Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biobased furandicarboxylic acids (FDCAs): effects of isomeric substitution on polyester synthesis and properties†
Shanmugam
Thiyagarajan
ab,
Willem
Vogelzang
ab,
Rutger
J. I. Knoop
ab,
Augustinus E.
Frissen
ab,
Jacco
van Haveren
ab and
Daan S.
van Es
*ab
aFood & Bio-based Research, Wageningen University and Research Centre, P.O. Box 17, 6700 AA Wageningen, The Netherlands. E-mail: daan.vanes@wur.nl; shanmugam.thiyagarajan@wur.nl; Fax: +31 317 483011; Tel: +31 317 481160
bDutch Polymer Institute, P.O. Box 902, 5600 AX, Eindhoven, The Netherlands
First published on 27th November 2013
Abstract
In this study we present the application of different isomers of furandicarboxylic acid, or FDCA, obtained from agro-residues, in polyester synthesis. New polyesters based on 2,4-FDCA and 3,4-FDCA isomers with (linear) diols were thoroughly characterised and compared with their as-synthesised 2,5-FDCA analogues. All polymers were obtained by melt polycondensation of linear diols with FDCA dimethyl esters and exhibit molecular weights in the range Mw = 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–65
000–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and polydispersities close to 2.0. Thermogravimetric analysis (TGA) of the new polyesters shows that they have comparable or even higher thermal stability compared to the 2,5-FDCA polyesters. Interestingly, the glass-transition temperatures (Tg) of 2,4-FDCA derived polyesters are similar to those of the 2,5-FDCA isomers. Both differential scanning calorimetry (DSC) and wide-angle X-ray diffraction (WAXD) analyses showed that 2,4-PEF is amorphous, while 2,5-PEF and 3,4-PEF are semi-crystalline materials.
000 and polydispersities close to 2.0. Thermogravimetric analysis (TGA) of the new polyesters shows that they have comparable or even higher thermal stability compared to the 2,5-FDCA polyesters. Interestingly, the glass-transition temperatures (Tg) of 2,4-FDCA derived polyesters are similar to those of the 2,5-FDCA isomers. Both differential scanning calorimetry (DSC) and wide-angle X-ray diffraction (WAXD) analyses showed that 2,4-PEF is amorphous, while 2,5-PEF and 3,4-PEF are semi-crystalline materials.
Introduction
Increasing awareness that the still growing use of fossil feedstocks and concomitant emissions of greenhouse gases (GHG) is affecting global climate change1 is one of the main drivers in the search for sustainable, renewable feedstocks for chemicals and materials. The increasing use of synthetic polymers can contribute to reducing GHG emissions due to the lower weight of those materials compared to e.g. steel, aluminum or glass. However, this trend can only become truly positive when these polymeric materials are based on renewable resources. Due to the high oxygen content of the most abundantly available biomass components like polysaccharides and lignin, one can debate whether it is viable to use such feedstock for the production of highly defunctionalised “drop-in” chemical building blocks like olefins. A different approach would be to transform biomass components into “new” chemical building blocks with unique structures and functionalities, following thermodynamically more favorable pathways.One such “new” biobased building block is furan-2,5-dicarboxylic acid (or 2,5-FDCA) which is widely propagated as an alternative to the ubiquitous terephthalic acid (TA).2,3 Recent reports on the application of 2,5-FDCA in a range of polyesters have shown that in comparison with TA, polymer properties are quite often (surprisingly) comparable.3–5 Furthermore, preliminary reports on gas barrier properties indicate that PEF synthesized from 2,5-FDCA has superior performance compared to polyethylene terephthalate (PET) derived from TA.3 Nevertheless, before 2,5-FDCA can become a viable alternative to TA, more challenges need to be met, including economic viability. Currently, most synthetic routes to 2,5-FDCA are based on the oxidation of 5-hydroxymethyl-2-furaldehyde (HMF)6 which can be obtained by cyclodehydration of C6 sugars like fructose and glucose.7 In order to avoid possible competition with the food chain we have investigated several other strategies based on the use of non-digestible agro-residues like sugar beet pulp or bagasse. Pectinic sugars like galacturonic acid can be converted into galactaric acid8 and subsequently into 2,5-FDCA. Agro-residues like sugar beet pulp or bagasse are also a source of C5 sugars. In the latter case xylans and arabinans in hemi-cellulose can be cyclodehydrated to furfural, which can be subsequently oxidized to 2-furoic acid.9,10 Solid state disproportionation of the latter in a Henkel-type reaction then furnishes 2,5-FDCA together with furan.10
However, as we recently reported, during the Henkel reaction, depending on the reaction conditions, not only the 2,5-isomer is formed (70%) but also the 2,4- (30%) and to a lesser extent the 3,4-isomer (<5%) (Scheme 1).9 Furthermore, we have also shown that separation of the 2,5- and 2,4-isomer is feasible. In order to assess the viability of the Henkel-route it is necessary to gain insight into the value of the separate isomers as polymer building blocks. Whereas there is a limited, yet steadily increasing amount of literature on the synthesis and properties of 2,5-FDCA based polyesters, surprisingly only a very few reports on either 2,4- or 3,4-FDCA polyesters can be found. In 1958, Hachihama et al. reported one example of the 2,4-FDCA based polyester with ethylene glycol (2,4-PEF); however, key properties like Tg and molecular weights were not described.11 A few decades later, Gandini et al. synthesized poly(butylene-furan-3,4-dicarboxylate) from 3,4-FDCA and 1,4-butanediol. These authors unfortunately only reported a glass-transition temperature.12
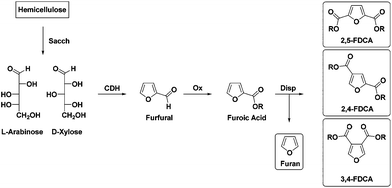 | ||
| Scheme 1 Synthesis of FDCAs via disproportionation reaction (Sacch = saccharification, CDH = cyclodehydration, Ox = oxidation, Disp = disproportionation). | ||
Hence, here we report a systematic comparison of polyesters containing 2,5-, 2,4- or 3,4-FDCA, and a series of homologous industrially relevant aliphatic diols. The obtained data allow for a structure–property analysis with regard to the furandicarboxylic acid substitution pattern, giving insight into the influence on
– Reactivity of the monomers (molecular weight build-up and isolated yield)
– Stability of the monomers (molecular weight build-up, polydispersity and colour)
– Thermal properties of the polymers (Tg, Tm)
– Thermal stability of the polymers (colour, mass loss)
– Degree of crystallinity and rate of crystallization.
Results and discussion
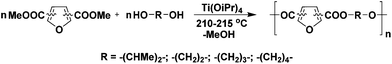
2,5-FDCA and 2,4-FDCA were prepared and purified (>98%) according to reported procedures.5,9,13 All polyesters were prepared according to the same protocol in order to compare the reactivity of the FDCA isomers. Melt polycondensation of the dimethyl esters of 2,5-, 2,4- or 3,4-FDCA with linear (potentially) biobased aliphatic diols (ethylene glycol (EG), 1,3-propanediol (PDO), 1,4-butanediol (1,4-BDO), and 2,3-butanediol (2,3-BDO)) yielded a set of 12 polyesters, of which 6 are novel (Scheme 2).The polycondensation was performed by a conventional two-step melt-polycondensation procedure using titanium(IV) isopropoxide as the catalyst (1.25 mol%). The pre-polymerization was performed at 160 °C for 12 h, followed by further polycondensation at 210–215 °C for 2 h under reduced pressure (0.02 mbar) in the second-step. The polyesters were obtained in excellent isolated yields (80–98%), while after precipitation from methanol all polyesters were obtained as white powders.
While the known polyesters based on 2,5-FDCA merely served as benchmark, all 2,4- and 3,4-FDCA polyesters (except for 2,4-PEF and 3,4-PBF) have not been described before. The structures of all the polyesters were confirmed by NMR (1H and 13C) (see ESI†) and FTIR spectroscopy. Gel permeation chromatography (GPC) of the furandicarboxylate polyesters showed that all polymers were obtained in medium to high molecular weights, with polydispersity index (PDI) values close to 2 (Table 1).
| Entry | Code | GPCa | Yielde (%) | Appearancef | ||
|---|---|---|---|---|---|---|
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (g mol−1) b (g mol−1) |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (g mol−1) c (g mol−1) |
PDId | ||||
| a Gel permeation chromatography performed on crude samples using HFIP as a solvent. b M n: number-average molecular weight. c M w: weight-average molecular weight. d PDI: polydispersity index. e Isolated yield (crude). f Appearance of the crude polyester in the reactor. g As-synthesised PET. h Commercial bottle-grade PET sample. | ||||||
| Ethylene glycol (EG) | ||||||
| 1 | 2,5-PEF | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.8 | 91 | Opaque |
| 2 | 2,4-PEF | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.9 | 88 | Translucent |
| 3 | 3,4-PEF | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.9 | 92 | Translucent |
| 1,3-Propane diol (PDO) | ||||||
| 4 | 2,5-PPF | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.8 | 89 | Translucent |
| 5 | 2,4-PPF | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.0 | 92 | Translucent |
| 6 | 3,4-PPF | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.8 | 93 | Translucent |
| 1,4-Butane diol (1,4-BDO) | ||||||
| 7 | 2,5-P14BF | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2.5 | 90 | Opaque |
| 8 | 2,4-P14BF | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.7 | 93 | Opaque |
| 9 | 3,4-P14BF | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.0 | 91 | Opaque |
| 2,3-Butane diol (2,3-BDO) | ||||||
| 10 | 2,5-P23BF | 8000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.5 | 87 | Translucent |
| 11 | 2,4-P23BF | 2700 | 5200 | 2.0 | 83 | Translucent |
| 12 | 3,4-P23BF | 9100 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.0 | 80 | Translucent |
| Polyethylene terephthalate | ||||||
| 13 | PETg | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.0 | 98 | Opaque |
| 14 | PETh | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.9 | — | Opaque |
Given the commercial importance of PET, the ethylene glycol (EG) polyesters or PEFs (Table 1, entries 1–3) are the main focus of this study. The reference polymer 2,5-PEF was obtained with an excellent Mn of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (PDI 1.8), which is comparable to literature values,5,14 thereby validating our polycondensation procedure. Polycondensation of either 2,4- or 3,4-FDCA with EG yielded materials with comparable molecular weights, polydispersities and crude isolated yields. This indicates that under comparable polycondensation conditions, the position of the carboxylic acid groups on the furan ring has no significant influence on reactivity. This is all the more striking in the case of 3,4-FDCA, since for the EG polyester of the analogous ortho-phthalic acid (PA) it was not possible to obtain high molecular weights by melt polycondensation.15
100 (PDI 1.8), which is comparable to literature values,5,14 thereby validating our polycondensation procedure. Polycondensation of either 2,4- or 3,4-FDCA with EG yielded materials with comparable molecular weights, polydispersities and crude isolated yields. This indicates that under comparable polycondensation conditions, the position of the carboxylic acid groups on the furan ring has no significant influence on reactivity. This is all the more striking in the case of 3,4-FDCA, since for the EG polyester of the analogous ortho-phthalic acid (PA) it was not possible to obtain high molecular weights by melt polycondensation.15
Furthermore, contrary to observations on the chemical reactivity of monomeric furans, the absence of high oxidation state substituents on C2 and or C5 of the furan ring does not seem to have any detrimental effect on chemical selectivity. One major difference in this series is that while 2,5-PEF is opaque after cooling down from the melt, the 2,4- and 3,4 analogues are translucent, indicative of a lower degree of crystallinity, or lower rate of crystallisation. For comparison, two PET examples are included in Table 1; an as-synthesised sample (polycondensation conditions are described in the Experimental section) and a commercial, bottle-grade PET sample. These reference materials will be discussed further in the thermal analysis section.
In the biobased 1,3-propanediol (PDO) series (Table 1, entries 4–6), the significantly higher molecular weight of the 2,5-PPF is striking, while for the other two FDCA isomers there is little difference with the corresponding EG polyesters. In this series all polyesters were obtained as translucent materials. Changing to 1,4-butanediol (1,4-BDO) appears to have more pronounced effects. While the Mn values of 2,5- and 2,4-P14BF are very similar, the former deviates with regard to PDI. In this series the 3,4-FDCA isomer yields the highest molecular weight polyester, while all polyesters were obtained as opaque materials from the melt.
The last diol included in this study is 2,3-butanediol (2,3-BDO). This secondary diol is less reactive than its primary isomer 1,4-BDO, which is obvious from the significantly lower Mws. While the reference 2,5-P23BF is obtained with a similar Mn as reported in the literature,16 the 2,4-analogue was obtained as low Mw material only. In contrast, the 3,4-analogue is comparable to the 2,5-isomer. From the data in Table 1 no obvious correlation can be found between the FDCA substitution pattern and reactivity as would follow from systematic differences in molecular weight build-up.
Fig. 1 shows the 1H-NMR spectra of the three isomeric PEF polymers (13C-NMR spectra are shown in the ESI†). Due to the symmetry of 2,5-FDCA, the 1H-NMR spectrum of 2,5-PEF shows only 1 furanic proton, and 1 broad singlet for the ethylene glycol residue (Fig. 1a). In the case of 2,4-PEF, 2 furanic singlets at 8.2 (H5) and 7.6 (H3) ppm illustrate the asymmetry of this compound. Furthermore, also the EG residue is split-up. While the chemical shift of the EG residue is similar to that of the 2,5-analogue, H3 has shifted downfield in 2,4-PEF, while H5 resonates even further downfield due to the neighboring oxygen group (Fig. 1b). The 3,4-isomer is symmetrical, resulting in singlets for the furanic proton and the EG residue respectively. H2/H5 shows a slight upfield shift compared to 2,4-PEF, while this is also observed for the EG signal (Fig. 1c).
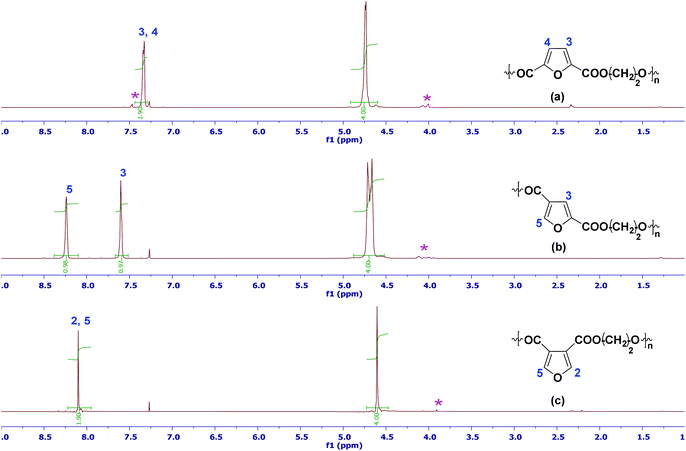 | ||
Fig. 1 The representative 1H-NMR spectra of precipitated (a) 2,5-PEF; (b) 2,4-PEF and (c) 3,4-PEF in CDCl3 and TFA-d (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The asterisk indicates end groups. 1). The asterisk indicates end groups. | ||
Detailed analysis of the EG-residue part of the 1H-NMR spectra of the PEF series shows besides end-groups (Mn values obtained by end-group analysis confirm GPC results) also the presence of signals that can be attributed to diethylene glycol (DEG) (see ESI, Fig. 31†). This is not surprising since the dehydration of ethylene glycol in the presence of acid catalyst leads to the formation of diethylene glycol which was identified as one of the most important side products in PET synthesis.17–20 The incorporation of DEG as a co-monomer in PET synthesis results in a decrease in melting temperature of about 5 °C (for each percent increase in DEG concentration). DEG incorporation also affects other properties like mechanical, hydrolytic and light stability as well as the thermal and oxidative degradation behavior of the material.21
We estimated the DEG incorporation in the PEF series to be less than 1% (based on 1H-NMR analysis, see ESI, Fig. 31†), which is lower than typically encountered during PET synthesis.22–26 Remarkably, DEG formation and incorporation into PEF has not been reported previously.5,27–29
Sousa et al. reported DEG incorporation of up to 5.2% in EG copolyesters derived from TA and 2,5-FDCA (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20).30 Furthermore, these authors showed that the DEG content decreases with increasing 2,5-FDCA content (1.7% DEG for 2,5-FDCA–TA, 90
20).30 Furthermore, these authors showed that the DEG content decreases with increasing 2,5-FDCA content (1.7% DEG for 2,5-FDCA–TA, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), which is in agreement with our findings for the FDCA–EG homopolyesters. NMR spectra of the other FDCA polyesters are given in the ESI.†
10), which is in agreement with our findings for the FDCA–EG homopolyesters. NMR spectra of the other FDCA polyesters are given in the ESI.†
Thermogravimetric analysis (TGA) of the FDCA polyesters shows that all new polyesters have comparable or even higher thermal stability compared to the 2,5-FDCA polyesters (Table 2). The consistently higher thermal stability of the 2,4-FDCA based polyesters is remarkable given the fact that these materials have on average the lowest Mws according to GPC (see Table 1).
| Entry | Code | TGAa | DSCd | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T 5% (°C) |
T
max![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (°C) c (°C) |
First heating | Second heating | ||||||
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (°C) e (°C) |
T
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f (°C) f (°C) |
ΔHm (J g−1) | T g (°C) | T m (°C) | ΔHm (J g−1) | ||||
| a TGA was performed on crude materials recorded from 30 to 600 °C at 10 °C min−1 under a N2 atmosphere. b Temperature at 5% weight loss. c Temperature at maximum rate of decomposition. d DSC was performed from −60 °C to 230 °C with two heating and cooling runs at 10 °C min−1. e Glass-transition temperature. f Melting temperature. g Melting temperature was determined after annealing. h No melting endotherm was observed up to the maximum temperature (230 °C). i As-synthesised PET. j Commercial bottle-grade PET. | |||||||||
| Polyethylene furandicarboxylate | |||||||||
| 1 | 2,5-PEF | 339 | 411 | 80 | 211 | 55 | 79 | 209 | 55 |
| 2 | 2,4-PEF | 345 | 429 | 71 | n.o.h | — | 73 | n.o.h | — |
| 3 | 3,4-PEF | 321 | 415 | 36 | 153 | 63 | 35 | 155g | 64 |
| Poly-1,3-propylene furandicarboxylate | |||||||||
| 4 | 2,5-PPF | 330 | 405 | 54 | 175 | 32 | 51 | 173 | 22 |
| 5 | 2,4-PPF | 342 | 415 | 46 | n.o.h | — | 46 | 83g | 0.5 |
| 6 | 3,4-PPF | 328 | 406 | −9 | n.o.h | — | −6 | n.o.h | — |
| Poly-1,4-butylene furandicarboxylate | |||||||||
| 7 | 2,5-P14BF | 304 | 367 | 51 | 173 | 39 | 38 | 170 | 39 |
| 8 | 2,4-P14BF | 328 | 402 | 29 | 68/100/116 | 5/1/10 | 34 | 79/103/117g | 5/2/8 |
| 9 | 3,4-P14BF | 312 | 395 | 4 | 55/70/79 | 0.7/10/5.5 | −6 | 55/69/80g | 1/6.2/1.5 |
| Poly-2,3-butylene furandicarboxylate | |||||||||
| 10 | 2,5-P23BF | 279 | 351 | 90 | —h | — | 87 | — | — |
| 11 | 2,4-P23BF | 279 | 365 | 88 | —h | — | 82 | — | — |
| 12 | 3,4-P23BF | 285 | 363 | 57 | —h | — | 52 | — | — |
| Polyethylene terephthalate | |||||||||
| 13 | PETi | 355 | 445 | 71 | 243 | 40 | 73 | 243 | 39 |
| 14 | PETj | 371 | 451 | 81 | 242 | 69 | 77 | 245 | 42 |
For all polyesters a single stage decomposition mechanism is observed between 350 and 425 °C. Representative TGA traces of poly(ethylene-furandicarboxylates) are shown in Fig. 2 (TGA curves for the other polymers are shown in the ESI†). Fig. 2 also includes the TGA traces of the two PET reference samples. The higher thermal stability of the commercial sample is probably due to the higher Mw and the presence of stabilisers. From Fig. 2 it is clear that the PEF series is intrinsically less thermally stable at temperatures in excess of 350 °C. Nevertheless, all polyfurandicarboxylates are stable up to 300 °C, which still allows for a very broad processing window for these materials.
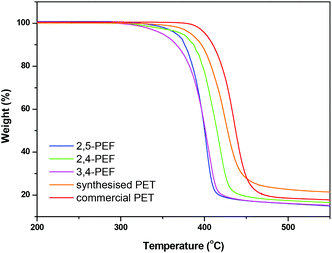 | ||
| Fig. 2 TGA traces of (crude) polyethylene furandicarboxylates and polyethylene terephthalates recorded from 30 to 600 °C at 10 °C min−1 under a N2 atmosphere. | ||
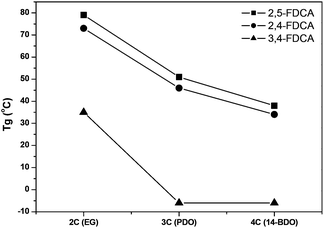
Whereas TGA analysis showed no dramatic differences between the FDCA polyesters, differential scanning calorimetry (DSC) revealed the remarkable effects of the position of the carboxylic groups in the FDCA isomers (Table 2). In the PEF series the Tg of 2,4-PEF (Table 2, entry 2) is only 6 °C lower than that of 2,5-PEF, while the Tg of 3,4-PEF is significantly lower (Table 2, entry 3). In the PPF series a similar trend can be observed. Again the difference in Tg between 2,5-PPF and the 2,4 isomer is small (5 °C). For 3,4-PPF the Tg is decreased significantly to −6 °C (Table 2, entry 6). Nevertheless, the observed drop in Tg (approx. 40 °C) is only slightly larger compared to the ones observed for the 2,5- and 2,4-isomers (approx. 30 °C). The 1,4-BDO series again displays a comparable trend; while the Tg decreases further, the difference between the 2,5- and 2,4-PBFs remains small, while no further decrease for the 3,4-isomer is observed. The observed values for 2,5-P14BF and 3,4-P14BF are in good agreement with those reported previously.5,12,39
All polyesters based on 2,3-BDO were, as expected, fully amorphous. 2,3-BDO is known to increase the Tg due to hindered rotation about the C2–C3 axis (compared to EG). The Tg value of 87 °C (Table 2, entry 10) found for the 2,5-P23BF corresponds to the one reported recently by Gubbels et al.16 Again, the Tg values of the 2,5 and 2,4 isomers are within a 5 °C range, while the 3,4-isomer shows a considerable increase in Tg. As the Mws of the 2,3-BDO series are significantly lower compared to EG series, the Tg increasing effect of 2,3-BDO is quite dramatic.
The effect of the position of the carboxylic acid groups on the Tg of the polyesters is graphically represented in Fig. 3, which makes it even more clear that the 2,4-analogue is highly comparable to the 2,5-isomer. With regard to melting transitions the position of the carboxylic acid groups in FDCA has a dramatic impact. The observed melting points for the 2,5-FDCA based polyesters are all in good agreement with those reported in the literature.5,14,39 Whereas 2,5-PEF already has a substantially lower Tm than PET (209 °C vs. 245 °C), no melting point was observed in the case of 2,4-PEF even after annealing at 90 °C for 5 h. In contrast, for 3,4-PEF a melting point of 155 °C was recorded after annealing at 100 °C for 5 h. The surprising absence of melting point of 2,4-PEF is in sharp contrast to its high Tg of 73 °C. The Tg/Tm ratio (in K) of 2,5- and 3,4-PEF are 0.72 (in line with those reported for other semi-aromatic EG polyesters).40 In the PPF series the Tm of the 2,4-FDCA polyester is approx. 90 °C lower than that of the 2,5-analogue. Again, only after annealing a clear melting endotherm could be observed. Despite several attempts, no melting transitions could be recorded for the 3,4-PPF. In contrast, all polyesters based on P14BF are semi-crystalline. The 2,4-FDCA based polyester showed multimodal melting behavior after annealing at 60 °C for 5 h, with the lowest melting point again approx. 90 °C lower than for the 2,5-analogue. Comparable multimodal melting behavior was observed for 3,4-P14BF after annealing at 40 °C for 5 h.
Fig. 4(a and b) shows the DSC heating and cooling curves of all three PEF isomers. The absence of cold crystallization in the case of 2,4- and 3,4-PEF (as compared to the 2,5-analogue) further indicates that these polymers have very low rates of crystallization.
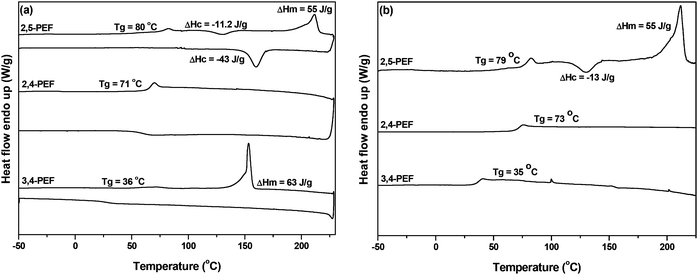 | ||
| Fig. 4 (a) First heating/cooling and (b) second heating DSC curves of poly(ethylene-furandicarboxylate)s derived from FDCA analogues. The heating and cooling rates are 10 °C per minute. | ||
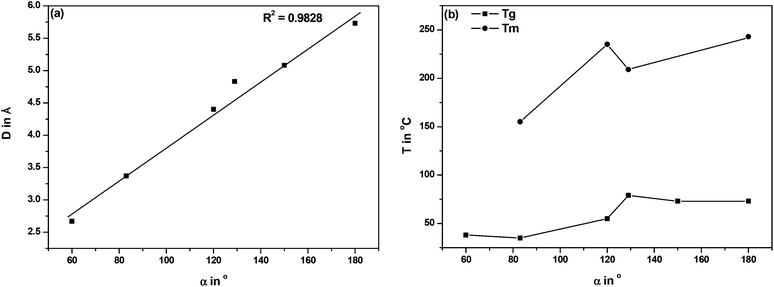
In order to find an explanation for the observed differences between the FDCA polyesters, we compared the structural characteristics of the FDCA isomers in relation to their phthalic acid analogues, as well as the thermal properties of the ensuing EG polyesters in Table 3. The data are arranged in descending order with respect to the interatomic distance (D) between the carboxylic acid groups in the respective diacids.
| Diacid | Symmetry | D (Å) | α (°) | EG polyester Tg (°C) | EG polyester Tm (°C) |
|---|---|---|---|---|---|
| a Interatomic distance between C1 and C6 (carboxylic acid groups). b Projected angle between the C1–C2 bond and the C5–C6 bond. | |||||
| TA31 | C i | 5.73 | 180 | 73 | 243 |
| 2,4-FDCA–Me9 | C 2/c | 5.08 | 150 | 73 | — |
| 2,5-FDCA32 | C s | 4.83 | 129 | 79 | 209 |
| IPA33 | C 2ν | 4.40 | 120 | 5534,35 | 235 |
| 3,4-FDCA-Me36,37 | P21/a | 3.37 | 83 | 35 | 155 |
| PA38 | C 2/c or Cc | 2.67 | 60 | 3815 | — |
With regard to symmetry, 2,4-FDCA is the least symmetrical diacid in the series under investigation. Comparison of the D and the projected angle between the C1–C2 bond and the C5–C6 bond (α) leads to the conclusion that contrary to common opinion in the literature the 2,5-FDCA isomer is not similar to TA, but rather to IPA. While the 2,4-isomer is closer to TA, the 3,4-isomer has characteristics comparable to PA (Fig. 5). A plot of D vs. α, as expected, shows a nearly linear relationship (Fig. 6a). In contrast, no unambiguous correlation can be found between α and Tg or Tm of the EG polyesters (Fig. 6b). Nevertheless, the 2,4-isomer clearly induces anomalous effects in the crystallinity. The main difference between 2,4-FDCA and the other diacids is the lower symmetry of the former. Other factors that could influence crystallinity are e.g. dipole moments and π-stacking. Further research is required here, but falls outside of the scope of the present study.
Fig. 7 shows the wide angle X-ray diffraction (WAXD) patterns of the isomeric PEFs which were used to calculate the degree of crystallinity of these polymers after precipitation from methanol (see also Table 4). The 2,5-isomer showed only a surprisingly low degree of crystallinity of 14% (after subtraction of the amorphous halo, see ESI†). Dividing the melting enthalpy of the first DSC heating run (corrected for cold crystallization) by the degree of crystallinity of the sample allows for estimating the heat of fusion of the 100% crystalline material. When this is done for the precipitated 2,5-PEF an unrealistically high value of 314 J g−1 is obtained (compared to reference values for e.g. PET and PBT, Table 4). This prompted us to anneal the sample (for 15 h at 175 °C), resulting in a significantly higher degree of crystallinity of 56%, and a heat of fusion of 109 J g−1 (DSC traces for annealed 2,5-PEF are shown in the ESI†). In the case of 2,4-PEF, no melting was observed in the DSC of the precipitated material, which was supported by the absence of crystalline reflections in the WAXD diffractogram (Fig. 7). Attempts to induce crystallinity in 2,4-PEF by annealing for up to 5.0 h at 90 °C were unsuccessful. In contrast, precipitated 3,4-PEF proved to be highly crystalline from the WAXD analysis (55%), giving an estimated 116 J g−1 for the heat of fusion at 100% crystallinity.
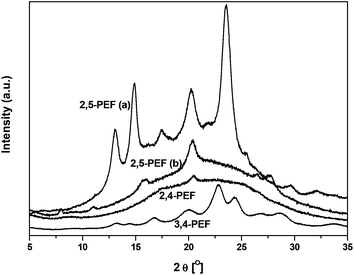 | ||
| Fig. 7 WAXD diffractograms of 2,5-PEF ((a) annealed at 175 °C for 15 h, (b) precipitated from MeOH) and precipitated 2,4-PEF and 3,4-PEF. | ||
Systematic comparison with literature values of analogous phthalate based polyesters is hampered by the surprisingly scarce data on these polymers. Nevertheless, the estimated heat of fusion of 2,5-PEF and 3,4-PEF are comparable to those reported for PET and PBT. Unfortunately, since poly(ethylene phthalate) or PEP is reported to be amorphous,15 no comparison can be made with this structural analogue. While no literature value is known for the heat of fusion of poly(ethylene isophthalate) (PEI), reported values for poly(butylene isophthalate) or PBI differ strongly, ranging from 115 to 192 J g−1,42,43 which makes it nearly impossible to compare.
Conclusions
We have successfully prepared and characterised a broad set of furandicarboxylic acid based polyesters by melt polycondensation, yielding medium to high molecular weight polymers. Three different FDCA isomers, products obtained by Henkel-type disproportionation of agro-residue based furoic acid, have been investigated. Contrary to expectations, the position of the carboxylic acid groups on the furan ring showed no clear correlation with regard to reactivity (yield, Mn) or selectivity (PDI, colour). TGA analysis showed that all FDCA polyesters had sufficient thermal stability, especially in relation to their respective melting points. Thermal analysis by DSC however revealed dramatic effects of the isomeric substitution. Whereas the Tgs of the 2,4-FDCA polyesters are very similar to those based on the 2,5-isomer or even TA, no melting point of the former was observed. Furthermore, DSC analysis indicates a very low rate of crystallisation, which is apparent from the need for extensive annealing in order to induce crystallinity. Preliminary attempts to identify the parameters responsible for the observed differences in polymer properties by means of a structure–property analysis did not reveal any clear correlations. WAXD analysis confirmed the semi-crystalline nature of the 2,5- and 3,4-PEF isomers, and allowed us to estimate their respective heat of fusion for the first time. Overall we have shown that 2,4- and 3,4-FDCA polyesters can be made in sufficient Mws by industrially applicable methods, and that these materials have (commercially) interesting, and sometimes outrightly intriguing properties. More in-depth analysis of these new materials, as well as application in specific product areas is part of ongoing investigations.Experimental section
Materials
The synthesis of dimethyl furan-2,5-dicarboxylate and furan-2,4-dicarboxylate was described in recent publications.5,9 Dimethyl furan-3,4-dicarboxylate (98%, Sigma-Aldrich), ethylene glycol (anhydrous, 99.8%, Sigma-Aldrich), 1,3-propane diol (≥99.6%, Sigma-Aldrich), 1,4-butane diol (≥99%, Sigma-Aldrich), 2,3-butane diol (≥99%, Sigma-Aldrich), titanium(IV) isopropoxide (≥97%, Sigma-Aldrich), toluene (anhydrous, 99.8%, Sigma-Aldrich), o-xylene (anhydrous, 97%, Sigma-Aldrich) trifluoroacetic acid (99%, Sigma-Aldrich), chloroform (Merck, p.a.), Methanol (Merck, p.a.) and chloroform-d (99.8 atom% D, Sigma-Aldrich). All the chemicals were used as received, unless denoted otherwise.Methods
Fourier transform infrared (FT-IR) spectra were obtained on a Varian Scimitar 1000 FT-IR spectrometer equipped with a Pike MIRacle ATR Diamond/ZnSe single reflection plate and a DTSG-detector. The measurement resolution was set at 4 cm−1, and the spectra were collected in the range 4000–650 cm−1 with 32 co-added scans. NMR spectra were recorded on a Bruker Avance III spectrometer operating at 400.17 MHz (1H) and 100.62 MHz (13C). Differential Scanning Calorimetry (DSC) measurements were conducted on a Perkin Elmer Diamond series DSC. The temperature range used was −60 °C up to 230 °C at a heating rate and cooling rate of 10 °C min−1. The thermal stabilities of the polyesters were determined by thermogravimetric analysis (TGA) with a STA 6000 (Simultaneous Thermal Analyser) from PerkinElmer Instrument. The samples were heated from 30 to 600 °C at a heating rate of 10 °C min−1 under a nitrogen flow of 40 mL min−1. The absolute molecular weights of the polyesters were determined by Gel Permeation Chromatography (GPC) on a Viscotek HP-SEC system, VE-2001 GPC max (pump and auto sampler) equipped with TDA305 Triple Detector Array (Right Angle Light Scattering (RALS) + Low Angle Light Scattering (LALS), Refractive Index (RI) Detector and Viscometer). 2X GPC column PSS PFG analytical linear M and guard column, molecular range ∼250–2.5 × 10−6 D (PMMA in HFIP). Data were calculated with OmniSEC™, Version 4.6 software. Hexafluoroisopropanol (HFIP) containing 0.02 M potassium trifluoroacetate was used as an eluent with a flow-rate of 0.7 ml min−1. Control measurements were performed with Easy vial PMMA standards from Agilent. Wide angle X-ray scattering (WAXS) powder diffractograms were recorded on a Philips PC-APD diffractometer in the reflection geometry in the angular range 4–40° (2θ), with a step size of 0.02° (2θ) and an acquisition time of 1.0 s per step. The Cu Kα1 radiation from the anode, generated at 40 kV and 30 mA, was monochromatized using a 15 μm Ni foil (λ = 0.1542 nm). The diffractometer was equipped with a 1° divergence slit, a 0.2 mm receiving slit, and a 1° scatter slit.General polycondensation procedure for FDCAs analogues
In a typical experiment, polycondensations were conducted in 100 mL three-neck round-bottom flasks equipped with a mechanical overhead stirrer, nitrogen inlet and water-condenser. Furandicarboxylic acid (1.5 g, 8.14 mmol) and diol (16.2 mmol) were charged into the reaction flask. The set-up was placed under vacuum and purged with nitrogen gas, and this cycle was repeated three times. The polycondensation method involves two stages. During the first stage, the reaction was carried out under nitrogen gas to form oligomers. The reaction mixture was heated in the silicone-oil bath at 115 °C for 15 min with constant stirring. After observing the complete melt of the mixture, the catalyst Ti(OiPr)4 (1.25 mol%) in 1 mL of toluene was added into the flask under a continuous flow of nitrogen gas. The temperature was now increased to 160 °C and stirred for 12 h, and finally to 200–215 °C for 1.5 h to complete the first stage of the pre-polymerization reaction. The methanol and toluene were collected in the cooling flask. During the second stage of the polycondensation to obtain high molecular weight polyesters, a high vacuum of 0.02 mbar was applied gradually to the polycondensation set-up at 210–215 °C for 2 h. After completion of the reaction, the reaction mixture was cooled down to room temperature under a nitrogen atmosphere. The polymer was purified by dissolving in 10 mL of chloroform–TFA mixture (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and precipitated in 100 mL of methanol, filtered and dried in vacuo at 40 °C for 12 h to yield a white powder.
1) and precipitated in 100 mL of methanol, filtered and dried in vacuo at 40 °C for 12 h to yield a white powder.
Polycondensation procedure for PET synthesis
In a typical experiment, polycondensations were conducted in 100 mL three-neck round-bottom flasks equipped with a magnetic stirrer, nitrogen inlet and water-condenser. DMT (10.6 g, 54.6 mmol) and diol (4.5 g, 72.5 mmol) were charged into the reaction flask. The set-up was placed under vacuum and purged with nitrogen gas, and this cycle was repeated three times. The polycondensation method involves two stages. During the first stage, the reaction was carried out under nitrogen gas to form oligomers. The reaction mixture was heated in a salt bath (a mixture of KNO3 (53 wt%), NaNO2 (40 wt%), and NaNO3 (7 wt%)) at 165 °C for 15 min with constant stirring. After observing the complete melt of the mixture, the catalyst Ti(OiPr)4 (0.02 mol%) in 2 mL of o-xylene was added into the flask under the continuous flow of nitrogen gas. The temperature was now increased to 165 °C and stirred for 12 h, and finally to 265 °C for 4 h to complete the first stage of the pre-polymerization reaction. The methanol and o-xylene were collected in the cooling flask. During the second stage of the polycondensation to obtain high molecular weight polyesters, a high vacuum of 0.1 mbar was applied gradually to the polycondensation set-up at 265 °C for 3 h. After completion of the reaction, the reaction mixture was cooled down to room temperature under a nitrogen atmosphere. The polymer was purified by dissolving in 10 mL of chloroform–TFA mixture (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and precipitated in 100 mL of methanol, filtered and dried in vacuo at 40 °C for 12 h to yield a white fibrous material.
1) and precipitated in 100 mL of methanol, filtered and dried in vacuo at 40 °C for 12 h to yield a white fibrous material.
Poly(ethylene-2,5-furandicarboxylate) (2,5-PEF)
FT-IR (neat): ν = 3126, 2966, 1722, 1582, 1269, 1021, 969, 829, 763 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 7.3 (s, 2H), 4.7 (s, 4H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 159.5, 146.1, 121.1, 63.7 ppm.Poly(ethylene-2,4-furandicarboxylate) (2,4-PEF)
FT-IR (neat): ν = 3142, 2960, 1726, 1591, 1264, 1080, 978, 868, 760 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 8.2 (s, 1H), 7.6 (s, 1H), 4.7 (d, 4H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 163.8, 160.0, 152.7, 144.8, 120.4, 118.8, 63.9, 63.6 ppm.Poly(ethylene-3,4-furandicarboxylate) (3,4-PEF)
FT-IR (neat): ν = 3144, 2948, 1726, 1545, 1268, 1064, 878, 835, 761 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 8.1 (s, 2H), 4.6 (s, 4H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 163.0, 150.8, 116.7, 63.2 ppm.Poly(propylene-2,5-furandicarboxylate) (2,5-PPF)
FT-IR (neat): ν = 3121, 2950, 1723, 1581, 1272, 1035, 967, 828, 765 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 7.5 (s, 2H), 4.7 (t, 4H), 2.4 (q, 2H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 154.6, 141.0, 114.6, 57.8, 22.0 ppm.Poly(propylene-2,4-furandicarboxylate) (2,4-PPF)
FT-IR (neat): ν = 3143, 2958, 1718, 1590, 1260, 1076, 978, 827, 760 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 8.2 (s, 1H), 7.5 (s, 1H), 4.5 (m, 4H), 2.2 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 163.7, 159.8, 151.9, 144.7, 120.4, 118.4, 62.9, 62.4, 27.4 ppm.Poly(propylene-3,4-furandicarboxylate) (3,4-PPF)
FT-IR (neat): ν = 3148, 2964, 1719, 1540, 1270, 1055, 932, 884, 754 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 8.0 (s, 2H), 4.4 (t, 4H), 2.2 (m, 2H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 163.8, 150.6, 116.9, 62.7, 27.2 ppm.Poly(1,4-butylene-2,5-furandicarboxylate) (2,5-P14BF)
FT-IR (neat): ν = 3120, 2965, 1730, 1579, 1274, 1023, 968, 824, 768 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 7.3 (s, 2H), 4.4 (m, 4H), 1.9 (m, 4H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 159.1, 145.3, 118.6, 65.3, 23.6 ppm.Poly(1,4-butylene-2,4-furandicarboxylate) (2,4-P14BF)
FT-IR (neat): ν = 3139, 2964, 1720, 1588, 1262, 1078, 979, 864, 761 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 8.2 (s, 1H), 7.5 (s, 1H), 4.4 (d, 4H), 1.9 (s, 4H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 163.8, 162.4, 150.1, 143.1, 118.7, 116.3, 64.4, 64.0, 23.0 ppm.Poly(1,4-butylene-3,4-furandicarboxylate) (3,4-P14BF)
FT-IR (neat): ν = 3146, 2954, 1730, 1538, 1273, 1063, 937, 886, 761 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 8.0 (s, 2H), 4.4 (s, 4H), 1.9 (s, 4H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 164.2, 150.5, 116.9, 65.9, 24.6 ppm.Poly(2,3-butylene-2,5-furandicarboxylate) (2,5-P23BF)
FT-IR (neat): ν = 3127, 2987, 1719, 1580, 1268, 1079, 964, 826, 764 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 7.42–7.08 (m, 2H), 5.60–5.20 (m, 2H), 1.60–1.19 (m, 6H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 156.44, 143.86, 117.25, 71.62, 13.04, 11.92 ppm.Poly(2,3-butylene-2,4-furandicarboxylate) (2,4-P23BF)
FT-IR (neat): ν = 3145, 2988, 1717, 1589, 1255, 1191, 976, 864, 758 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 8.31–8.00 (m, 1H), 7.53 (t, J = 4.0 Hz, 1H), 5.36 (ddqd, J = 19.0, 12.7, 6.8, 6.4, 3.0 Hz, 2H), 1.42 (p, J = 5.4, 4.7 Hz, 6H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 162.12, 158.30, 151.09, 144.05, 119.73, 119.62, 117.36, 73.07, 72.63, 14.72, 14.72, 13.66 ppm.Poly(2,3-butylene-3,4-furandicarboxylate) (3,4-P23BF)
FT-IR (neat): ν = 3431, 2987, 1723, 1539, 1267, 1050, 1000, 843, 755 cm−1; 1H NMR (400 MHz, CDCl3 + CF3COOD): δ = 8.15–7.93 (m, 2H), 5.28 (hept, J = 5.3, 4.0 Hz, 2H), 1.37 (t, J = 5.7 Hz, 6H) ppm; 13C NMR (100 MHz, CDCl3 + CF3COOD): δ = 161.02, 149.07, 115.85, 71.91, 14.16, 13.00 ppm.Acknowledgements
L. Gootjes, BSc is gratefully acknowledged for NMR analysis, W. Teunissen and H. de Beukelaer, BSc for GC-MS and DSC analyses. This work is part of the research program of the Dutch Polymer Institute (DPI), # 737 EXPLOROMA.Notes and references
- Climate Change 2013: The Physical Science Basis.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- E. d. Jong, M. A. Dam, L. Sipos and G. J. M. Gruter, in Biobased Monomers, Polymers, and Materials, American Chemical Society, 2012, pp. 1–13 Search PubMed.
- D. S. van Es, J. Renew. Mater., 2013, 1, 61–72 CAS.
- R. J. I. Knoop, W. Vogelzang, J. van Haveren and D. S. van Es, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4191–4199 CrossRef CAS.
- J. N. Chheda, Y. Roman-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342–350 RSC.
- R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- F. van der Klis, A. E. Frissen, J. van Haveren and D. S. van Es, ChemSusChem, 2013, 6, 1640–1645 CAS.
- S. Thiyagarajan, A. Pukin, J. van Haveren, M. Lutz and D. S. van Es, RSC Adv., 2013, 3, 15678–15686 RSC.
- T. Pan, J. Deng, Q. Xu, Y. Zuo, Q.-X. Guo and Y. Fu, ChemSusChem, 2013, 6, 47–50 CrossRef CAS PubMed.
- Y. Hachihama, T. Shono and K. Hyono, Technol. Rep. Osaka Univ., 1958, 8, 475–480 CAS.
- A. Chaabouni, S. Gharbi, M. Abid, S. Boufi, R. E. Gharbi and A. Gandini, J. Soci T Chimique Tunisie, 1999, 4, 547–558 CAS.
- I. W. Ashworth, M. C. Bowden, B. Dembofsky, D. Levin, W. Moss, E. Robinson, N. Szczur and J. Virica, Org. Process Res. Dev., 2002, 7, 74–81 CrossRef.
- M. Gomes, A. Gandini, A. J. D. Silvestre and B. Reis, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3759–3768 CrossRef CAS.
- B. Lee, J. W. Lee, S. W. Lee, J. Yoon and M. Ree, Polym. Eng. Sci., 2004, 44, 1682–1691 CAS.
- E. Gubbels, L. Jasinska-Walc and C. E. Koning, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 890–898 CrossRef CAS.
- L. H. Buxbaum, Angew. Chem., Int. Ed., 1968, 7, 182–190 CrossRef CAS.
- L. H. Buxbaum, Angew. Chem., Ger. Ed., 1968, 80, 225–233 CrossRef.
- R. Janssen, H. Ruysschaert and R. Vroom, Makromol. Chem., 1964, 77, 153–158 CrossRef CAS.
- H. Zimmerman and N. T. Kim, Polym. Eng. Sci., 1980, 20, 680–683 CAS.
- J. M. Besnoin and K. Y. Choi, J. Macromol. Sci., C, 1989, 29, 55–81 Search PubMed.
- S. G. Hovenkamp and J. P. Munting, J. Polym. Sci., Part A: Polym. Chem., 1970, 8, 679–682 CAS.
- J.-W. Chen and L.-W. Chen, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 3073–3080 CrossRef CAS.
- J.-W. Chen and L.-W. Chen, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1797–1803 CrossRef CAS.
- L.-W. Chen and J.-W. Chen, J. Appl. Polym. Sci., 2000, 75, 1229–1234 CrossRef CAS.
- L.-W. Chen and J.-W. Chen, J. Appl. Polym. Sci., 2000, 75, 1221–1228 CrossRef CAS.
- A. Gandini, A. J. D. Silvestre, C. P. Neto, A. F. Sousa and M. Gomes, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 295–298 CrossRef CAS.
- J. Ma, Y. Pang, M. Wang, J. Xu, H. Ma and X. Nie, J. Mater. Chem., 2012, 22, 3457–3461 RSC.
- M. Jiang, Q. Liu, Q. Zhang, C. Ye and G. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1026–1036 CrossRef CAS.
- A. F. Sousa, M. Matos, C. S. R. Freire, A. J. D. Silvestre and J. F. J. Coelho, Polymer, 2013, 54, 513–519 CrossRef CAS PubMed.
- M. Bailey and C. J. Brown, Acta Crystallogr., 1967, 22, 387–391 CrossRef CAS.
- E. Martuscelli and C. Pedone, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1968, 24, 175–179 CrossRef CAS.
- R. Alcala and S. Martinez-Carrera, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 28, 1671–1677 CrossRef CAS.
- Y. S. Hu, A. Hiltner and E. Baer, J. Appl. Polym. Sci., 2005, 98, 1629–1642 CrossRef CAS.
- S. W. Lee, M. Ree, C. E. Park, Y. K. Jung, C. S. Park, Y. S. Jin and D. C. Bae, Polymer, 1999, 40, 7137–7146 CrossRef CAS.
- Y. Okada, K. Nakatsu and A. Shimada, Bull. Chem. Soc. Jpn., 1971, 44, 928–933 CrossRef CAS.
- D. E. Williams and R. E. Rundle, J. Am. Chem. Soc., 1964, 86, 1660–1666 CrossRef CAS.
- T. G. D. Van Schalkwyk, Acta Crystallogr., 1954, 7, 775–775 CrossRef CAS.
- J. Zhu, J. Cai, W. Xie, P.-H. Chen, M. Gazzano, M. Scandola and R. A. Gross, Macromolecules, 2013, 46, 796–804 CrossRef CAS.
- W. A. Lee and G. J. Knight, Brit. Poly. J., 1970, 2, 73–80 CrossRef CAS.
- B. Wunderlich, Thermal Analysis of Polymeric Materials, Springer, 1990 Search PubMed.
- M. C. Righetti, M. Pizzoli and A. Munari, Macromol. Chem. Phys., 1994, 195, 2039–2047 CrossRef CAS.
- H. K. Yip and H. L. Williams, J. Appl. Polym. Sci., 1976, 20, 1209–1215 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, TGA traces, DSC curves and WAXD profiles of all the polyesters. See DOI: 10.1039/c3gc42184h |
| This journal is © The Royal Society of Chemistry 2014 |