Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Olefin cross-metathesis as a valuable tool for the preparation of renewable polyesters and polyamides from unsaturated fatty acid esters and carbamates†
Matthias
Winkler
and
Michael A. R.
Meier
*
Laboratory of Applied Chemistry, Institute of Organic Chemistry, Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany. E-mail: m.a.r.meier@kit.edu; Web: http://www.meier-michael.com
First published on 22nd April 2014
Abstract
Olefin cross-metathesis of unsaturated fatty acid methyl ester (FAME) derived benzyl carbamates with methyl acrylate is described. The obtained by-product, an α,β-unsaturated ester, was further modified via thia-Michael addition reactions in order to synthesize branched AA-type or AB-type monomers for the preparation of polyesters, which are tuneable by oxidation. Cross-metathesis of fatty acid derived carbamates was used as a novel approach to prepare linear AB-type monomers, which can be used for the preparation of renewable polyamides PA11, PA12 and PA15. The necessary fatty acid carbamates were prepared by applying a catalytic Lossen rearrangement procedure. The presented synthesis strategy has potential for the bio-sourced preparation of monomers for the production of polyamides. All prepared polymers were fully characterized by NMR, SEC, and DSC analyses. Additionally, the Young's modulus of the prepared long-chain polyamide PA15 was determined.
Introduction
The price, availability, and environmental impact are the main reasons for the chemical industry to move away from fossil resources. However, the polymer industry is still strongly depending on the use of petroleum derived monomers. In order to substitute these monomers, efficient synthetic routes utilizing renewable resources are needed and are of high interest. Moreover, processes making use of the synthetic potential of nature and producing little to no waste are highly desirable. Recognized as one of the most important and the most frequently used renewable raw materials, plant oils are valuable alternatives not only for the synthesis of diverse fine chemicals, but also for polymer chemistry.1,2 Many efficient catalytic modification reactions of fatty acids or fatty acid methyl esters (FAMEs) to prepare monomers or monomer precursors have been developed. Herein, methoxycarbonylation,3,4 ketonization,5–7 acetoxylation,8,9 or enzymatic transformations10–12 offer interesting modification possibilities. Olefin metathesis proved to be an especially powerful tool in this context and was used to prepare different monomers from diverse plant oils in an efficient catalytic manner.1,13–15 In 2007, our group reported on the cross-metathesis of FAMEs and methyl acrylate.16 All metathesis reactions were performed in bulk using an excess of the methyl acrylate and the Hoveyda–Grubbs 2nd generation catalyst. Full conversion of the FAMEs and a very good cross-metathesis selectivity were obtained using a catalyst loading of <0.5 mol%. Moreover, self-metathesis (SM) of fatty acids to prepare diesters is described as a catalytic procedure to obtain renewable monomer precursors. In the case of undecenoic acid (a castor oil derivative),17 self-metathesis was used to generate the C20 diacid, an AA-type monomer to prepare polyesters by polycondensation.18 Employing oleic acid or erucic acid for SM reactions revealed to be slightly more challenging since the metathesis equilibrium cannot be shifted by simply removing ethylene as a by-product. However, also several procedures to prepare the oleic or erucic acid derived diacids via metathesis reactions are known.19–21 Furthermore, the metathesis reaction of FAME with diverse functional compounds allows the synthesis of functionalized FAMEs. Herein, the CM of FAMEs with allyl alcohol, allyl chloride, diethyl maleate or cis-butene-1,4-diyl diacetate is well investigated.22–25 The above mentioned approaches demonstrate the versatility of the (cross-)metathesis reaction in order to synthesize valuable functionalized fatty acid derivatives or fatty acid based monomers. However, the synthesis of polyamide monomers using metathesis reactions is barely described. Such an efficient catalytic synthesis procedure, however, is highly interesting since polyamides represent a class of highly valuable engineering plastics. The (cross-)metathesis of amine or amide functionalized substrates still remains a great challenge. Usually, either the amine function is protected prior to the metathesis reaction or the metathesis reaction is performed in acetic media in order to in situ protonate the amine and prevent catalyst deactivation.26,27 Robinson and coworkers reported the SM of undecenylamine to prepare the corresponding diamine, a valuable and renewable AA-type monomer for the preparation of polyamides. Bruneau et al. reported the cross-metathesis of 10-undecenenitrile or acrylonitrile with methyl 10-undecenoate or methyl acrylate, respectively.28 The presented tandem procedure provides a sustainable and very interesting synthetic route to linear amino esters as useful polyamide precursors.These findings prompted us to search for novel catalytic procedures allowing the preparation of fatty acid based amino esters, which can be used to prepare renewable polyamides.
Thus, the olefin cross-metathesis of unsaturated fatty acid methyl ester (FAME) derived carbamates with methyl acrylate was investigated for the first time in order to synthesize renewable polyamides and polyesters. Moreover, utilizing all products of the respective metathesis reactions, the cross-metathesis by-products were also converted to valuable monomers and subsequently polymerized.
Results and discussion
In an earlier publication, we reported on the efficient olefin cross-metathesis of methyl acrylate and unsaturated fatty acid methyl esters (FAMEs).16 The cross-metathesis reactions were performed under solvent-free conditions using low catalyst loadings and selectively provided the corresponding diesters and an α,β-unsaturated ester. The presented catalytic synthesis procedure offers sustainable access to polyester precursors.These findings prompted us to search for a possibility to perform efficient cross-metathesis reactions of fatty acid based substrates in order to obtain renewable polyamide precursors. In principle, instead of employing FAMEs in the cross-metathesis with methyl acrylate, the use of the corresponding amine would directly provide the desired amino FAME. However, cross-metathesis reactions of compounds having an amine moiety are still quite challenging and usually the amine function is protected.26 In this context, Bruneau and colleagues reported the cross-metathesis of 10-undecenenitrile with methyl acrylate.28 Subsequent hydrogenation of the cross-metathesis product yielded the desired AB-type monomer as a polyamide precursor. Recently, we reported the synthesis of fatty acid derived carbamates, which can be obtained in an environmentally friendly fashion via a catalytic Lossen rearrangement.29 The prepared methyl carbamates can be used to synthesize the corresponding amines by simple carbamate cleavage under basic conditions. Thus, a cross-metathesis of such carbamates and subsequent carbamate cleavage would enable the synthesis of the desired AB-type monomers as illustrated in Fig. 1. In order to enable a mild deprotection of the carbamate and hydrogenation of the double bond in one step, we used fatty acid derived benzyl carbamates as starting materials for the cross-metathesis reaction with methyl acrylate (Fig. 1). The catalytic Lossen rearrangements were performed according to our previously reported procedure utilizing TBD as a catalyst, methanol and dibenzyl carbonate.29 It is noteworthy that dibenzyl carbonate was synthesized by simple trans-esterification of dimethyl carbonate (a renewable and untoxic reagent), according to the procedure reported by our group earlier.30 An advantage of the Lossen rearrangement performed with dibenzyl carbonate and benzyl alcohol is the reaction efficiency. Thus, higher yields were obtained as in the synthesis of the corresponding methyl carbamates.29 Moreover, the excess of the required alcohol and carbonate was significantly reduced. On the other hand, the regeneration of the used benzyl alcohol and dibenzyl carbamate in order to perform the Lossen rearrangement in a sustainable manner is not as straightforward. However, the use of a Kugelrohr distillation apparatus allowed for a simple and efficient recycling of the benzyl alcohol and dibenzyl carbamate by distillation. It has to be noted that all Lossen rearrangements were performed with 20 mol% of the TBD catalyst. Interestingly, the reaction can also be carried out with lower catalyst loadings (5–10 mol%), though longer reaction times are needed.
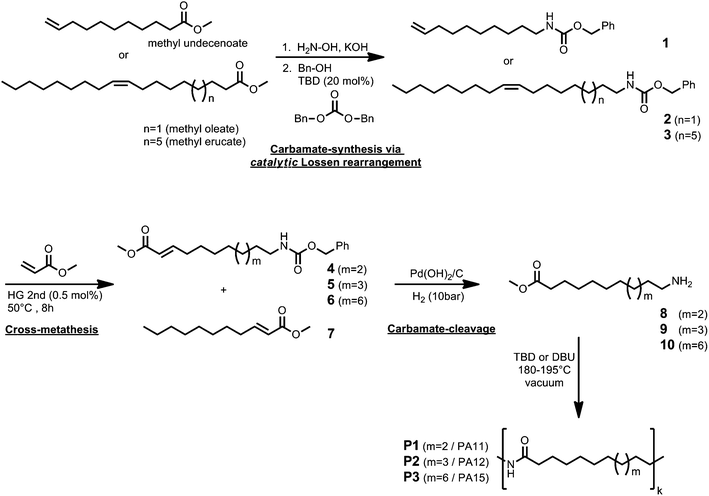 | ||
| Fig. 1 Synthesis of renewable AB-type monomers via Lossen rearrangement, cross-metathesis, and subsequent deprotection. | ||
The cross-metathesis reactions were performed similar to a known procedure.16 Thus, all metathesis reactions were performed in bulk using the Hoveyda–Grubbs second generation catalyst (0.5 mol%) and an excess of the methyl acrylate. The desired products 4–6 were obtained in good yields (up to 91%) and were subsequently used for the preparation of the ω-amino FAMEs. In the case of the cross-metathesis of benzyl carbamate 3, a white precipitate was formed after a short reaction time, caused by self-metathesis, and only rather low yields (47–50%) were obtained. Improved yields of up to 80% were obtained if the reaction was performed at higher temperatures (60–65 °C, similar to the procedure reported by Slugovc et al. using ethyl acrylate at a reaction temperature of 75–80 °C26). Yields of up to 80% were also obtained if the reaction was performed on a smaller scale (1.5 g/3.4 mmol) under vigorous stirring. As a by-product, methyl undec-2-enoate 7 is formed, which can be further derivatized as described below (Fig. 3). The hydrogenation of the double bond and the cleavage of the benzyl carbamate were performed in one step. First, we used Pd/C as a convenient catalyst for heterogeneous hydrogenations and cleavage of benzyl carbamates. Although we employed a pressure of 20 bar of hydrogen at a temperature of 35 °C, the benzyl carbamate was not cleaved after two days. If Pearlman's catalyst (Pd(OH)2/C) was used instead, the hydrogenation as well as the cleavage of the carbamate was completed after 16 hours, providing the ω-amino FAMEs in very good yields and in high purity without further purification. The solvent used for the carbamate-cleavage/hydrogenation reaction appeared to be of crucial importance. The desired product was only obtained in high purity if alcohols, such as methanol or ethanol, were used. As an example, the 1H-NMR spectra of the benzyl carbamate 3, the cross-metathesis product 6, and the final amino FAME 10 are shown in Fig. 2, demonstrating the overall successful transformation of methyl erucate to the desired AB-type monomer. Subsequently, the prepared amino FAMEs were used in polycondensation reactions to prepare polyamides (PAs) with a varying carbon chain length using TBD or DBU as the catalyst. SEC analysis of P1, P2 and P3 revealed molecular weights of 14.9 kDa to 22.6 kDa and a dispersity of 1.73 to 2.20 (molecular weights were determined relative to narrow poly(methylmethacrylate) standards, see also ESI†). The melting points were determined by differential scanning calorimetry (DSC) and ranged from 169 °C to 186 °C (Table 1).
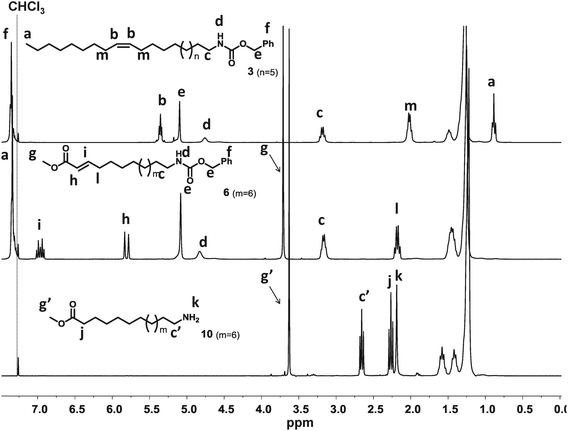 | ||
| Fig. 2 Comparison of the 1H-NMR spectra of the benzyl carbamate 3, the cross-metathesis product 6, and the amino FAME 10. | ||
Compared to commercially available polyamides, the melting points are in the expected range. For commercially available PA11 and PA12, melting points of 183 °C and 180 °C are reported, which are similar to the measured melting points of FAME based polyamides P1 and P2 (Table 1).32 Compared to PA11 and PA12, the amide frequency of PA15 is lower, and thus the melting point of the fatty acid derived polyamide P3 (Tm = 169 °C) is lower. When compared to PA16 with a melting point of 166 °C, the melting point of P3 is slightly higher due to an increased amide frequency.31
Both polymers P3 and PA16 are polyamides having an extended aliphatic segment and represent a class of interesting materials.33 Besides DSC, NMR and SEC analyses, we performed stress–strain measurements of the prepared long-chain polyamide P3 to determine the Young's modulus and compared the results to commercial PA11 and PA12 (for experimental details of the stress–strain measurements see ESI†). The measurements revealed a Young's modulus for P3 of 1480 MPa, which is lower than the modulus for the commercial PA11 and PA12. Apparently, the longer chain polyamides PA15 and PA16 having a lower amide group frequency have lower Young's modulus due to reduced hydrogen bonding. On the other hand, PA11 has a higher amide group frequency, thus having a stronger hydrogen bond interaction, but its Young's modulus is lower than that of polyamide 12 having a similar molecular weight. Herein, the slightly longer aliphatic chain segments have a significant impact on the Young's modulus. This effect can be explained by the different crystallinity of odd- and even-nylons.34 The same effect can be observed when the PA15 and P16 are compared with each other, although the difference in the Young's moduli is less pronounced.
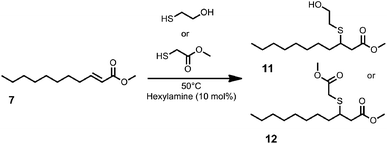
As a by-product of the cross-metathesis of the fatty acid derived benzyl carbamates, an α,β-unsaturated ester, methyl undec-2-enoate 7, is obtained, which appeared to be an interesting starting material for further modifications. As is known, α,β-unsaturated carbonyl compounds undergo Michael type addition reactions; thus, the use of 7 as a Michael acceptor appears to be an appropriate synthetic strategy to transform the methyl undec-2-enoate to value-added compounds. In order to obtain monomers, we used 2-mercaptoethanol and methyl thioglycolate as Michael-donors to obtain monomers 11 and 12, respectively (Fig. 3).
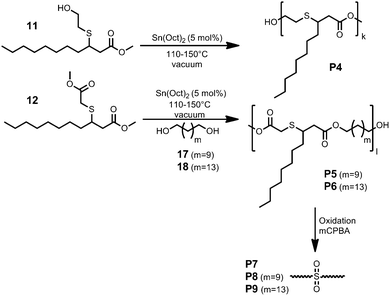
Thia-Michael additions performed in bulk and with a small excess of the thiol (1.2 equivalents) provided the best results. Full conversion of 7 was achieved after stirring for 5 h at 50 °C using hexylamine (10 mol%) as the catalyst. The obtained monomer 11 was directly used in polycondensation reactions. The monomer 12 can be used in polycondensation reactions as well, if an appropriate diol or diamine is used. Thus, we transformed the obtained diesters from the metathesis of methyl oleate or -erucate to the corresponding diols 17 and 18 by standard reduction procedures (see ESI†) and used them as co-monomers (Fig. 4). In this way, we were able to use all products of the respective cross-metathesis reaction for the synthesis of renewable monomers. In previous studies, 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) showed very good performance as an organocatalyst for the synthesis of diverse polyesters and polyamides.35–37 In the case of monomers 11 and 12, this catalyst did not work since elimination of the thiol moiety occurred. However, using tin ethylhexanoate or titanium isopropoxide as a catalyst yielded the desired polyesters without the occurrence of elimination side reactions (Fig. 4). Size exclusion chromatography (SEC) revealed polymers with a molecular weight of 17.0–19.0 kDa and a dispersity of 1.52–1.90. The thermal properties of the prepared polyesters were studied using DSC, revealing melting points of −18.6 °C–15.5 °C.
As expected, the branched polyesters show low melting points due to their dangling side-chains. The polymer P6 displayed the highest melting point of 15.5 °C, since the employed long-chain C15 diol, derived from methyl erucate, provided increased crystallinity. The polymer P4 showed a Tg of 8 °C (Table 2).
| Polymer | M n [kDa] | Đ | T m [°C] |
|---|---|---|---|
| P4 | 17.1 | 1.52 | T g = 8 |
| P5 | 18.9 | 1.74 | −18.6 |
| P6 | 18.0 | 1.90 | 15.5 |
| P7 | — | — | 21 |
| P8 | — | — | −5 |
| P9 | — | — | 25 |
To modify the thiol containing polyesters (P4–P6), we performed some oxidation reactions with meta-chloroperoxybenzoic acid (mCPBA) in order to convert the sulfur atoms in the backbone of these polyesters to sulfones (P7–P9, Fig. 5, Table 2). SEC analysis of the oxidized polymers was not possible, since after the oxidation the polymers were not soluble in common SEC solvents (e.g. CHCl3, THF, DMAC, DMF, HFIP, or toluene).
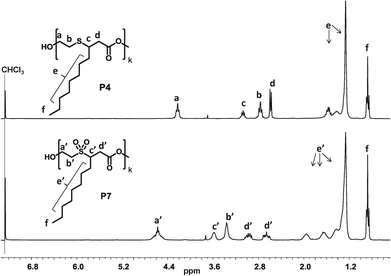 | ||
| Fig. 5 Comparison of the 1H-NMR spectra before (top) and after (bottom) the oxidation of the sulfur containing polyester P4. | ||
Nevertheless, the slight solubility of the prepared polymers in chloroform allowed for 1H-NMR analysis of the oxidized products, which revealed the full oxidation of the sulfur, while no degradation of the polymer was observed (Fig. 5). DSC analysis of the polymers showed that the melting point of the polyesters increased due to this oxidation (Table 2).
Conclusions
The synthesis of plant oil derived renewable polyesters and polyamides is described. Based on the products obtained by cross-metathesis of the FAME derived carbamates with methyl acrylate, diverse monomers were prepared. Methyl undec-2-enoate (as by-product) was utilized in thia-Michael additions to synthesize an AB- or AA-type monomer. On the other hand, the obtained diesters from the cross-metathesis were reduced to the corresponding diols and used for polymerizations with the prepared AA-type monomer. The synthesized sulfur containing polyesters were oxidized to the corresponding sulfone-polyesters by a simple oxidation procedure, which led to increased melting points and significantly different solubilities of the polymers. On the other hand, in order to synthesize FAME based renewable polyamides, a novel efficient and catalytic strategy including the cross-metathesis of FAME derived benzyl carbamates is introduced. These carbamates were prepared via a catalytic Lossen rearrangement utilizing dibenzyl carbonate and benzyl alcohol. High yields were achieved in the cross metathesis of these benzyl carbamates with methyl acrylate leading to AB-type monomer precursors, while using low catalyst loadings (0.5 mol%) and performing the reaction under bulk conditions. Under very mild reaction conditions (ambient temperature and atmospheric hydrogen pressure), the carbamate was cleaved and the remaining double bond was hydrogenated in one step, leading to the amino FAMEs in quantitative yields. The corresponding polyamides were prepared in organocatalyzed polycondensation reactions yielding PA11, PA12 or PA15. The tensile measurement of the long-chain PA15 revealed a high Young's modulus, further demonstrating the potential use of this synthesis strategy for the preparation of new bio-sourced polyamides with good properties.Acknowledgements
Financial support for this project from the German Federal Ministry of Food, Agriculture and Consumer Protection, represented by the Fachagentur Nachwachsende Rohstoffe FNR (FKZ: 22006311) is kindly acknowledged. The authors would like to also thank Daniel Zimmermann and Helena Hörig (KIT) for experimental support.Notes and references
- L. Montero de Espinosa and M. A. R. Meier, Eur. Polym. J., 2011, 47, 837–852 CrossRef CAS PubMed.
- M. A. R. Meier, J. O. Metzger and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1788–1802 RSC.
- F. Stempfle, D. Quinzler, I. Heckler and S. Mecking, Macromolecules, 2011, 44, 4159–4166 CrossRef CAS.
- C. Jiménez-Rodriguez, G. R. Eastham and D. J. Cole-Hamilton, Inorg. Chem. Commun., 2005, 8, 878–881 CrossRef PubMed.
- I. Hermans, K. Janssen, B. Moens, A. Philippaerts, B. Van Berlo, J. Peeters, P. A. Jacobs and B. F. Sels, Adv. Synth. Catal., 2007, 349, 1604–1608 CrossRef CAS.
- B. Morandi, Z. K. Wickens and R. H. Grubbs, Angew. Chem., Int. Ed., 2013, 52, 2944–2948 CrossRef CAS PubMed.
- M. Winkler and M. A. R. Meier, Green Chem., 2014, 16, 1784–1788 RSC.
- M. von Czapiewski, O. Kreye, H. Mutlu and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2013, 115, 76–85 CrossRef CAS.
- E. N. Frankel, W. K. Rohwedder, W. E. Neff and D. Weisleder, J. Org. Chem., 1975, 40, 3247–3253 CrossRef CAS.
- J.-W. Song, E.-Y. Jeon, D.-H. Song, H.-Y. Jang, U. T. Bornscheuer, D.-K. Oh and J.-B. Park, Angew. Chem., Int. Ed., 2013, 52, 2534–2537 CrossRef CAS PubMed.
- J. O. Metzger and U. Bornscheuer, Appl. Microbiol. Biotechnol., 2006, 71, 13–22 CrossRef CAS PubMed.
- W. Lu, J. E. Ness, W. Xie, X. Zhang, J. Minshull and R. A. Gross, J. Am. Chem. Soc., 2010, 132, 15451–15455 CrossRef CAS PubMed.
- S. Chikkali and S. Mecking, Angew. Chem., Int. Ed., 2012, 51, 5802–5808 CrossRef CAS PubMed.
- R. Malacea, C. Fischmeister, C. Bruneau, J.-L. Dubois, J.-L. Couturier and P. H. Dixneuf, Green Chem., 2009, 11, 152–155 RSC.
- X. Miao, C. Fischmeister, C. Bruneau and P. H. Dixneuf, ChemSusChem, 2009, 2, 542–545 CrossRef CAS PubMed.
- A. Rybak and M. A. R. Meier, Green Chem., 2007, 9, 1356–1361 RSC.
- H. Mutlu and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2010, 112, 10–30 CrossRef CAS.
- J. Trzaskowski, D. Quinzler, C. Bährle and S. Mecking, Macromol. Rapid Commun., 2011, 32, 1352–1356 CrossRef CAS PubMed.
- C. Vilela, A. J. D. Silvestre and M. A. R. Meier, Macromol. Chem. Phys., 2012, 213, 2220–2227 CrossRef CAS.
- P. B. Dam, M. C. Mittelmeijer and C. Boelhouwer, J. Am. Oil Chem. Soc., 1974, 51, 389–392 CrossRef.
- H. Ngo, K. Jones and T. Foglia, J. Am. Oil Chem. Soc., 2006, 83, 629–634 CrossRef CAS PubMed.
- M. A. R. Meier, A. Rybak and D. Geisker, Hydroxy- and aldehyde functional compounds, WO, 2010/083934, 2009 Search PubMed.
- A. Behr, J. P. Gomes and Z. Bayrak, Eur. J. Lipid Sci. Technol., 2011, 113, 189–196 CrossRef CAS.
- A. Behr and J. Pérez Gomes, Beilstein J. Org. Chem., 2011, 7, 1–8 CrossRef CAS PubMed.
- T. Jacobs, A. Rybak and M. A. R. Meier, Appl. Catal., A, 2009, 353, 32–35 CrossRef CAS PubMed.
- M. Abbas and C. Slugovc, Monatsh. Chem., 2012, 143, 669–673 CrossRef CAS.
- C. P. Woodward, N. D. Spiccia, W. R. Jackson and A. J. Robinson, Chem. Commun., 2011, 47, 779–781 RSC.
- X. Miao, C. Fischmeister, P. H. Dixneuf, C. Bruneau, J. L. Dubois and J. L. Couturier, Green Chem., 2012, 14, 2179–2183 RSC.
- O. Kreye, S. Wald and M. A. R. Meier, Adv. Synth. Catal., 2013, 355, 81–86 CrossRef CAS.
- H. Mutlu, J. Ruiz, S. C. Solleder and M. A. R. Meier, Green Chem., 2012, 14, 1728–1735 RSC.
- N. Kolb, M. Winkler, C. Syldatk and M. A. R. Meier, Eur. Polym. J., 2014, 51, 159–166 CrossRef CAS PubMed.
- K. Marchildon, Macromol. React. Eng., 2011, 5, 22–54 CrossRef CAS.
- M. Ehrenstein, S. Dellsperger, C. Kocher, N. Stutzmann, C. Weder and P. Smith, Polymer, 2000, 41, 3531–3539 CrossRef CAS.
- N. S. Murthy, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1763–1782 CrossRef CAS.
- R. C. Pratt, B. G. G. Lohmeijer, D. A. Long, R. M. Waymouth and J. L. Hedrick, J. Am. Chem. Soc., 2006, 128, 4556–4557 CrossRef CAS PubMed.
- M. Winkler, M. Steinbiß and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2014, 116, 44–51 CrossRef CAS.
- D. Tang, D.-J. Mulder, B. A. J. Noordover and C. E. Koning, Macromol. Rapid Commun., 2011, 32, 1379–1385 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed description of all experimental procedures. See DOI: 10.1039/c4gc00273c |
| This journal is © The Royal Society of Chemistry 2014 |