Laccase-catalyzed synthesis of catechol thioethers by reaction of catechols with thiols using air as an oxidant†
Heba T.
Abdel-Mohsen
,
Jürgen
Conrad
and
Uwe
Beifuss
*
Bioorganische Chemie, Institut für Chemie, Universität Hohenheim, Garbenstr. 30, Stuttgart, D-70599, Germany. E-mail: ubeifuss@uni-hohenheim.de; Fax: (+49)711 459 22951; Tel: (+49)711 459 22171
First published on 30th October 2013
Abstract
The laccase-catalyzed reaction between catechols and thiols using aerial oxygen as the oxidant delivers the corresponding catechol thioethers with yields up to 96% under mild reaction conditions.
The development of selective, efficient, economic and sustainable oxidations presents a major challenge to synthetic organic chemistry.1 Against this background, enzyme-catalyzed oxidations using aerial oxygen as the oxidant are receiving particular attention.2 Laccases, which belong to the class of multicopper oxidases (benzenediol: O2 oxidoreductase E.C. 1.10.3.2.), are among the most attractive enzymes in this respect2,3 since they are characterized by a number of features and advantages: laccases are easily accessible; they are capable of catalyzing the oxidation of numerous substrates in aqueous solvent systems under mild reaction conditions (temperature, pH, pressure) with oxygen; the oxidation of the substrates is linked to the formation of water as the only byproduct; and the substrate scope of laccase-catalyzed reactions can be broadened by using laccase/mediator systems allowing for the oxidation of substrates with higher oxidation potentials.
So far, laccase-catalyzed oxidations have mostly been used for oxidizing several functional groups4 and for oxidative couplings of electron rich phenolic substrates.5 One particular promising aspect of the laccase-catalyzed transformations is the potential for combining the laccase-catalyzed generation of highly reactive molecules with subsequent non-oxidative chemical transformations to new domino processes. This approach has been used for the formation of quinoid systems and their subsequent reactions with C-nucleophiles.6 The nucleophilic 1,4-addition of different N-nucleophiles to o- and p-quinones obtained by laccase-catalyzed oxidation of catechol and hydroquinone, respectively, has also been explored.3b,7 However, little is known about the laccase-catalyzed reaction between hydroquinones or catechols and thiols.8 So far, it has been reported that 3-substituted 1,2,4-triazolo[4,3-b](4,1,2)benzothiadiazine-8-ones can be synthesized by laccase-catalyzed in situ generation of p-benzoquinone followed by reaction with 5-substituted 4-amino-3-mercapto-1,2,4-triazoles.8d Wellington et al. have reported on the laccase-catalyzed reaction between 1,4-dihydroxynaphthalenes and thiols to form various naphthoquinone sulfides.8b,c Even less is known about enzyme-catalyzed reactions between catechols and thiols. The only exception is the laccase-initiated domino reaction between catechols and 6-substituted 1,2,3,4-tetrahydro-4-oxo-2-thioxo-5-pyrimidinecarbonitriles for the synthesis of pyrimidobenzothiazole derivatives.8a The 1,4-addition of simple thiols to o-benzoquinones generated by enzyme-catalyzed oxidation of catechols to deliver the corresponding catechol thioethers is still unexplored.
Catechol thioethers are known to exhibit various biological (e.g., antibacterial and antioxidant) activities.9 That is why their synthesis is of interest. Aromatic thioethers can be synthesized by a number of methods, including the Pd-, Cu- and Rh-catalyzed reaction of aryl halides with thiols under basic conditions.10 Most catechol thioethers have been obtained by oxidation of catechols in the presence of the corresponding thiols. The oxidation of the catechols to o-benzoquinones has been achieved either by using K3[Fe(CN)6] as the oxidant11 or by anodic oxidation.12 Clearly, both methods face some problems, such as the use of at least stoichiometric amounts of an inorganic oxidant or the use of special equipment not available in many laboratories.
Against this background, we considered the laccase-initiated domino reaction between catechols and thiols as a highly attractive alternative for the synthesis of catechol thioethers (Scheme 1).
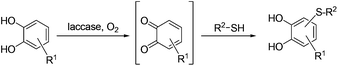 | ||
| Scheme 1 Synthesis of catechol thioethers by laccase-catalyzed reaction between catechols and thiols. | ||
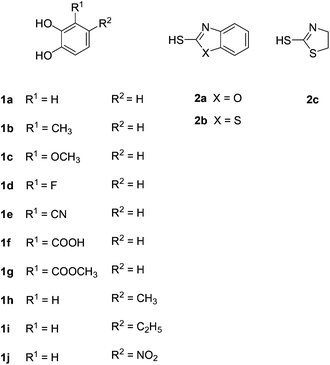
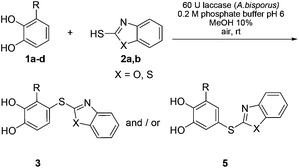
The catechols and the heterocyclic thiols used for the synthesis of the catechol thioethers are depicted in Fig. 1. The reaction between catechol (1a) and 2-mercaptobenzoxazole (2a) on a 1 mmol scale was selected as a model reaction (Table 1). When equimolar amounts of 1a and 2a were reacted in the presence of 300 U laccase from Agaricus bisporus (6 U mg−1) in 0.2 M phosphate buffer at pH 6.0/MeOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 20 h at rt, the corresponding catechol thioether 3a was isolated in 77% (Table 1, entry 1). The yield of 3a could be improved to 93% when the reaction was performed with a slight excess (1.25 equiv.) of 1a (Table 1, entry 2). It should be emphasized that the thioether 3a was formed exclusively; no disulfide formation was observed.4a This result confirms the observation that the laccase-catalyzed generation of o-benzoquinones from catechols does not require a mediator.6a–d,f,g,i–k It is also remarkable that only the monothioether was formed; not a trace of the corresponding bisthioether could be detected.
1) for 20 h at rt, the corresponding catechol thioether 3a was isolated in 77% (Table 1, entry 1). The yield of 3a could be improved to 93% when the reaction was performed with a slight excess (1.25 equiv.) of 1a (Table 1, entry 2). It should be emphasized that the thioether 3a was formed exclusively; no disulfide formation was observed.4a This result confirms the observation that the laccase-catalyzed generation of o-benzoquinones from catechols does not require a mediator.6a–d,f,g,i–k It is also remarkable that only the monothioether was formed; not a trace of the corresponding bisthioether could be detected.
| Entry | Laccase (U) | T | Time (h) | Yield of 3a (%) |
|---|---|---|---|---|
| a 1 mmol 1a and 1 mmol 2a were reacted. b 1.25 mmol 1a and 1 mmol 2a were reacted. c 0.63 mmol 1a and 0.50 mmol 2a were reacted. d Incomplete conversion. e No reaction. | ||||
| 1a | 300 | rt | 20 | 77 |
| 2b | 300 | rt | 15 | 93 |
| 3b | 150 | rt | 16 | 95 |
| 4b | 120 | rt | 16 | 93 |
| 5c | 60 | rt | 16 | 93 |
| 6b | 120 | 50 °C | 12 | 84 |
| 7b | 100 | rt | 17 | —d |
| 8b | 75 | rt | 24 | —d |
| 9b | — | rt | 24 | —e |
Further experiments devoted to the influence of the amount of the laccase from A. bisporus clearly demonstrated that the reaction of 1a with 2a tolerates a stepwise reduction of the catalyst load from 300 U to 120 U without any loss of yield (Table 1, entries 3 and 4). A further decrease did not pay off, since with 100 or 75 U laccase the transformations did not run to completion (Table 1, entries 7 and 8). The experiment with 120 U laccase was also performed at 50 °C; however, the yield dropped from 93 to 84% (Table 1, entry 6). Notably, the reaction could be run successfully on a 0.50 mmol scale with as little as 60 U laccase (Table 1, entry 5). Therefore, all further experiments were performed with 0.50 mmol thiol.‡ Finally, a control experiment was conducted to show that the formation of the thioether 3a does not proceed in the absence of the laccase (Table 1, entry 9).
| Entry | 1 | R | 2 | X | Time (h) | Product |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5b 5b |
Yield (%) |
|---|---|---|---|---|---|---|---|---|
| a 0.63 mmol 1 and 0.5 mmol 2 were reacted. b Ratio determined from the 1H NMR spectrum of the crude product. | ||||||||
| 1 | a | H | a | O | 16 | 3a | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
93 |
| 2 | a | H | b | S | 17 | 3b | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
87 |
| 3 | b | CH3 | a | O | 24 | 3c, 5c | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
90 |
| 4 | b | CH3 | b | S | 17 | 3d, 5d | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
95 |
| 5 | c | OCH3 | a | O | 17 | 3e, 5e | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
96 |
| 6 | c | OCH3 | b | S | 17 | 3f, 5f | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83 83 |
95 |
| 7 | d | F | a | O | 17 | 3g, 5g | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
90 |
| 8 | d | F | b | S | 17 | 3h, 5h | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
86 |
| Entry | 1 | R | Time (h) | 8 | Yield (%) |
|---|---|---|---|---|---|
| a 0.63 mmol 1 and 0.5 mmol 2c were reacted. | |||||
| 1 | a | H | 20 | a | 83 |
| 2 | h | CH3 | 17 | b | 74 |
| 3 | i | C2H5 | 17 | c | 82 |
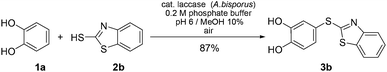
The reaction was not restricted to 2-mercaptobenzoxazole (2a) as a thiol, but could also be performed with 2-mercaptobenzothiazole (2b). When 2b was reacted with unsubstituted catechol (1a) under the conditions developed for the transformation with 2a (Table 1, entry 5), the catechol thioether 3b§ was obtained exclusively in 87% yield (Scheme 2).
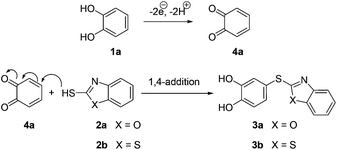
It is assumed that the process starts with the laccase-catalyzed oxidation of catechol (1a) to the corresponding o-benzoquinone (4a), followed by the intermolecular nucleophilic 1,4-addition of 2-mercaptobenzoxazole (2a) or 2-mercaptobenzothiazole (2b) to produce the corresponding catechol thioethers 3a, b (Scheme 3).
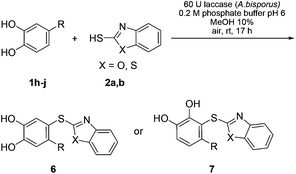
Then, we focused on the reactions between 2a, b and monosubstituted catechols 1b–j. In a first set of experiments, 2a, b were reacted with 3-substituted catechols 1b–d under the conditions described in Table 1, entry 5. Depending on whether the intermolecular nucleophilic attack of the heterocyclic thiols 2a, b occurs at C-4 or C-5 of the corresponding o-benzoquinones 4b–d (Scheme 4), either products of type 3 or type 5 were formed. The reaction between 3-methylcatechol (1b) and 2-mercaptobenzoxazole (2a) gave 90% of a 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 mixture of the regioisomers 3c and 5c (Table 2, entry 3). A similar result was observed with 2-mercaptobenzothiazole (2b) (Table 2, entry 4). The isomers arising from the C-4 attack also preferentially formed in the reactions of 3-fluorocatechol (1d): with 2a as the nucleophile, a 63
31 mixture of the regioisomers 3c and 5c (Table 2, entry 3). A similar result was observed with 2-mercaptobenzothiazole (2b) (Table 2, entry 4). The isomers arising from the C-4 attack also preferentially formed in the reactions of 3-fluorocatechol (1d): with 2a as the nucleophile, a 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 mixture of 3g and 5g was obtained (Table 2, entry 7); with 2b the isomers 3h and 5h were formed in a 69
37 mixture of 3g and 5g was obtained (Table 2, entry 7); with 2b the isomers 3h and 5h were formed in a 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 ratio (Table 2, entry 8). The only exceptions were the transformations between 3-methoxycatechol (1c) and 2a, b. With both nucleophiles, the 5-substituted catechol thioethers 5e and 5f were formed as the major isomers (Table 2, entries 5 and 6). It should be noted that the C-4 addition of 2a, b to 4b and 4d (quinones) was unexpected, since laccase-catalyzed 1,4-additions of C-nucleophiles are known to proceed at C-5.6a–d,f,g,i–k However, the laccase-catalyzed 1,4-additions of thiols to the o-quinone resulting from 3-methylcatechol (1b) show a close resemblance to some of the results from reactions run under electrochemical conditions.12b,c Thioethers were not formed when 3-substituted catechols having an electron withdrawing group at the 3-position, i.e.1e (R = CN), 1f (R = COOH) and 1g (R = COOMe), were used as substrates.
31 ratio (Table 2, entry 8). The only exceptions were the transformations between 3-methoxycatechol (1c) and 2a, b. With both nucleophiles, the 5-substituted catechol thioethers 5e and 5f were formed as the major isomers (Table 2, entries 5 and 6). It should be noted that the C-4 addition of 2a, b to 4b and 4d (quinones) was unexpected, since laccase-catalyzed 1,4-additions of C-nucleophiles are known to proceed at C-5.6a–d,f,g,i–k However, the laccase-catalyzed 1,4-additions of thiols to the o-quinone resulting from 3-methylcatechol (1b) show a close resemblance to some of the results from reactions run under electrochemical conditions.12b,c Thioethers were not formed when 3-substituted catechols having an electron withdrawing group at the 3-position, i.e.1e (R = CN), 1f (R = COOH) and 1g (R = COOMe), were used as substrates.
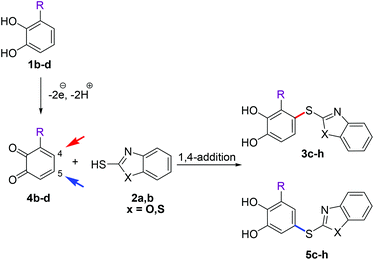 | ||
| Scheme 4 Regioselectivity of the laccase-catalyzed reactions between 3-substituted catechols 1b–d and thiols 2a, b. | ||
Next, the reactions between 2-mercaptobenozoxazole (2a) and 2-mercaptobenzothiazole (2b) with a number of 4-substituted catechols were performed under the reaction conditions given in Table 1, entry 5. It was interesting to see that the reactions with 4-methylcatechol (1h) and 4-ethylcatechol (1i) exclusively delivered the catechol thioethers 6a–d resulting from the nucleophilic attack of 2a, b at C-5 of the corresponding o-benzoquinone intermediates 4h,i (Scheme 5) with yields ranging from 83 to 90% (Table 3, entries 1–4). However, with 4-nitrocatechol (1j) as the substrate, the 1,4-addition selectively took place at C-3 of the corresponding o-benzoquinone 4j to deliver the thioether 7a exclusively in 85% yield (Scheme 5, Table 3, entry 5).
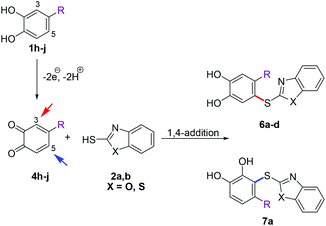 | ||
| Scheme 5 Regioselectivity of the laccase-catalyzed reactions between 4-substituted catechols 1h–j and thiols 2a, b. | ||
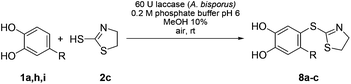
Finally, 2-mercaptothiazoline (2c) was reacted with unsubstituted catechol (1a) and the 4-substituted catechols 1h, i under the conditions displayed in Table 1, entry 5. In accordance with the results of 2a and 2b, the corresponding catechol thioethers 8a–c were formed exclusively with yields ranging from 74 to 83% (Table 4).
The newly developed laccase-catalyzed reaction between catechols 1 and thiols 2 complies with the principles of green chemistry13 for a number of reasons: the reaction is a highly selective enzyme-catalyzed transformation that allows the selective preparation of catechol thioethers in a single step with yields up to 96%. No toxic byproducts were formed. The reactions were performed with atmospheric oxygen as the oxidant, avoiding any toxic reagents. Atmospheric oxygen is not only one of the cheapest oxidants, but it is also considered to be environmentally benign. During the course of the reaction, oxygen is reduced to completely safe and nontoxic water, which is the only byproduct of the entire process. Using the laccase-catalyzed reaction between catechol (1a) and 2-mercaptobenzoxazole (2a) as an example, the E-factor (kg waste per kg product)1a,14 of the process is 7.88 kg kg−1. This value compares well with the E-factors of other synthetic methods for the synthesis of catechol thioethers. The atom economy15 of the presented method is high (94%). A mixture of phosphate buffer (pH 6.0) and 10 vol% methanol, which is a safe and environmentally preferred solvent,1c,16 was used as the reaction medium. The laccase-catalyzed domino reaction can be carried out under mild reaction conditions (room temperature, atmospheric pressure, pH 6.0). The laccase-catalyzed formation of catechol thioethers is characterized by high turnover numbers (TON), e.g., in the reaction between 1a and 2a, it amounts to 4512. This value underlines the high catalytic efficiency of the enzyme-catalyzed reaction. The turnover frequencies of the process are good; in the example mentioned above the TOF amounts to 282 h−1.
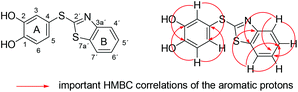
Structures of all compounds were unambiguously elucidated by mass spectrometry and NMR spectroscopy including 2D NMR for the full assignment of the 1H and 13C chemical shifts. For example, analysis of the gCOSY spectrum of compound 3b (Fig. 2) revealed two scalar coupled 1H spin systems, one consisting of protons 3-H, 5-H and 6-H (ring A) and the other of aromatic protons 4′-H, 5′-H, 6′-H and 7′-H (ring B). Their corresponding directly bonded carbons were identified by gHSQC. The quaternary carbons C-1, C-2 and C-4 (ring A) were assigned by gHMBC optimized for JCH = 8 Hz. A strong 3J-HMBC correlation between 3-H as well as 5-H and the phenolic carbon at δ 148.66 ppm established the C-1 position of the latter. The chemical shifts of the remaining A ring carbons (C-2/C-4) at δ 146.61 and 116.66 ppm respectively were deduced by the strong 3J-HMBC correlations to 6-H. However, the unambiguous assignment of the C-2 or C-4 position to the observed values and thus the substitution pattern at C-2 and C-4 was not possible even with a gHMBC experiment optimized for JCH = 1 Hz. Therefore, the 13C NMR chemical shifts were computed by DFT quantum mechanical DFT calculations at the DFT GIAO mPW1PW91/6-311+G(2d,p)//mPW1PW91/6-31G(d) level of theory.17 On the basis of computationally calculated 13C chemical shifts, the quaternary carbons C-2 and C-4 were assigned to be at δ 146.61 (δ calcd 142.71 ppm) and 116.66 (δ calcd 119.25 ppm), respectively. Similarly, the quaternary carbons C-3a′ and C-7a′ of ring B were assigned at δ 153.71 (δ calcd 153.07 ppm) and δ 134.75 (δ calcd 139.77 ppm), respectively. The most downfield shifted carbon at δ 171.99 ppm was tentatively assigned to the C-2′ position.
Conclusion
To summarize, a simple-to-perform, efficient and sustainable method for the synthesis of substituted catechol thioethers has been developed. It relies on the laccase-catalyzed oxidation of a catechol to the corresponding o-benzoquinone which in turn undergoes an intermolecular 1,4-addition with a thiol as a nucleophile. The reactions can be performed under mild reaction conditions with air as the oxidant, and the products are formed with yields ranging between 74 and 96%. Depending on the substituent on the catechol moiety, different regioisomers are formed.Acknowledgements
We thank Ms S. Mika for recording NMR spectra and Dr. A. Baskakova as well as Dipl.-Ing. J. Trinkner and Ms K. Wohlbold (Institut für Organische Chemie, Universität Stuttgart) for recording mass spectra. H. T. A.-M. is grateful to Deutscher Akademischer Austauschdienst (DAAD) for financial support.Notes and references
- (a) R. A. Sheldon, I. W. C. E. Arends and U. Hanefeld, Green Chemistry and Catalysis, Wiley-VCH, Weinheim, 2007 Search PubMed; (b) P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, New York, 2000 Search PubMed; (c) K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31 RSC; (d) D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. L. Leazer, Jr., R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411 RSC.
- For reviews, see: (a) D. Monti, G. Ottolina, G. Carrea and S. Riva, Chem. Rev., 2011, 111, 4111 CrossRef CAS PubMed; (b) F. Hollmann, I. W. C. E. Arends, K. Buehler, A. Schallmey and B. Bühler, Green Chem., 2011, 13, 226 RSC; (c) E. I. Solomon, U. M. Sundaram and T. E. Machonkin, Chem. Rev., 1996, 96, 2563 CrossRef CAS PubMed.
- (a) S. Witayakran and A. J. Ragauskas, Adv. Synth. Catal., 2009, 351, 1187 CrossRef CAS; (b) A. Mikolasch and F. Schauer, Appl. Microbiol. Biotechnol., 2009, 82, 605 CrossRef CAS PubMed; (c) A. Kunamneni, S. Camarero, C. García-Burgos, F. J. Plou, A. Ballesteros and M. Alcalde, Microb. Cell Fact., 2008, 7, 32 CrossRef PubMed.
- (a) H. T. Abdel-Mohsen, K. Sudheendran, J. Conrad and U. Beifuss, Green Chem., 2013, 15, 1490 RSC; (b) H. T. Abdel-Mohsen, J. Conrad and U. Beifuss, Green Chem., 2012, 14, 2686 RSC; (c) H. Leutbecher, M.-A. Constantin, S. Mika, J. Conrad and U. Beifuss, Tetrahedron Lett., 2011, 52, 604 CrossRef; (d) A. Coniglio, C. Galli, P. Gentili and R. Vadalà, J. Mol. Catal. B: Enzym., 2008, 50, 40 CrossRef CAS; (e) F. d'Acunzo, P. Baiocco and C. Galli, New J. Chem., 2003, 27, 329 RSC; (f) M. Fabbrini, C. Galli, P. Gentili and D. Macchitella, Tetrahedron Lett., 2001, 42, 7551 CrossRef CAS; (g) A. Potthast, T. Rosenau, C.-L. Chen and J. S. Gratzl, J. Org. Chem., 1995, 60, 4320 CrossRef CAS.
- (a) M.-A. Constantin, J. Conrad and U. Beifuss, Green Chem., 2012, 14, 2375 RSC; (b) M.-A. Constantin, J. Conrad, E. Merişor, K. Koschorreck, V. B. Urlacher and U. Beifuss, J. Org. Chem., 2012, 77, 4528 CrossRef CAS PubMed; (c) M.-A. Constantin, J. Conrad and U. Beifuss, Tetrahedron Lett., 2012, 53, 3254 CrossRef CAS; (d) B. Pickel, M.-A. Constantin, J. Pfannstiel, J. Conrad, U. Beifuss and A. Schaller, Angew. Chem., Int. Ed., 2010, 49, 202 CrossRef CAS PubMed; (e) S. Ncanana, L. Baratto, L. Roncaglia, S. Riva and S. G. Burton, Adv. Synth. Catal., 2007, 349, 1507 CrossRef CAS; (f) C. Ponzoni, E. Beneventi, M. R. Cramarossa, S. Raimondi, G. Trevisi, U. M. Pagnoni, S. Riva and L. Forti, Adv. Synth. Catal., 2007, 349, 1497 CrossRef CAS; (g) S. Ciecholewski, E. Hammer, K. Manda, G. Bose, V. T. H. Nguyen, P. Langer and F. Schauer, Tetrahedron, 2005, 61, 4615 CrossRef CAS; (h) F. d'Acunzo, C. Galli and B. Masci, Eur. J. Biochem., 2002, 269, 5330 CrossRef; (i) T. Shiba, L. Xiao, T. Miyakoshi and C.-L. Chen, J. Mol. Catal. B: Enzym., 2000, 10, 605 CrossRef CAS.
- (a) S. Emirdağ-Öztürk, S. Hajdok, J. Conrad and U. Beifuss, Tetrahedron, 2013, 69, 3664 CrossRef; (b) M. Kidwai, A. Jain, A. Sharma and R. C. Kuhad, Catal. Sci. Technol., 2013, 3, 230 RSC; (c) J. Pietruszka and C. Wang, Green Chem., 2012, 14, 2402 RSC; (d) J. Pietruszka and C. Wang, ChemCatChem, 2012, 4, 782 CrossRef CAS; (e) S. Hajdok, J. Conrad and U. Beifuss, J. Org. Chem., 2012, 77, 445 CrossRef CAS PubMed; (f) H. Leutbecher, G. Greiner, R. Amann, A. Stolz, U. Beifuss and J. Conrad, Org. Biomol. Chem., 2011, 9, 2667 RSC; (g) S. Hajdok, J. Conrad, H. Leutbecher, S. Strobel, T. Schleid and U. Beifuss, J. Org. Chem., 2009, 74, 7230 CrossRef CAS PubMed; (h) H. Leutbecher, S. Hajdok, C. Braunberger, M. Neumann, S. Mika, J. Conrad and U. Beifuss, Green Chem., 2009, 11, 676 RSC; (i) S. Witayakran, L. Gelbaum and A. J. Ragauskas, Tetrahedron, 2007, 63, 10958 CrossRef CAS; (j) S. Hajdok, H. Leutbecher, G. Greiner, J. Conrad and U. Beifuss, Tetrahedron Lett., 2007, 48, 5073 CrossRef CAS; (k) H. Leutbecher, J. Conrad, I. Klaiber and U. Beifuss, Synlett, 2005, 3126 CAS.
- (a) S. Herter, D. Michalik, A. Mikolasch, M. Schmidt, R. Wohlgemuth, U. Bornscheuer and F. Schauer, J. Mol. Catal. B: Enzym., 2013, 90, 91 CrossRef CAS; (b) K. W. Wellington and N. I. Kolesnikova, Bioorg. Med. Chem., 2012, 20, 4472 CrossRef CAS PubMed; (c) S. Herter, A. Mikolasch, D. Michalik, E. Hammer, F. Schauer, U. Bornscheuer and M. Schmidt, Tetrahedron, 2011, 67, 9311 CrossRef CAS; (d) V. Hahn, T. Davids, M. Lalk, F. Schauer and A. Mikolasch, Green Chem., 2010, 12, 879 RSC; (e) A. Mikolasch, A. Matthies, M. Lalk and F. Schauer, Appl. Microbiol. Biotechnol., 2008, 80, 389 CrossRef CAS PubMed.
- (a) H. T. Abdel-Mohsen, J. Conrad and U. Beifuss, J. Org. Chem., 2013, 78, 7986 CrossRef CAS PubMed; (b) K. W. Wellington, G. E. R. Gordon, L. A. Ndlovu and P. Steenkamp, ChemCatChem, 2013, 5, 1570 CrossRef CAS; (c) K. W. Wellington, R. Bokako, N. Raseroka and P. Steenkamp, Green Chem., 2012, 14, 2567 RSC; (d) U. T. Bhalerao, C. Muralikrishna and B. R. Rani, Tetrahedron, 1994, 50, 4019 CrossRef CAS.
- H. Adibi, A. Rashidi, M. M. Khodaei, A. Alizadeh, M. B. Majnooni, N. Pakravan, R. Abiri and D. Nematollahi, Chem. Pharm. Bull., 2011, 59, 1149 CrossRef CAS PubMed.
- (a) C.-S. Lai, H.-L. Kao, Y.-J. Wang and C.-F. Lee, Tetrahedron Lett., 2012, 53, 4365 CrossRef CAS; (b) R. S. Schwab, D. Singh, E. E. Alberto, P. Piquini, O. E. D. Rodrigues and A. L. Braga, Catal. Sci. Technol., 2011, 1, 569 RSC; (c) L. Cai, J. Cuevas, Y.-Y. Peng and V. W. Pike, Tetrahedron Lett., 2006, 47, 4449 CrossRef CAS; (d) W. Deng, Y. Zou, Y.-F. Wang, L. Liu and Q.-X. Guo, Synlett, 2004, 1254 CAS; (e) F. Y. Kwong and S. L. Buchwald, Org. Lett., 2002, 4, 3517 CrossRef CAS PubMed; (f) U. Schopfer and A. Schlapbach, Tetrahedron, 2001, 57, 3069 CrossRef CAS.
- E. Tammari, N. Mirazi and D. Nematollahi, Mendeleev Commun., 2006, 16, 285 CrossRef.
- (a) T. Kashiwagi, F. Amemiya, T. Fuchigami and M. Atobe, Chem. Commun., 2012, 48, 2806 RSC; (b) A. R. Fakhari, S. S. H. Davarani, H. Ahmar, K. Hasheminasab and H. R. Khavasi, J. Heterocycl. Chem., 2009, 46, 443 CrossRef CAS; (c) C.-C. Zeng, F.-J. Liu, D.-W. Ping, L.-M. Hu, Y.-L. Cai and R.-G. Zhong, Tetrahedron, 2009, 65, 4505 CrossRef CAS; (d) A. R. Fakhari, S. S. H. Davarani, H. Ahmar and S. Makarem, J. Appl. Electrochem., 2008, 38, 1743 CrossRef CAS; (e) M. M. Khodaei, A. Alizadeh and N. Pakravan, J. Org. Chem., 2008, 73, 2527 CrossRef CAS PubMed; (f) S. S. H. Davarani, D. Nematollahi and M. Shamsipur, Heteroat. Chem., 2007, 18, 644 CrossRef CAS; (g) D. Nematollahi and E. Tammari, J. Org. Chem., 2005, 70, 7769 CrossRef CAS PubMed.
- (a) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC; (b) S. L. Y. Tang, R. L. Smith and M. Poliakoff, Green Chem., 2005, 7, 761 RSC; (c) R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437 RSC; (d) R. A. Sheldon, Chem. Commun., 2008, 3352 RSC.
- (a) R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC; (b) R. A. Sheldon, CHEMTECH, 1994, 24, 38 CAS; (c) R. A. Sheldon, Chem. Ind., 1997, 12 CAS.
- B. M. Trost, Angew. Chem., Int. Ed., 1995, 34, 259 CrossRef CAS.
- (a) R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854 RSC; (b) P. G. Jessop, Green Chem., 2011, 13, 1391 RSC; (c) C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, GAUSSIAN 03 (Revision E.01), Gaussian, Inc., Wallingford, CT, 2004 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental data and copies of the 1H NMR and 13C NMR spectra. See DOI: 10.1039/c3gc41968a |
‡ General procedure for the laccase-catalyzed synthesis of catechol thioethers: a 100 mL round bottomed flask with a magnetic stir bar was charged with a solution or suspension of the catechol 1 (0.63 mmol) and the thiol 2 (0.5 mmol) in methanol (3 mL). Phosphate buffer (0.2 M, pH 6, 27 mL) and laccase from A. bisporus (10 mg, 6 U mg−1) were added and the mixture was stirred under air at rt for the time given (see Tables 1–4). The reaction mixture was acidified with 2 M HCl to pH ∼ 4 and saturated with solid NaCl. The precipitated product was filtered with suction using a Buchner funnel. The filter cake was washed with aq. NaCl (15%, 25 mL) and water. The crude products obtained after drying exhibit a purity of 90–95% (NMR). Analytically pure products were obtained by column filtration (CH2Cl2–EtOAc 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1 1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the crude products. 1) of the crude products. |
§ Selected analytical data of 4-(benzo[d]thiazol-2′-ylthio)benzene-1,2-diol (3b): pale yellow solid; mp 198–200 °C; Rf = 0.53 (CH2Cl2–EtOAc = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); λmax(MeCN)/nm 284 (log ε, 4.60) and 206 (4.20); 1); λmax(MeCN)/nm 284 (log ε, 4.60) and 206 (4.20); ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) max (atr)/cm−1 3426 (br), 3054 (C–H), 1593, 1415, 1270 and 1030; δH (300 MHz; DMSO-d6) 6.90 (1H, d, 3J5-H,6-H 8.1 Hz, 6-H), 7.07 (1H, dd, 3J5-H,6-H 8.4 Hz, 4J3-H,5-H 2.1 Hz, 5-H), 7.13 (1H, d, 4J3-H,5-H 2.1 Hz, 3-H), 7.29 (1H, ddd, 3J5′-H,6′-H 7.2 or 7.9 Hz, 3J6′-H,7′-H 7.2 or 7.9 Hz, 4J4′-H,6′-H 1.1 Hz, 6′-H), 7.42 (1H, ddd, 3J4′-H,5′-H 7.3 or 8.2 Hz, 3J5′-H,6′-H 7.3 or 8.2 Hz, 4J5′-H,7′-H 1.3 Hz, 5′-H), 7.79 (1H, ddd, 3J4′-H,5′-H 8.1 Hz, 4J4′-H,6′-H 1.2 or 0.6 Hz, 5J4′-H,7′-H 1.2 or 0.6 Hz, 4′-H), 7.89 (1H, ddd, 3J6′-H,7′-H 8.0 Hz, 4J5′-H,7′-H 1.3 or 0.7 Hz, 5J4′-H,7′-H 1.3 or 0.7 Hz, 7′-H) and 9.63 (2H, br, 1-OH and 2-OH); δC (75 MHz; DMSO-d6) 116.66 (C-4), 117.01 (C-6), 121.12 (C-4′), 121.64 (C-7′), 122.45 (C-3), 124.13 (C-6′), 126.27 (C-5′), 127.66 (C-5), 134.75 (C-7a′), 146.61 (C-2), 148.66 (C-1), 153.71 (C-3a′) and 171.99 (C-2′); m/z (EI, 70 eV) 275 (M+, 100%), 242 (M+ − SH, 5), 167 (C7H5S2N+, 8) and 115 (15); HRMS (EI, M+) found 275.0051 calcd for C13H9S2NO2: 275.0075. max (atr)/cm−1 3426 (br), 3054 (C–H), 1593, 1415, 1270 and 1030; δH (300 MHz; DMSO-d6) 6.90 (1H, d, 3J5-H,6-H 8.1 Hz, 6-H), 7.07 (1H, dd, 3J5-H,6-H 8.4 Hz, 4J3-H,5-H 2.1 Hz, 5-H), 7.13 (1H, d, 4J3-H,5-H 2.1 Hz, 3-H), 7.29 (1H, ddd, 3J5′-H,6′-H 7.2 or 7.9 Hz, 3J6′-H,7′-H 7.2 or 7.9 Hz, 4J4′-H,6′-H 1.1 Hz, 6′-H), 7.42 (1H, ddd, 3J4′-H,5′-H 7.3 or 8.2 Hz, 3J5′-H,6′-H 7.3 or 8.2 Hz, 4J5′-H,7′-H 1.3 Hz, 5′-H), 7.79 (1H, ddd, 3J4′-H,5′-H 8.1 Hz, 4J4′-H,6′-H 1.2 or 0.6 Hz, 5J4′-H,7′-H 1.2 or 0.6 Hz, 4′-H), 7.89 (1H, ddd, 3J6′-H,7′-H 8.0 Hz, 4J5′-H,7′-H 1.3 or 0.7 Hz, 5J4′-H,7′-H 1.3 or 0.7 Hz, 7′-H) and 9.63 (2H, br, 1-OH and 2-OH); δC (75 MHz; DMSO-d6) 116.66 (C-4), 117.01 (C-6), 121.12 (C-4′), 121.64 (C-7′), 122.45 (C-3), 124.13 (C-6′), 126.27 (C-5′), 127.66 (C-5), 134.75 (C-7a′), 146.61 (C-2), 148.66 (C-1), 153.71 (C-3a′) and 171.99 (C-2′); m/z (EI, 70 eV) 275 (M+, 100%), 242 (M+ − SH, 5), 167 (C7H5S2N+, 8) and 115 (15); HRMS (EI, M+) found 275.0051 calcd for C13H9S2NO2: 275.0075. |
| This journal is © The Royal Society of Chemistry 2014 |