- M. E. Kaighn, K. S. Narayan, Y. Ohnuki, J. F. Lechner and L. W. Jones, Invest. Urol., 1979, 17, 16–23 CAS.
- G. L. Wright Jr., C. Haley, M. L. Beckett and P. F. Schellhammer, Urol. Oncol., 1995, 1, 18–28 CrossRef CAS.
- S. M. DePorter, I. Lui, U. Mohan and B. R. McNaughton, Chem. Biol., 2013, 20, 434–444 CrossRef CAS PubMed.
- S. M. Fuchs and R. T. Raines, Biochemistry, 2004, 43, 2438–2444 CrossRef CAS PubMed.
- M. Mammen, S. K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2755–2794 CrossRef CAS.
- N. Thorne, J. Inglese and D. S. Auldl, Chem. Biol., 2010, 17, 646–657 CrossRef CAS PubMed.
- M. P. Hall, J. Unch, B. F. Binkowski, M. P. Valley, B. L. Butler, M. G. Wood, P. Otto, K. Zimmerman, G. Vidugiris, T. Machleidt, M. B. Robers, H. A. Benink, C. T. Eggers, M. R. Slater, P. L. Meisenheimer, D. H. Klaubert, F. Fan, L. P. Encell and K. V. Wood, ACS Chem. Biol., 2012, 7, 1848–1857 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental protocols, protein sequences used in this work. See DOI: 10.1039/c3mb70429g |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |