 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrothermal decarboxylation of amino acid derived imidazolium zwitterions: a sustainable approach towards ionic liquids†
Sarah
Kirchhecker
,
Markus
Antonietti
and
Davide
Esposito
*
Max Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry, 14424 Potsdam, Germany. E-mail: davide.esposito@mpikg.mpg.de
First published on 8th May 2014
Abstract
Ionic liquids were prepared via hydrothermal decarboxylation of zwitterionic imidazolium compounds derived from amino acids and carbohydrate related dicarbonyl compounds. Two of these ionic liquids were used successfully as solvents in the Heck reaction and for the dissolution of cellulose.
Due to the environmental and societal problems associated with the use of fossil feedstocks, there is an ever growing demand to switch not only fuel production, but also that of fine chemicals to sustainable processes using renewable resources. Imidazolium moieties are found in a variety of fine chemicals, and in recent years they played a prominent role within the field of ionic liquids (ILs). At first, this new class of solvents was hailed as green without hesitation, due to properties such as negligible vapor pressure, high thermal and chemical stability compared to traditional molecular solvents, as well as their tuneability to specific tasks. This still holds true, but it is now known that many ionic liquids are toxic,1 and they are often produced from petroleum-derived chemicals via relatively long quaternisation routes.2 For ionic liquids to become greener, toxicity issues have to be addressed, and at the same time more sustainable synthetic approaches need to be designed. In this regard, ionic liquids based wholly or partially on natural compounds have been reported.3 For instance, amino acid ionic liquids have been designed, in which amino acids are used without much modification either as cation or anion, or both.4 Nevertheless, a sustainable approach towards imidazolium cation based ILs is still missing.
We recently reported the sustainable synthesis of a small library of homosubstituted imidazolium zwitterions (IMzw) based on a modification of the Debus–Radziszewski reaction, in which amino acids are incorporated into the imidazolium ring via the amino group.5 With the same efficiency aliphatic amines can replace amino acids in such a methodology, leading to the formation of standard ionic liquids. The generation of amines from amino acids has been considered as a sustainable alternative to petrochemicals. Substantial quantities of protein waste are being generated by the food and biotechnology industries and could become a cheap source of amino acids. Decarboxylation of amino acids however is not trivial and is usually performed with the help of enzymes.6 Alternatively, conjugated carbonyl compounds have been employed as catalysts mimicking enzymatic processes, albeit at high temperatures. In these cases, decarboxylation proceeds via the formation of a conjugate imine that stabilises the developing negative charge at the site of decarboxylation. In principle, the imidazolium moiety in the novel IMzw could act in analogy to a conjugate imine and promote decarboxylation of the incorporated amino acid in a similar fashion. In this way, imidazolium based ionic liquids could be obtained using exclusively amino acids and dicarbonyl compounds derived from biomass in a simple two-step process (Fig. 1).
 | ||
| Fig. 1 Hydrothermal decarboxylation of imidazolium zwitterions. R1, R2 = H, CH3; R = amino acid side chain. | ||
Recently, hydrothermal processes have gained interest as convenient and green methods for the processing of biomass7 with interesting advantages as reaction media for the upgrading of cellulose8 and fatty acids.9 In addition, continuous processing has been recognised by fine chemical manufacturers as one of the major opportunities to improve the sustainability of industrial syntheses.10 Microreactors enable more efficient mixing and heat transfer, thereby reducing reaction times and increasing safety. They also allow for working in the near- and supercritical regions, which opens up new possibilities for green synthesis, making use of the unique properties of benign solvents such as water under these conditions.11
In this work we present a simple strategy to synthesise halogen free imidazolium ILs via continuous hydrothermal decarboxylation of IMzw. As the internal negative charge of the zwitterion is removed during decarboxylation, an external counterion is required for the formation of the IL. Initially we chose acetic acid as a benign and renewable source of anion.
Hydrothermal decarboxylation of pyruvaldehyde-derived IMzw with two equivalents of acetic acids in flow gave the corresponding imidazolium acetates in good yields ranging between 65 and 90% (Table 1). Only for the leucine-derived compound 4 the yield was lower (41%) (Table 1, entry 4). Free amino acids could not be decarboxylated under the same conditions, confirming the beneficial role of the imidazolium ring during decarboxylation.
| Entry | Amino acid | Product | Reaction conditions | NMR yield [%] | Isolated yield [%] | T g [°C] | T m [°C] |
|---|---|---|---|---|---|---|---|
Reaction conditions:a 300 °C, 150 bar, 4 min, H2O.b 250 °C, 120 bar, 4 min, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 H2O–EtOH.c Direct injection of a mixture of amino acid, acetic acid, formaldehyde and dicarbonyl compound.d Calculated after metathesis with LiN(Tf)2; n/d none detected down to −150 °C. 50 H2O–EtOH.c Direct injection of a mixture of amino acid, acetic acid, formaldehyde and dicarbonyl compound.d Calculated after metathesis with LiN(Tf)2; n/d none detected down to −150 °C. |
|||||||
| 1 | Gly |

|
0.25 M | ||||
| 100 | 90.0 | 65.1 | |||||
| 93 | |||||||
| 2 | Ala |

|
0.125 M | ||||
| 69 | 65.4d | −88.5 | |||||
| 46 | |||||||
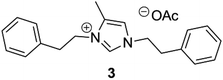
| 3 | Phe |
|
0.05 M | ||||
| 99 | 89.4 | −20.0 | |||||
| 90 | |||||||
| 4 | Leu |

|
0.03 M | ||||
| 41 | 17.9d | n/d | n/d | ||||
| 5 | Gly |
|
0.25 M | ||||
| 100 | 95.9 | −51.7 | |||||
| 6 | Gly |
|
0.125 M | ||||
| 100 | 98.0 | 125.9 | |||||
| 7 | Ala |

|
0.125 M | ||||
| 21 | 12.5d | −13.4 | |||||
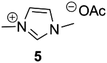
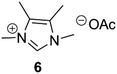
With the aim to expand the scope of the methodology, we also performed the decarboxylation on imidazolium compounds obtained using glyoxal- as well as 2,3-butadione as the dicarbonyl compound. Like pyruvaldehyde, glyoxal can be derived from biomass,12 while butadione is produced by fermentation, and it is present in dairy products such as butter and alcoholic beverages.13 The glyoxal- and butadione- derived compounds could be obtained with comparable yields using the same method applied for the preparation of pyruvaldehyde derived IMzw (see ESI†). Such compounds were then efficiently decarboxylated (entries 5 and 6), with exception of the glyoxal-derived alanine compound, which to our surprise afforded 7 in only 21% yield.
Since the imidazolium zwitterions are produced within minutes in a simple one-pot reaction of amino acids, dicarbonyl compound, formaldehyde and a slight excess of acetic acid as catalyst, we also evaluated the direct hydrothermal treatment of such a reaction mixture without any intermediate purification of the in situ formed IMzw. Indeed, for the glycine- and phenylalanine-derived compounds 1 and 3 such an approach gave similar yields (93% instead of 100% and 90% instead of 99% respectively), whereas for alanine-derived ionic liquid 2 the yield was slightly lower (46% compared to 69%).
ILs with different counterions can be readily produced by decarboxylation of the zwitterions in the presence of salts or acids of the corresponding anion. Apart from the acetate, we also obtained the chloride of compound 3 in 47.6% yield using 2 equivalents of NaCl during decarboxylation. Anions can also be exchanged by classical metathesis, which can serve at the same time as an easy and efficient way of purification, when applied to the crude reaction solution. For instance, the acetate of compounds 2, 4 and 7 could easily be exchanged by anion metathesis using lithium bis(trifluoromethane)sulfonimide (LiN(Tf)2).
All the pyruvaldehyde-derived compounds qualify as ionic liquids with melting points or glass transition temperatures below 100 °C, the majority of them being room temperature ionic liquids (Table 1). Noteworthily, ILs generated in this way are assumed to be halogen-free, as no quaternisation reactions have been employed during their synthesis. Moreover, all starting materials are almost quantitatively incorporated into the final product. C2 substitution on the imidazolium ring is known to increase the melting point and viscosity of ionic liquids, and one could expect a similar effect by introducing substituents on C4 and C5.14 Accordingly, a clear trend is seen towards increasing phase transition temperatures going from 5 with a Tg of −51.7 °C, to 1 (Tm = 65.1 °C) and finally 6 (Tm = 125.9 °C).
We further evaluated some physicochemical properties for a selected subsection of the newly obtained ILs. We focused on alanine derived compounds due to some structural similarity to the commercially available Emim derivatives. Interestingly, the viscosities of compounds 2 and 8 (Table 2) were measured to be very similar to the ones reported for the asymmetrical Emim OAc and bis(trifluoromethane)sulfonamide, anticipating their potential application as solvents. To demonstrate the usability of the so generated ionic liquids, we chose to employ them as solvents for the Heck reaction and the dissolution of cellulose, two applications where ionic liquids show unique advantages compared to other solvents. For the Heck reaction we chose the hydrophobic IL 2 and for the cellulose dissolution the more hydrophilic compound 8. Since under the current microreactor setup we could only synthesise ILs on a scale of ca. 500 μL, compounds 2 and 8 were prepared on a multigramme scale by the Debus–Radziszewski reaction using pyruvaldehyde and ethylamine (see ESI†). Analysis of the spectral data confirmed that the compounds synthesised in this way were identical to the ones obtained via the decarboxylation route. The Heck reaction is a very important carbon–carbon bond forming reaction.15 Traditionally, this is performed with homogeneous palladium catalysts containing phosphine ligands. To reduce cost, there have been efforts to simplify the catalyst and improve its recyclability. Recently, ligand-free Heck protocols for the Heck reaction have appeared in the literature,16 with different approaches for the recycling of the formed Pd black after each cycle. The benefit of using ionic liquids for this reaction is that the Pd catalyst is immobilised in the ionic liquid phase and stabilised in the form of Pd nanoparticles, while the reaction products can be easily extracted and the catalyst can be reused, thereby reducing cost and environmental impact.17 For our Heck reaction we chose the hydrophobic ionic liquid 2 as the solvent and a palladium loading of 0.2 mol% with respect to iodobenzene. The reaction between iodobenzene and methyl acrylate in the presence of triethylamine proceeded with full conversion (as assessed by GC-MS and NMR), with an average isolated yield of 94% (Fig. 2). Transmission electron microscopy showed the formation of uniform Pd-nanoparticles stabilised in the IL (Fig. 3) in agreement with previously published literature.18 To verify the recyclability of the catalyst, the ionic liquid phase was reused after extraction of the product with toluene over five reaction cycles (Fig. 2). After the 3rd run, the large build-up of triethylammonium iodide resulted in a decreased activity. In this case, unreacted iodobenzene was detected by GC-MS analysis and the isolated yield resulted to be lower (85%). However, washing of the ionic liquid-Pd phase with water followed by drying restored the full catalytic activity for consecutive runs 4 and 5 (Fig. 2).
A second important area of application for ionic liquids is the field of biomass processing. ILs are among the very few compounds able to solubilise cellulose and even wood.19 To date, one of the most efficient ILs is Emim acetate, which has been reported to dissolve more than 20 wt% of cellulose.20 An extensive network of inter- and intramolecular hydrogen bonds between strands prevents dissolution of cellulose in traditional solvents. Dissolution in ILs is assumed to work via the disruption of this network and the formation of new hydrogen bonds between the IL ions and cellulose strands. This is accompanied by a transition from the native cellulose type I crystallinity pattern to type II.21 To demonstrate that the renewable ionic liquid 8 can be considered as an efficient alternative to Emim OAc and other ILs, dissolution experiments with microcrystalline cellulose (MCC) were performed. Dissolution was assessed visually and confirmed by light microscopy (see ESI†), revealing that 8 could solubilise 16.9 weight percent of microcrystalline cellulose at 100 °C. Powder X-ray diffraction analysis of a sample of cellulose dissolved in compound 8, precipitated with water and dried was additionally used to confirm dissolution, and showed the loss of the pattern of microcrystalline cellulose with the formation of a more amorphous structure (Fig. 4).
In conclusion, we have demonstrated a sustainable and efficient method to convert fully renewable amino acid derived imidazolium zwitterions into ionic liquids. Employing a continuous hydrothermal process, we generated a library of halogen free ILs via formal decarboxylation of the amino acids. Differently substituted molecules were tested and the methodology proved to be general, within the limits of the study. The applicability of representative compounds was demonstrated by using them as solvents for the Heck reaction and for the dissolution of cellulose. The reported method represents a valuable quaternisation-free alternative route for the preparation of ILs in which the nitrogen substitution is formally obtained via amino acid decarboxylation. Finally, we expect to expand the scope of this decarboxylative methodology by applying it to other amino acid-derived systems other than the imidazolium ion, in order to generate a broader family of ionic liquids. The possible implementation of this method on a multigramme preparative scale is currently under investigation.
References
- D. Zhao, Y. Liao and Z. Zhang, Clean: Soil, Air, Water, 2007, 35, 42–48 CrossRef CAS.
- B. Clare, A. Sirwardana and D. R. Macfarlane, Top. Curr. Chem., 2010, 290, 1–40 CrossRef.
- Y. Fukaya, Y. Iizuka, K. Sekikawa and H. Ohno, Green Chem., 2007, 9, 1155 RSC; K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2005, 127, 2399–2399 CrossRef CAS PubMed; S. T. Handy, Chem. – Eur. J., 2003, 9, 2938–2944 CrossRef; S. T. Handy, M. Okello and G. Dickenson, Org. Lett., 2003, 5, 2513–2515 CrossRef PubMed; G.-h. Tao, L. He, W.-s. Liu, L. Xu, W. Xiong, T. Wang and Y. Kou, Green Chem., 2006, 8, 639 RSC.
- H. Ohno and K. Fukumoto, Acc. Chem. Res., 2007, 40, 1122–1129 CrossRef CAS PubMed.
- D. Esposito, S. Kirchhecker and M. Antonietti, Chem. – Eur. J., 2013, 19, 15097–15100 CrossRef CAS PubMed.
- C. O. Tuck, E. Perez, I. T. Horvath, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS PubMed; Z. Xiang, J. Mol. Struct., 2013, 1049, 149–156 CrossRef PubMed.
- B. Hu, K. Wang, L. Wu, S. H. Yu, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS PubMed.
- D. Esposito and M. Antonietti, ChemSusChem, 2013, 6, 989–992 CrossRef CAS PubMed.
- N. Mo and P. E. Savage, ACS Sustainable Chem. Eng., 2014, 2, 88–94 CrossRef CAS.
- C. Jiménez-González, P. Poechlauer, Q. B. Broxterman, B.-S. Yang, D. am Ende, J. Baird, C. Bertsch, R. E. Hannah, P. Dell'Orco, H. Noorman, S. Yee, R. Reintjens, A. Wells, V. Massonneau and J. Manley, Org. Process Res. Dev., 2011, 15, 900–911 CrossRef.
- S. Kobayashi, Sub- and supercritical water, Science of Synthesis, Water in Organic Synthesis, Georg Thieme Verlag, Stuttgart, Germany, 2012 RSC; S. G. Newman and K. F. Jensen, Green Chem., 2013, 15, 1456 RSC; T. Adschiri, Y.-W. Lee, M. Goto and S. Takami, Green Chem., 2011, 13, 1380 RSC; T. Wirth, Microreactors in Organic Chemistry and Catalysis, Wiley-VCH, 2nd edn, 2013 Search PubMed.
- Donald S. Scott, International Pat., WO88/00935, 1988 CAS; R. H. Venderbosch and W. Prins, Biofuels, Bioprod. Biorefin., 2010, 4, 178–208 CAS.
- D. G. Peterson and G. A. Reineccius, Flavour Fragrance J., 2003, 18, 215–220 CrossRef CAS; G. Zgherea, C. Stoian and S. Peretz, J. Liq. Chromatogr. Relat. Technol., 2011, 34, 1268–1282 CrossRef.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2nd edn, 2007 RSC; C. Liao, N. Shao, K. S. Han, X. G. Sun, D. E. Jiang, E. W. Hagaman and S. Dai, Phys. Chem. Chem. Phys., 2011, 13, 21503–21510 RSC.
- N. T. S. Phan, M. Van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609–679 CrossRef CAS; I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef PubMed.
- A. H. M. de Vries, J. M. C. A. Mulders and J. H. Mommers, Org. Lett., 2003, 5, 3285–3288 CrossRef CAS PubMed.
- M. H. Prechtl, J. D. Scholten and J. Dupont, Molecules, 2010, 15, 3441–3461 CrossRef CAS PubMed.
- J. Scholten and J. D. Dupont, Chem. Soc. Rev., 2010, 39, 1780–1804 RSC.
- N. Sun, M. Rahman, Y. Qin, M. L. Maxim, H. Rodríguez and R. D. Rogers, Green Chem., 2009, 11, 646 RSC; A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550 RSC.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC; H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Green Chem., 2008, 10, 696 RSC.
- F. Boissou, A. Mühlbauer, K. De Oliveira Vigier, L. Leclercq, W. Kunz, S. Marinkovic, B. Estrine, V. Nardello-Rataj and F. Jérôme, Green Chem., 2014, 16, 2463 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc00564c |
| This journal is © The Royal Society of Chemistry 2014 |





