Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Titania-catalysed oxidative dehydrogenation of ethyl lactate: effective yet selective free-radical oxidation†
Enrique V.
Ramos-Fernandez
*,
Norbert J.
Geels
,
N. Raveendran
Shiju
* and
Gadi
Rothenberg
Van 't Hoff Institute for Molecular Sciences, University of Amsterdam, P.O. Box 94157, 1090 GD Amsterdam, The Netherlands. E-mail: e.v.ramosfernandez@uva.nl; n.r.shiju@uva.nl; Web: http://hims.uva.nl/hcsc
First published on 15th May 2014
Abstract
We research here the catalytic oxidative dehydrogenation of ethyl lactate, as an alternative route to ethyl pyruvate. Testing various solid catalysts (Fe2O3, TiO2, V2O5/MgO–Al2O3, ZrO2, CeO2 and ZnO), we find that simple and inexpensive TiO2 efficiently catalyses this reaction under mild conditions. Furthermore, molecular oxygen was used as the terminal oxidant. Importantly, this reaction runs well also using inexpensive commercial solvent mixtures. Both the desired reaction and the by-products formation follow a free-radical mechanism. Remarkably, adding activated carbon, a solid radical scavenger, hardly affects the catalytic activity, but enhances the product selectivity. This is because this solid radical scavenger hampers the formation of undesired products in solution, without suppressing the oxidation at the catalyst surface.
Introduction
Pyruvic acid and its esters are used as intermediates for perfumes, food additives, and electronic materials. They are also attractive as raw materials for various bioactive substances such as antiviral drugs.1,2 Currently, pyruvic acid is produced via dehydrative decarboxylation of tartaric acid. The reaction typically runs in liquid phase, but was also adapted for vapor-phase flow operations using a silica-supported pyrosulfate catalyst (K2S2O7/SiO2), giving ethyl pyruvate continuously in good yields (60%), albeit at the high temperature of 300 °C.3 The problem is that this reaction requires excess KHSO4 as a dehydrating agent, leading to an expensive and wasteful process. Pyruvate can also be obtained by a microbial process, based primarily on strains of yeast and E. coli. However, both strains require precise regulation of media composition during fermentation and complex supplements.4Alternatively, pyruvic acid or esters can be synthesised via the catalytic oxidation of lactic acid/esters, e.g. converting ethyl lactate 1 to ethyl pyruvate 2 (eqn (1)).5 In this case, the whole process would be based on biomass-derived feedstocks, and the reaction could in theory proceed directly from lactic acid to pyruvic acid. Practically, however, the main problem with this approach is there is no efficient catalyst for it.
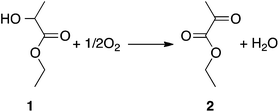 | (1) |
Due to the importance of pyruvic acid and its derivatives, these reactions were studied by several groups, using both gas and liquid phases. In gas-phase reactions, various solid catalysts were used, including TeO2 and MoO3.5–9 These processes give high yields of pyruvate, but they are energy-intensive. Conversely, the liquid-phase option needs no vaporizing. Hayashi et al. reported catalytic conversions of lactic acid to pyruvic acid in an aqueous phase.5,7,10 They found that, while pyruvic acid was not formed on plain Pd/C, doping the catalyst by either lead, bismuth or tellurium gave spectacular results at 90 °C.5,9,10 That said, this reaction used also an excess of NaOH, adjusting the pH to 8, and practically starting from the lactate ion. Ideally, one would prefer a reaction at low temperature, using an inexpensive catalyst (no noble metals), in absence any strong base or solvent. But this is much easier said than done.
Addressing this challenge, we screened various simple oxides with the aim of finding a catalyst that can work in the oxidative dehydrogenation of lactic acid. After several experiments, we chose titania (TiO2) as a catalyst. Several intriguing properties make titania not only a useful support but also an active phase. Titania can chemisorb alcohols at low temperatures dissociatively.11,12 It can also strongly adsorb oxygen (∼50 kJ mol−1) at room temperature,13,14 and it has a rich surface chemistry, which can support free-radical processes.
However, until now titania has mainly been studied as a support of active phases, photocatalysts or oxidation catalysts using H2O2. Here we report that titania is an effective noble metal-free catalyst for selective oxidation of ethyl lactate into ethyl pyruvate using molecular oxygen under mild conditions. We study the reaction conditions and explain the reasons for high product selectivity.
Experimental
Materials and instrumentation
TiO2 (125 m2 g−1) and activated carbon (960 m2 g−1)15 were supplied by Eurotitania and Calgon, respectively. Other chemicals were purchased from Sigma-Aldrich or Air Liquide and used as received. Adsorption isotherms and differential heats of adsorption are accessed directly by a manometric set-up combined with a micro-calorimeter (Calvet C80, Setaram).16,17 The calorimeter used for these experiments is of the Calvet type. It measures the heat flux in and/or out of the sample cell, and can be operated isothermally at a fixed temperature. Gas or vapour can be fed into the system by a piston, that can introduce a full, 1/2 stroke or 1/4 stroke. The introduction pressure cannot be higher than 100 kPA. Two pressure transducers with different sensitivities allow an accurate measurement of pressure, of the gas phase in contact with the sample, from 0 kPa to 10 kPa and from 0 kPa to 1000 kPa. The system has two independent data acquisition systems, one for the manometric (isotherm) data, the other for the calorimetric data. A detailed description of the experimental system is given elsewhere.18,19 Reaction samples were analysed on a gas chromatograph (Interscience GC8000) equipped with a FID and a capillary DB-1 column, using He as carrier gas, and an external standard.Procedure for catalytic oxidative dehydrogenation
The liquid phase oxidation of ethyl lactate 1 was carried out in a 400 ml stirred autoclave (Biometa, fitted with a system for liquid sampling) at 403 K and at constant pressure of 1 MPa of pure oxygen. The catalyst (2 g) was immersed in 200 g of ethyl lactate. In experiments using a solvent, mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (solvent
1 (solvent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) reactant) was used. After flushing the reactor with pure oxygen thrice, the temperature was raised to 403 K. Zero time was taken at this moment and stirring was switched on. Reaction progress was monitored by GC.
reactant) was used. After flushing the reactor with pure oxygen thrice, the temperature was raised to 403 K. Zero time was taken at this moment and stirring was switched on. Reaction progress was monitored by GC.
Results and discussion
First, we tested several metal oxides in the oxidative dehydrogenation of ethyl lactate (TiO2, Fe2O3, V2O5/MgO–Al2O3, ZrO2, CeO2 and ZnO) as catalysts. The performance of all the catalysts were tested in a batch reactor, monitoring the consumption of ethyl lactate 1 and the production of ethyl pyruvate 2 (eqn (1)). In all cases no solvent was used and the catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) substrate ratio was kept constant at 100
substrate ratio was kept constant at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w. Four materials, Fe2O3, ZrO2, CeO2 and ZnO, showed <5% conversion after 24 h, and were therefore discarded. Another, V2O5/MgO–Al2O3 showed good catalytic activity in the first run. However, control experiments showed that this was due to leaching of vanadium species into solution, and no conversion was found in subsequent runs. Happily, we found that titania was both active and selective at low conversions. Thus, we decided to focus on this catalyst.
1 w/w. Four materials, Fe2O3, ZrO2, CeO2 and ZnO, showed <5% conversion after 24 h, and were therefore discarded. Another, V2O5/MgO–Al2O3 showed good catalytic activity in the first run. However, control experiments showed that this was due to leaching of vanadium species into solution, and no conversion was found in subsequent runs. Happily, we found that titania was both active and selective at low conversions. Thus, we decided to focus on this catalyst.
We then investigated the textural properties of the titania. It is a mesoporous material with a mean pore size of 3.6 nm and a surface area of 125 m2 g−1. Powder X-ray diffraction showed sharp and well-defined peaks. The anatase phase is predominant (>50%), but brookite and rutile are also present.
Intrigued by the fact that titania was working where supposedly similar oxides were not, we decided to measure the strength of the interactions of alcohols on the catalyst surface. This was done using microcalorimetry (see Experimental section for details). However, since ethyl lactate has a high boiling point (151–155 °C), direct measurement of its interaction with TiO2 calorimetrically is difficult. Instead, we measured the enthalpies of adsorption of low boiling point alcohols with different chemical properties. By comparing these properties with those of ethyl lactate, we could estimate the interaction between ethyl lactate and titania. Fig. 1 shows the vapour adsorption isotherms for t-butanol, i-propanol and methanol. For all three, the majority of the adsorption takes place at low relative pressures (p/p0 < 0.1), indicating strong absorbate–absorbent interaction. This is especially pronounced in the case of methanol. The differential heats of adsorption profiles for the alcohols were similar, starting with a high heat (115–140 kJ mol−1) and then decreasing slowly until reaching the saturation of the sample, and thereafter decreasing rapidly. The initial heats of adsorption vary, with t-BuOH > i-PrOH > MeOH. In all cases, the heats of adsorption are high in comparison with pure physisorption of alcohols in an inert apolar porous solid, such as activated carbon, (ΔH ∼ 50 kJ mol−1), again indicating a strong adsorption.11
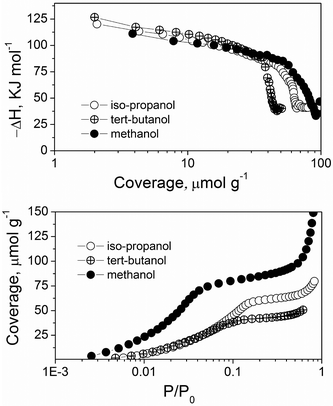 | ||
| Fig. 1 Differential heat of adsorption against the coverage for different alcohols on TiO2 (top) and vapour adsorption isotherms of different alcohols on TiO2 (bottom). | ||
This strong adsorption of the alcohol is significant, because an enthalpy of 115–125 kJ mol−1 is of the same order of magnitude as the energy needed to activate the alcohol O–H bond.20 Thus, we can hypothesise that the ethyl lactate, which is quite similar in structure to i-PrOH, can chemisorb on the surface and thus start the dehydrogenation reaction. We also measured the heat of adsorption of acetone (see ESI†). The initial heat of adsorption is 102 kJ mol−1, which is significantly lower than that of alcohols (∼120 kJ mol−1). This indicates that alcohols have better affinity for TiO2 than ketones. Thus, ethyl lactate is adsorbed most likely by a hydroxyl group interaction.21
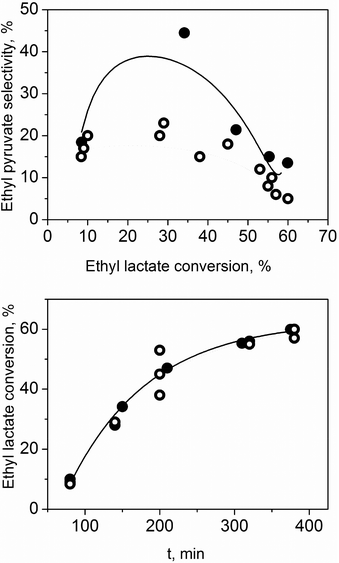
To ensure that our catalytic results are valid and reflect the chemical reaction and not any other effects, we ran extensive blank experiments, hot filtration experiments and reusability tests (details in the ESI†). Particularly important were the results found when we checked the mass transfer limitations. Fig. 2 shows the catalytic performance of TiO2 on the oxidative dehydrogenation of ethyl lactate (no solvent was added to this reaction). The conversion increased with time reaching 60% after 5 h. We found exactly the same reaction rate when the stirring speed was doubled. This demonstrates that we are in the chemical kinetic regime (no oxygen mass transfer limitations).
However, the stirring speed influences the selectivity towards ethyl pyruvate. Higher stirring speed induced lower selectivity. This means that the oxidation is the first step and it is not limited by oxygen concentration. Conversely, the formation of by-products is a liquid phase reaction in which the oxygen is not involved, and it is enhanced by the mixing. Note that only ethyl pyruvate and polymeric species were detected.22
In order to avoid the polymerization, we followed different strategies. Firstly, we used a solvent, decreasing the concentrations and thereby minimizing the polymerization. For this particular strategy, high boiling point solvents are needed since the reaction is performed at 130 °C. We tested chlorobenzene, DMSO, water, diethylsuccinate, and two commercial dibasic esters DBE-0 and DEB-9, all using a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w substrate
1 w/w substrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio.
catalyst ratio.
The solvent influences the reaction pathways. Thus, water induces a high reaction rate but it also leads to hydrolysis products. We could not detect ethyl pyruvate since the ester bond is easily hydrolysed under these hydrothermal conditions. Using chlorobenzene, we did not see any catalytic activity even after 24 h. Chlorobenzene is apolar, and the oxygen solubility is limited. The reaction does not proceed, since the oxygen can not reach the catalyst surface. The case of DMSO is particularly interesting. It is a polar solvent in which oxygen can be dissolved and hence one would expect catalytic activity. However, we did not find products even after 24 h. This may be because DMSO is a good radical scavenger and the ethyl lactate oxidation follows a free radical mechanism (see discussion below).
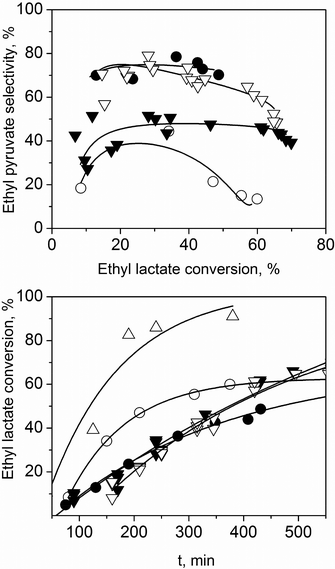
Then, we moved to a less traditional high boiling point solvent, diethyl succinate. Fig. 3 shows the results. The reaction is slower compared to the reaction in the absence of a solvent. However, the selectivity increases, reaching 75% at 50% conversion. Thereafter, it decreases due to polymerization. The dilution effect of the solvent is the key. To further check it, we also analysed concentration effects. Fig. 4 shows that increasing the ethyl lactate concentration, the reaction rate is faster, which indicates that the reaction order is positive with respect to the ethyl lactate.
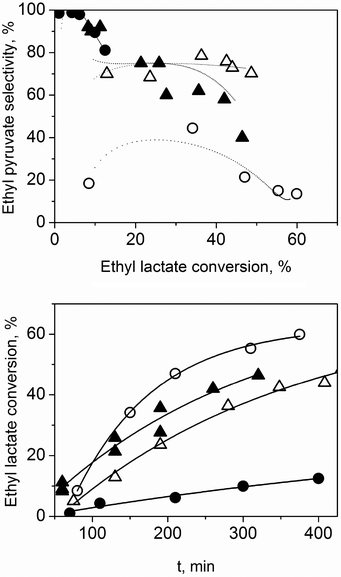
Note that adding a solvent increases the selectivity towards ethyl pyruvate. The higher the concentration of the initial solution, the faster the selectivity decreases as conversion increases (at higher ethyl pyruvate concentrations, polymerisation dominates). But while diethyl succinate is an acceptable solvent for lab-scale reactions, it is too expensive for larger scale. We therefore examined “dibasic ester” mixtures (DBE). These are widely available, with many large-scale applications (paints strippers, plasticisers, drilling fluids). We studied DBE-0 and DBE-9 (Sigma-Aldrich; mixtures of dimethyl glutarate, dimethyl succinate and dimethyl adipate). Fig. 3 shows the results. In terms of activity, all the solvents behave similarly. DBE-9 gives better selectivity compared to no solvent, but still lower than diethyl succinate, and DBE-0 is as good as diethyl succinate.
This is already an economical improvement, but from a green chemistry point of view the best would be to use no solvent at all. That would be cleaner, cheaper, and give higher reactor space-time yields. From the above experiments, we knew three things: (i) TiO2 selectively catalyses the oxidation of ethyl lactate to ethyl pyruvate; (ii) the decrease in selectivity is due to polymerization of ethyl pyruvate in the liquid phase (iii) The oxidation follows a free-radical mechanism. The last point is supported by a recent report, that states that TiO2 can generate radicals in the absence of light.21 Armed with this knowledge, we addressed the challenge of using no solvent yet still reaching high selectivity in this free-radical reaction.
First, we tried suppressing the polymerization by adding p-benzoquinone (in all the experiments with polymer inhibitors, no solvent was used). After 24 h, no conversion was observed. This may be due to the radical inhibition of the p-benzoquinone at the surface of the catalyst, suppressing the oxidation of ethyl lactate by preventing free-radical formation at the surface. Thus, we needed an inhibitor that slows the polymerization of ethyl pyruvate in solution, but doesn't affect the oxidation at the catalyst surface.
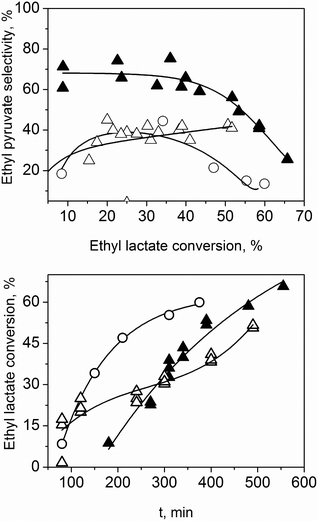
Considering that solid–solid kinetics are much slower than solid–liquid ones,23,24 activated carbon is an interesting radical scavenger.25 In theory, it can selectively hinder the formation of free-radicals in solution, since its solid–solid contact with TiO2 is less likely. Fig. 5 shows how activated carbon affects the reaction. There is an induction period, which may be due to the partial suppression of the radical formation at the catalyst surface. Once the reaction starts, however, the kinetics are similar to those of the reaction without solvent. The selectivity towards ethyl pyruvate increases up to 70%.
To understand better this induction period, we ran additional tests. The reaction was run for 2 h without any inhibitor. Then activated carbon was added. The results (Fig. 5) are striking. The moment one adds the activated carbon the reaction is suppressed, but the selectivity remains unchanged, i.e., no polymerization taken place. The activated carbon inhibits the ethyl pyruvate polymerization in the liquid phase. Thanks to this, we can avoid using solvents and still get high selectivity.
Combining our results with previous studies,21 we can propose a reaction mechanism (Scheme 1). The calorimetric measurements show that alcohols are strongly adsorbed at the surface of TiO2, with heats of adsorption twice that in inert porous solids. Moreover, alcohols adsorb more strongly than ketones. This indicates chemisorption. Indeed, this agrees with published infrared and STM studies,14,26 which detected titanium alkoxide and proton as products of the interaction of TiO2 and alcohols.27,28 This proton binds to one of the electrophilic oxygens near by the Ti alkoxide. The hydroxyl generated by the ethyl lactate deprotonation is very reactive due to the dislodging effect of the nucleophilic alkoxide and can react with O2 to form –OOH species. The decomposition of this species leads to OH˙ radical, which abstracts a second proton of the alcohol to form ethyl pyruvate and water (see Scheme 1).
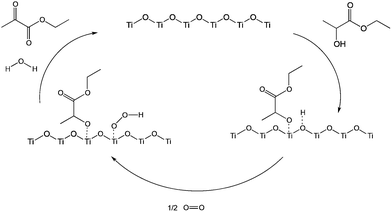 | ||
| Scheme 1 Proposed reaction mechanisms for the surface-catalysed oxidative dehydrogenation of ethyl lactate to ethyl pyruvate. | ||
Though ceria and zirconia are oxides similar to TiO2, it is not clear now what makes TiO2 unique. One reason may be the weaker interaction of alcoholic group with ceria and zirconia compared to TiO2. Secondly, ceria and zirconia may not be able to generate free radicals thus making them less effective for this reaction.
Conclusion
In free-radical reactions, it may seem that the two Green Chemistry principles of “avoid using solvents” and “focus on selective reactions” are mutually exclusive, as one trades off the other. Yet by understanding the reaction kinetics you can tune the selectivity in this titania-catalysed oxidation of ethyl lactate to ethyl pyruvate. Adding a small amount of active carbon avoids the use of solvent and keeps the high product selectivity. This is because the activated carbon decouples the two free-radical processes in the system. By combining a solid catalyst with a solid free-radical scavenger we can selectively slow down the unwanted polymerisation of the product, ethyl pyruvate. We hope that our fellow scientists will find this useful in other free-radical catalytic oxidations.Notes and references
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- E. D. Morgan, H. Goddard and G. A. Thomas, J. Chem. Educ., 1977, 54, 782–782 CrossRef CAS.
- S. Sugiyama, S. Fukunaga, K. Ito, S. Ohigashi and H. Hayashi, J. Catal., 1991, 129, 12–18 CrossRef CAS.
- T. B. Causey, K. T. Shanmugam, L. P. Yomano and L. O. Ingram, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 2235–2240 CrossRef CAS.
- H. Hayashi, N. Shigemoto, S. Sugiyama, N. Masaoka and K. Saitoh, Catal. Lett., 1993, 19, 273–277 CrossRef CAS.
- H. Hayashi, S. Sugiyama, N. Masaoka and N. Shigemoto, Ind. Eng. Chem. Res., 1995, 34, 135–139 CrossRef CAS.
- H. Hayashi, S. Sugiyama, N. Kokawa and K. Koto, Appl. Surf. Sci., 1997, 121, 378–381 CrossRef.
- K. Li, X. Ge, Y. W. Han and J. Y. Shen, Chin. J. Inorg. Chem., 1999, 15, 826–830 CAS.
- H. Hayashi, S. Sugiyama, Y. Katayama, K. Sakai, M. Sugino and N. Shigemoto, J. Mol. Catal., 1993, 83, 207–217 CrossRef CAS.
- S. Sugiyama, T. Kikumoto, H. Tanaka, K. Nakagawa, K.-I. Sotowa, K. Maehara, Y. Himeno and W. Ninomiya, Catal. Lett., 2009, 131, 129–134 CrossRef CAS.
- H. Kowalczyk, G. Rychlicki and A. P. Terzyk, Pol. J. Chem., 1993, 67, 2019–2028 CAS.
- A. G. Thomas and K. L. Syres, Chem. Soc. Rev., 2012, 41, 4207–4217 RSC.
- C. Lun Pang, R. Lindsay and G. Thornton, Chem. Soc. Rev., 2008, 37, 2328–2353 RSC.
- Y. Du, N. A. Deskins, Z. Zhang, Z. Dohnalek, M. Dupuis and I. Lyubinetsky, J. Phys. Chem. C, 2008, 113, 666–671 Search PubMed.
- J. M. Martin-Martinez and M. C. Mittelmeijer-Hazeleger, Langmuir, 1993, 9, 3317–3319 CrossRef CAS.
- A. F. P. Ferreira, M. C. Mittelmeijer-Hazeleger, M. A. Granato, V. F. D. Martins, A. E. Rodrigues and G. Rothenberg, Phys. Chem. Chem. Phys., 2013, 15, 8795–8804 RSC.
- E.-J. Ras, M. J. Louwerse, M. C. Mittelmeijer-Hazeleger and G. Rothenberg, Phys. Chem. Chem. Phys., 2013, 15, 4436–4443 RSC.
- A. F. P. Ferreira, M. C. Mittelmeijer-Hazeleger, J. van der Bergh, S. Aguado, J. C. Jansen, G. Rothenberg, A. E. Rodrigues and F. Kapteijn, Microporous Mesoporous Mater., 2013, 170, 26–35 CrossRef CAS PubMed.
- A. F. P. Ferreira, M. C. Mittelmeijer-Hazeleger, A. Bliek and J. A. Moulijn, Microporous Mesoporous Mater., 2008, 111, 171–177 CrossRef CAS PubMed.
- C. R. Moylan and J. I. Brauman, J. Phys. Chem., 1984, 88, 3175–3176 CrossRef CAS.
- I. Fenoglio, G. Greco, S. Livraghi and B. Fubini, Chem. – Eur. J., 2009, 15, 4614–4621 CrossRef CAS PubMed.
- D. J. Jenkins, A. M. S. Alabdulrahman, G. A. Attard, K. G. Griffin, P. Johnston and P. B. Wells, J. Catal., 2005, 234, 230–239 CrossRef CAS PubMed.
- S. Mukhopadhyay, G. Rothenberg, H. Wiener and Y. Sasson, New J. Chem., 2000, 24, 305–308 RSC.
- G. Rothenberg, A. P. Downie, C. L. Raston and J. L. Scott, J. Am. Chem. Soc., 2001, 123, 8701–8708 CrossRef CAS PubMed.
- S. Barrientos-Ramirez, G. M. de Oca-Ramirez, E. V. Ramos-Fernandez, A. Sepulveda-Escribano, M. M. Pastor-Blas, A. Gonzalez-Montiel and F. Rodriguez-Reinoso, Appl. Catal., A, 2011, 397, 225–233 CrossRef CAS PubMed.
- G. A. M. Hussein, N. Sheppard, M. I. Zaki and R. B. Fahim, J. Chem. Soc., Faraday Trans., 1991, 87, 2661–2668 RSC.
- S. Tan, Y. Ji, Y. Zhao, A. Zhao, B. Wang, J. Yang and J. G. Hou, J. Am. Chem. Soc., 2011, 133, 2002–2009 CrossRef CAS PubMed.
- W. Zeng, T. Liu, Z. Wang, S. Tsukimoto, M. Saito and Y. Ikuhara, Mater. Trans., 2010, 51, 171–175 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Graphs showing the reaction profiles for leaching tests and catalyst reuse experiments. See DOI: 10.1039/c4gc00191e |
| This journal is © The Royal Society of Chemistry 2014 |