Research highlights: microfluidic point-of-care diagnostics
Westbrook
Weaver
,
Harsha
Kittur
,
Manjima
Dhar
and
Dino
Di Carlo
*
Department of Bioengineering, California NanoSystems Institute, Jonsson Comprehensive Cancer Center, University of California Los Angeles, 420 Westwood Plaza, 5121 Engineering V, Box 951600, Los Angeles, California 90095, USA. E-mail: diacrlo@seas.ucla.edu
First published on 2nd May 2014
Abstract
In this issue we highlight point-of-care (POC) diagnostic technologies to analyze cells, proteins, and small molecules from blood and other body fluids.
Key challenges that are important to address in a POC device, such as operating on a small sample size, performing complex sample preparation, and achieving a simple readout are addressed by the highlighted works in unique ways. New trends in POC analysis include the use of off-the-shelf consumer electronics1 (e.g. smart phones, google glasses, tablets, and scanners) for simplified readouts, paper based substrates to control fluid flow,2,3 and all electronic readouts. Additionally, researchers are now addressing clinical needs to analyze body fluids beyond blood – including ocular fluids, which have extremely limited sample sizes and are well suited to microscale approaches. The convergence of consumer technology, the networked world, and big data health informatics systems are expected to lead to continued fruitful endeavors in POC diagnostic development.
Depletion and differential counting of lymphocytes for HIV diagnostics
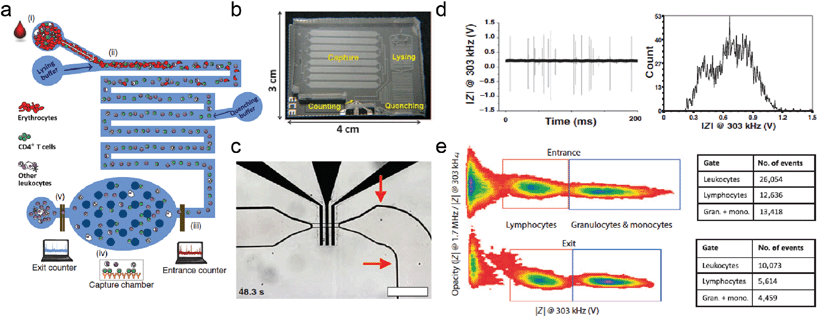
Simple and effective POC diagnostic devices have the capability to revolutionize patient care in resource-limited settings. Microfluidic platforms are well suited for POC devices, however POC analysis of cells can be more challenging when compared to more common colorimetric or fluorescence indicators of molecules. Another challenge is creation of a robust device that can operate on small volumes of whole blood (e.g. from a finger pinprick). Watkins et al.4 have combined microfluidic liquid handling and impedance based electrical readouts to create a simple and robust device for fast counting of both CD4+ and CD8+ T-lymphocytes from only 10 μl of whole blood (Fig. 1). The CD4/CD8 ratio is a number that directly influences clinical decision-making concerning initiation of antiretroviral therapy. All sample preparation is handled on chip, and the readout is impedance based, negating the need for any imaging or auxiliary optical equipment.Three important components have been skillfully implemented in this device: (i) on chip red blood cell lysis and quenching, (ii) dual frequency impedance measurement of the remaining leukocytes in flow, and (iii) capture of either CD4+ or CD8+ cells utilizing adsorbed anti-CD4 or anti-CD8 antibodies. Red blood cell lysis is critical to remove this interfering cell population that makes up the majority of the cellular component of blood (~5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per μL). Dual frequency impedance measurement (at 303 kHz and 1.7 MHz) similar to the methodology of Holmes et al.5 allows Watkins et al. to not only count the total number of cells that pass by the electrodes, but also to identify the leukocyte subpopulations (e.g. distinguish between granulocytes, monocytes and lymphocytes) in frequency space by effectively measuring membrane capacitance as well as size. The combination of this measurement with antibody capture allows finer subpopulation determination of the absolute number of both CD4+ and CD8+ T-lymphocytes in a single run. This is accomplished by simply subtracting two counts of the lymphocyte population after and before passing the capture chamber.
000 per μL). Dual frequency impedance measurement (at 303 kHz and 1.7 MHz) similar to the methodology of Holmes et al.5 allows Watkins et al. to not only count the total number of cells that pass by the electrodes, but also to identify the leukocyte subpopulations (e.g. distinguish between granulocytes, monocytes and lymphocytes) in frequency space by effectively measuring membrane capacitance as well as size. The combination of this measurement with antibody capture allows finer subpopulation determination of the absolute number of both CD4+ and CD8+ T-lymphocytes in a single run. This is accomplished by simply subtracting two counts of the lymphocyte population after and before passing the capture chamber.
Watkins et al. measured the performance of the device in both healthy patients and patients infected with HIV and undergoing antiretroviral therapy. CD4+ and CD8+ T-cell counts were compared against flow cytometry measurements in the same patients. The microfluidic electrical differential counting technique proved to be a strong performer in both healthy and infected patients, with low error compared to the gold standard flow cytometry counts, and high experimental and biological repeatability. In healthy patients, the technique was biased by 24 cells μl−1 and 9 cells μl−1 for CD4+ and CD8+ cells, respectively, whereas for infected patients the bias was 12 cells μl−1 and −55 cells μl−1 for CD4+ and CD8+ cells. Overall, this resulted in percentage errors in the calculations of 2.9% and 1.6% for CD4+ and CD8+ cells in healthy patients, and 5.3% and 7.4% in infected patients. The diagnostic CD4/CD8 ratio error was 1.9% in healthy patients and 11.9% in infected patients. The technical repeatability was 4.5% and the biological repeatability was 2.1% for CD8 counts and 1.4% for CD4 counts. Importantly, the total test time for 10 μl of undiluted blood was only 15 minutes to a complete readout.
Overall, this platform shows significant promise for use in low resource POC settings. The device is simple in operation, only requiring an electric readout (both low cost and robust), and not requiring any surface chemistry to immobilize capture antibodies, although the use of antibodies in general can lower the shelf life of any device. Further, this electrical counting technique performs comparably to the more time-consuming and less portable flow cytometry (requiring 4 antibodies conjugated with fluorophores, an expensive machine, and a highly trained technician). We expect this type of platform to enable more effective antiretroviral therapy administration in low resource areas, which could be accelerated by the relationship with Daktari Diagnostics, a company with which two of the authors are affiliated.
Paper-based ELISA for lucid detection of ocular ischemia
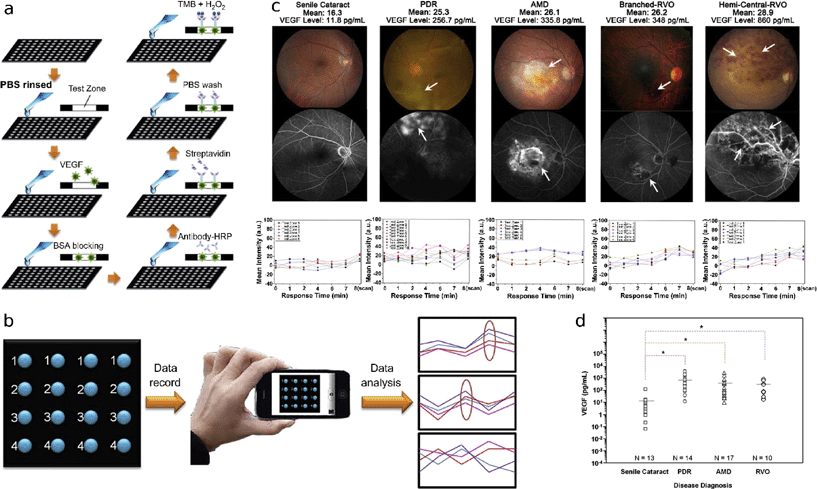
Vascular endothelial growth factor (VEGF) increases vascular permeability and neovascularization, therefore it is a useful biomarker for detection and treatment of several ocular diseases that result from both ischemia (and subsequent attempts of the body to increase oxygen delivery through neovascularization) and hypervascularization, both of which can lead to eventual loss of vision. Several tools have been developed to detect ocular ischemic ailments and diagnose appropriate anti-VEGF treatments including traditional ELISA and other multiplexed immunoassays. VEGF is commonly extracted from the aqueous humor, which has a sampling volume restriction of 200 μL to avoid chamber collapse, making a miniaturized assay particularly applicable. To overcome this limitation, Hsu et al. developed an affordable paper-based ELISA system (P-ELISA) in conjunction with a modified antibody against VEGF to be able to detect VEGF levels using only 2 μL of aqueous humor.6The P-ELISA protocol makes use of paper, specialized antibodies, and a cell phone readout (Fig. 2). Using the wax printing method,7 a 96-well paper plate was fabricated. Aqueous humor sample was loaded and blocked with BSA to prevent nonspecific interactions with proteins in subsequent steps. Next, the authors added modified bevacizumab (Avastin), a highly specific monoclonal antibody against all forms of VEGF-A. Bevacizumab was conjugated to horseradish peroxidase (HRP), which served as a colorimetric signal and greatly increased the sensitivity of the technology. Streptavidin was added to enhance signal readout. Finally, tetramethylbenzidine (TMB) and hydrogen peroxide (H2O2) were added, allowing HRP to oxidize clear TMB to its blue diimine form. Data acquisition and analysis were performed using a smartphone camera and image software (Fig. 2b). As a method of quality control of proper signal development, all readouts were subdivided into one of three patterns (Fig. 2b right panel), where any intensities that fell into the third category of irregular fluctuations were excluded.
The authors obtained aqueous humour samples from patients with ischemic conditions and assayed them using P-ELISA. From the colorimetric readouts (Fig. 2c lower panel), they applied their calibration curves to determine the mean VEGF levels. Kruskal–Wallis post-hoc analysis of variance confirmed that patients with proliferative diabetic retinopathy (PDR), age-related macular degeneration (AMD), and retinal vein occlusion (RVO) had significantly higher VEGF levels than those with senile cataracts (experimental control) (Fig. 2d), clearly displaying the P-ELISA's capability for acute detection of the ischemic condition.
P-ELISA was shown to perform with significantly greater efficiency than its conventional analog. It has a sensitivity of ~33.7 fg mL−1, greater than that of standard ELISA (~5 pg mL−1). The authors discuss that the orders of magnitude improvement in sensitivity may be related to use of the finely-engineered therapeutic antibody. Furthermore, the P-ELISA's total implementation time is ~44 minutes, more than 4 times quicker than conventional ELISA (~213 minutes); this is especially important for real-time diagnosis, since fresh aqueous humor samples are found to retain higher VEGF levels than frozen samples.8 From a clinical diagnostic perspective, this level of sensitivity may allow tracking of cytokines and growth factors other than VEGF, which may permit diagnosis of other ocular impairments. Potentially, it may be sensitive enough to track VEGF levels in cortical tears, providing a simple, affordable, non-invasive method of monitoring ocular ischemia at regular patient checkups. As a tool for drug therapy, the incredibly high sensitivity of this assay would enable the determination of precise anti-VEGF doses, as well as screening for any complications, such as neovascularization, that may arise after treatment. Taken together, P-ELISA is a powerful, inexpensive point-of-care diagnostic tool that is useful for monitoring VEGF levels, and has potential applications in detection of a variety of biomarkers and compatibility with alternative clinical sampling methods with small fluid volumes.
Bioelectronic nose sniffs for lung cancer
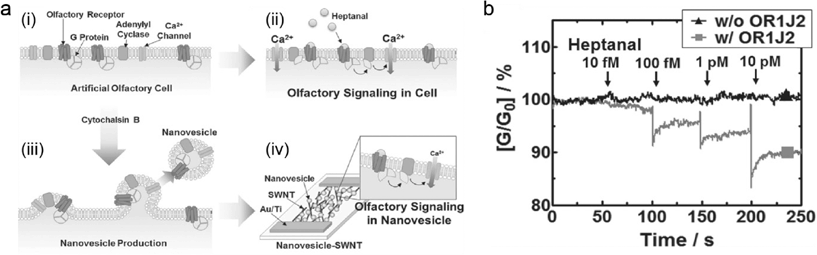
Most biomarkers used to diagnose disease are large biomolecules, such as proteins or nucleic acids. Small molecules and metabolites can also be indicative of a disease state, however, specific recognition is often more difficult in these cases. Recent work addresses this challenge by mimicking the human olfactory receptor (OR) system.9 These receptors are naturally highly selective towards specific small molecules and chemical moieties. ORs were coupled to a single walled carbon nanotube based field effect sensor system that converted the chemical binding into an electronic signal through an electrostatic mechanism associated with calcium influx.9 Lim et al. adopted this system to make a potential lung cancer diagnostic system that has the advantages of minimal sample processing, and rapid detection (Fig. 3).10Clinical studies have shown that non small cell lung cancer (NSCLC) patient blood has 10 fold higher concentrations of heptanal than control blood.11 Lim et al. tested 30 different human olfactory receptors (hOR) and found that OR1J2 specifically responds to heptanal. It is located on the neural membrane; when OR is stimulated, it results in an influx of Ca2+ ions.
They induced expression of OR1J2 on human embryonic kidney cells (HEK-293) and extracted OR1J2 containing nanovesicles from these cells. They tested the responsiveness of these hOR expressing cells to heptanal by using a fluorescent marker (Fura-2) that binds to intracellular Ca2+. Fura-2 shifts its excitation maximum from 380 nm to 340 nm when bound to Ca2+. When the OR1J2 is stimulated the influx of Ca2+ increased the fluorescence emission at 340 nm excitation.
These negatively charged nanovesicles adsorbed onto poly-D-lysine coated single walled nanotube field effect transistors (SWNT-FETs). Influx of Ca2+ ions into the nanovesicles provide a positive potential to the SWNT-FETs, resulting in a conductance change. Addition of only 0.5 μL heptanal containing samples decreased the conductance.
Lim et al. applied diluted blood plasma to the SWNT-FET to show that conductance is not significantly affected by non-heptanal compounds. Additionally, they found the system to have a detection limit of 10−14 M, with the capacity to recognize conductance changes when an additional 10−13 M heptanal was added to the sample.
The simple electrical readout of this bioelectronic platform enables it to be translated into a portable unit for point of care diagnosis. The system exhibits a clinically relevant detection limit to selectively distinguish heptanal levels in diseased blood, and future work should focus on plasma from lung cancer patients of various stages. The approach requires small volumes (0.5 μL) to yield a readout, further making it an attractive avenue for POC devices.
References
- S. Feng, R. Caire, B. Cortazar, M. Turan, A. Wong and A. Ozcan, ACS Nano, 2014, 8, 3069–3079 CrossRef CAS PubMed.
- N. R. Pollock, J. P. Rolland, S. Kumar, P. D. Beattie, S. Jain, F. Noubary, V. L. Wong, R. A. Pohlmann, U. S. Ryan and G. M. Whitesides, Sci. Transl. Med., 2012, 4, 152ra129 Search PubMed.
- S. J. Vella, P. Beattie, R. Cademartiri, A. Laromaine, A. W. Martinez, S. T. Phillips, K. A. Mirica and G. M. Whitesides, Anal. Chem., 2012, 84, 2883–2891 CrossRef CAS PubMed.
- N. N. Watkins, U. Hassan, G. Damhorst, H. Ni, A. Vaid, W. Rodriguez and R. Bashir, Sci. Transl. Med., 2013, 5, 214ra170 CrossRef PubMed.
- D. Holmes, D. Pettigrew, C. H. Reccius, J. D. Gwyer, C. van Berkel, J. Holloway, D. E. Davies and H. Morgan, Lab Chip, 2009, 9, 2881–2889 RSC.
- M.-Y. Hsu, C.-Y. Yang, W.-H. Hsu, K.-H. Lin, C.-Y. Wang, Y.-C. Shen, Y.-C. Chen, S.-F. Chau, H.-Y. Tsai and C.-M. Cheng, Biomaterials, 2014, 35, 3729–3735 CrossRef CAS PubMed.
- S.-J. Lo, S.-C. Yang, D.-J. Yao, J.-H. Chen, W.-C. Tu and C.-M. Cheng, Lab Chip, 2013, 13, 2686–2692 RSC.
- S. Balaiya, S. Grover, R. K. Murthy and K. V. Chalam, Clin. Ophthalmol., 2011, 5, 81–85 Search PubMed.
- B. R. Goldsmith, J. J. Mitala, J. Josue, A. Castro, M. B. Lerner, T. H. Bayburt, S. M. Khamis, R. A. Jones, J. G. Brand, S. G. Sligar, C. W. Luetje, A. Gelperin, P. A. Rhodes, B. M. Discher and A. T. C. Johnson, ACS Nano, 2011, 5, 5408–5416 CrossRef CAS PubMed.
- J. H. Lim, J. Park, E. H. Oh, H. J. Ko, S. Hong and T. H. Park, Adv. Healthcare Mater., 2014, 3, 360–366 CrossRef CAS PubMed.
- N. Li, C. Deng, X. Yin, N. Yao, X. Shen and X. Zhang, Anal. Biochem., 2005, 342, 318–326 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |