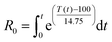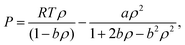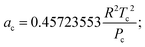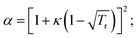The CO2-assisted autohydrolysis of wheat straw
Sara P.
Magalhães da Silva
ab,
Ana Rita C.
Morais
a and
Rafał
Bogel-Łukasik
*a
aLaboratório Nacional de Energia e Geologia, Unidade de Bioenergia, 1649-038 Lisboa, Portugal. Fax: +351217163636; Tel: +351210924600 ext. 4224
bDepartamento de Química, Universidade de Aveiro, 3810-193 Aveiro, Portugal. E-mail: rafal.lukasik@lneg.pt
First published on 16th September 2013
Abstract
The CO2-assisted autohydrolysis was used for wheat straw treatments at temperatures ranging from 180 to 210 °C and an initial CO2 pressure of 60 bar. The study was performed using three different mixture loadings, such as 250 g of H2O/25 g of wheat straw, 150 g of H2O/15 g of wheat straw and 75 g of H2O/7.5 g of wheat straw. The in situ formed carbonic acid was found to result in a higher dissolution of xylose as well as XOS (xylo-oligosaccharides) in comparison to CO2-free pre-treatments under the same conditions (temperature and LSR). The effect of CO2 concentration was also investigated to address the issue of CO2 involved in the reaction that allows to significantly increase the XOS content. At 210 °C with a mixture loading of 75 g of H2O/7.5 g of wheat straw, XOS were present in the liquor at a concentration of 15.75 g L−1. However, with more severe conditions more degradation products (mainly furfural) were detected (in the liquor and the recovered gas phase from depressurization after the reaction). Glucan was mainly retained in the solid phase (containing up to 64%) together with Klason lignin (maximum dissolution of 18%). The dissolved XOS in the liquid phase are proposed to be used in other applications, either directly, such as prebiotic ingredients, or indirectly, after post-hydrolysis to biofuel production through C5 sugars’ fermentation.
1. Introduction
Lignocellulosic feedstock is mainly composed of cellulose (35–50%), hemicellulose (20–35%) and lignin (5–30%),1 wherein the composition is dependent upon several parameters. Due to the complex macroscopic structure of lignocellulosic materials, several pre-treatment technologies are currently employed to overcome this resistance against chemical and microbial attacks. The pre-treatment methods can be categorised according to various criteria.2,3 Pre-treatments can be segregated between conventional (dilute acid hydrolysis, alkali4), hydrothermal (steam-explosion,5 wet oxidation,6 microwaves,7 and autohydrolysis8) and alternative methods (ionic liquids,9,10 sub- and supercritical fluids, mostly water and CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11–13).
11–13).
The autohydrolysis process uses compressed hot water (pressure above saturation point) with a general range of temperature between 150 and 230 °C and various reaction times from seconds to hours according to the operation mode applied.4 Hydronium ions generated in situ by water autoionization and acetic acid from dissolution of acetyl substituents of hemicelluloses have the capability to act as catalysts formed in situ in the autohydrolysis processes. A high recovery of hemicellulose in the liquid-fraction (mainly in oligomeric form) and of cellulose and lignin in the solid fraction with negligible losses are generally reported. The hemicellulose-rich liquor can be a source of value-added products. Xylooligosaccharides (XOS) are one of these products and can be obtained directly from autohydrolysis pre-treatment.8 Xylitol, important due to its application in the food, pharmaceutical and cosmetic industries, can be produced after the bioconversion of hemicellulose liquor as well.14 Moreover, production of reducing sugars, organic acids (such as acetic acid or propionic acid), bio-composites, furfural and other miscellaneous compounds can be developed too.15 On the other hand, the solid fraction obtained is mainly converted to sugar monomers and further to bioethanol along with a possibility to produce value-added commodities.
ScCO2 is a non-toxic, non-flammable and inexpensive reagent,16 and its employment generally lowers the temperature of the process leading to minor generation of degradation products and a higher yield of the reaction.4,17 Technologies involving the use of sub- (near its critical conditions) and supercritical treatments have been investigated for lignocellulosic material pre-treatments. In these processes sub- or supercritical water and/or supercritical CO2 were commonly used. Considering economic efficiency supercritical water or liquid hot water (LHW) treatments seem to be superior due to the water facilitated feasibility of hydrolysis that provides an acidic environment at high temperatures.18 Particularly in the subcritical range of temperature and pressure (P < 210 bar, T < 380 °C), the ionic product and the hydrolysis capacity of H2O increase due to the increased temperature. At 250 °C the ionic product for water, Kw, reaches a maximum of 6.34 × 10−12, resulting in a pH of 5.5 for water at 220 °C.18 Thus hemicellulose could be completely separated from lignocellulose and the enzymatic digestibility of cellulose can be significantly enhanced by treating the lignocellulosic material under the ascribed conditions.19,20 Recent studies on the sub-pressurized water effect of scCO2 in biomass pre-treatment13,18 showed that the presence of water favours the separations in such a manner that in the presence of CO2, the mixture becomes more acidic due to the in situ formation and two step dissociation of carbonic acid according to the following equation:
| CO2 + H2O ↔ HCO3− + H3O+; HCO3− + H2O ↔ CO32− + H3O+. |
By the dissolution of CO2, the pH of the water–CO2 mixture decreases to approximately 3.0 (depending on pressure and temperature) making the environment strongly acidic, thus facilitating the biomass hydrolysis.21
Compared to the conventional hydrothermal treatments, the combined sub- or supercritical processes demonstrate a much higher reaction rate; thus, neither the use of an additional catalyst nor inhibition of the reaction towards intermediates is required.22,23 The use of scCO2 can overcome the drawbacks resulting from the conventional pre-treatments with organic acids due to the CO2 practical neutralisation caused by a pressure reduction. The use of scCO2 was reported to not cause a significant change in the microscopic morphology of wood.24 Studies aimed at checking the scCO2 effect on raw lignocellulosic materials with different moisture contents under various pre-treatment conditions (temperature, time and pressure)11 demonstrated that an increase in moisture content to 73% (w/w) at 214 bar and 165 °C resulted in a significant increase of the final sugar yields from the enzymatic hydrolysis. An important conclusion on a pronounced effect of the moisture content in pre-treatments with scCO2 was drawn. Addition of CO2 to the autohydrolysis pre-treatment of beech wood showed an increase of xylan hydrolysis.25 On the other hand, CO2 applied in the autohydrolysis process at 100 bar did not enhance the degree of biomass dissolution12 due to the unfavourable acidic water/CO2 system.
The present work was devoted to examine the effect of CO2 on the autohydrolysis pre-treatment of wheat straw, in order to enhance the selectivity of the dissolved hemicellulose fraction. Moreover, this work aimed at evaluation of the temperature and non-isothermal operational mode effects on the composition of both the liquid and solid fractions as a function of the severity factor (log R0)26 (according to the following equation:
Additionally, the influence of CO2 density was also studied using the Peng–Robinson equation of state.27
2. Materials and methods
2.1 Materials
Wheat straw was kindly supplied by INIAV, I.P. – Estação Nacional de Melhoramento de Plantas (Elvas, Portugal). The material was ground using a knife mill (IKA® WERKE, MF 10 basic, Germany) to particles smaller than 1.5 mm, and stowed at room temperature. The wheat straw moisture was determined to be 8%. CO2 with a purity ≥99.99% bought from Air Liquide, AlphaGaz™ gamma, Paris, France was used. For post-processing filtrations, paper filters (Ø = 150 mm, no. 1238) from Filter-Lab, Microchip Technology Inc., Arizona, USA were used. For all experiments the following reagents were used: distilled water (17 MΩ cm−1) produced by the PURELAB Classic Elga system, and 72% (w/w) H2SO4 aqueous solution prepared from concentrated H2SO4 solution (96% purity) supplied by Panreac Química, Barcelona, Spain. In addition, ethanol at 96% purity (v/v) for gas phase capturing was acquired from Carlo Erba Group, Arese, Italy.2.2 CO2-assisted autohydrolysis of wheat straw
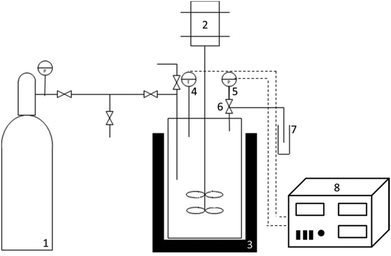
The CO2-assisted autohydrolysis treatments of wheat straw were performed in a stainless steel 600 mL reactor (series 4560, Parr Instruments Company, Moline, Illinois, USA). The reactor was equipped with two four-blade turbine impellers, and the temperature and pressure were controlled using a Parr PID controller, model 4842. An external fabric mantle was used to heat the reactor, while an internal stainless steel loop was used to cool the system with cold water. Fig. 1 illustrates a scheme of the apparatus used.The CO2-assisted autohydrolyses of wheat straw were carried out at three temperatures, namely 180, 200 and 210 °C, selected based on the literature data.8 An initial pressure of 60 bar at room temperature and an agitation speed of 70 rpm were maintained constant in all experiments. Different mixture loadings were used: 250 g of H2O/25 g of wheat straw; 150 g of H2O/15 g of wheat straw and 75 g of H2O/7.5 g of wheat straw. When the final desired temperature was attained, the reactor was rapidly cooled down to quench the reaction. A slow depressurization (2 bar minute−1) of the reaction mixture was executed when the temperature was lower than 20 °C to minimise the presence of volatile compounds in the vapour phase. The depressurised gas phase passed through a vial filled with a known amount of ethanol placed in the ice at a temperature of 0 °C. This procedure allows for dissolution of volatile compounds for posterior qualitative and quantitative analyses. The liquid (liquor) and solid fractions were recovered by vacuum filtration. The qualitative and quantitative analyses of all fractions were performed using the procedures presented below.
2.3 Chemical analyses
The liquor sample was subjected to hydrolysis with 4% (w/w) H2SO4 at 121 °C for 1 h in an autoclave (Uniclave, Portugal) to convert soluble oligosaccharides from hemicellulose into its constituent sugar monomers.30 After post-hydrolysis, oligosaccharides’ concentrations were expressed as an increase in sugar monomers determined by HPLC.
3. Results
3.1 Feedstock composition
The chemical composition of the wheat straw used in the CO2-assisted autohydrolysis pre-treatment was characterised. These data are compiled in Table 1. The level of the wheat straw moisture was determined to be 8%. A total of 63% of wheat straw biomass was polysaccharides among which 38.5% was cellulose (estimated as glucan). Wheat straw hemicellulose was composed of a β-D-(1,4)-linked xylopyranosyl backbone, substituted with arabinofuranose, 4-O-methylglucuronic acid, acetyl groups, xylose and phenolic acids.8 The total hemicellulose, 24.9%, was measured as the sum of xylose, arabinose and acetyl group content. In relation to the Klason lignin content, the obtained value was corrected for the ash content of acid insoluble residue and it was determined to be 17.7%. The obtained data are in good agreement with those reported by Carvalheiro et al.8| Component | This worka | Carvalheiro et al.8 |
|---|---|---|
| a Average of two replicates. b Determined as glucan. c Determined by difference. | ||
| Celluloseb | 38.5 ± 0.1 | 38.9 ± 0.2 |
| Hemicellulose | 24.9 | 23.5 |
| Xylan | 19.1 ± 0.6 | 18.1 ± 0.3 |
| Arabinan | 3.0 ± 0.1 | 3.0 ± 0.2 |
| Acetyl groups | 2.7 ± 0.2 | 2.5 ± 0.1 |
| Klason lignin | 17.7 ± 0.1 | 18.0 ± 0.5 |
| Ash | 10.7 ± 0.1 | 9.70 ± 0.03 |
| Protein | 4.7 ± 0.1 | 4.5 ± 0.5 |
| Othersc | 3.5 | 5.5 |
3.2 Composition of the liquors
The composition of the liquors was one of the parameters examined in this study. The wheat straw CO2-assisted autohydrolyses resulted in liquors containing a mixture of sugar oligomers (mainly XOS), monosaccharides (glucose, xylose and arabinose), acetic acid (from acetyl groups present in hemicellulose) and sugar decomposition products, namely HMF and furfural. According to the literature reports, the formation of these chemicals depends on the severity of pre-treatment conditions, namely temperature. The composition of liquors obtained from the CO2-assisted autohydrolysis under various conditions is depicted in Table 2.| Reaction | CO2-freea | CO2-assisted | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass loadingb | 250/25 | 250/25 | 150/15 | 75/7.5 | |||||||||||
| T (°C) | 210 | 210 | 180 | 200 | 210 | 180 | 200 | 210 | |||||||
| log R0 | 3.83 | 3.54 | 2.58 | 3.16 | 3.44 | 2.60 | 3.08 | 3.48 | |||||||
| pH | 4.32 | 3.85 | 4.38 | 4.13 | 3.93 | 4.55 | 4.39 | 4.03 | |||||||
| Composition/yields | g L−1 | g L−1 | g 100 g−1 | g L−1 | g 100 g−1 | g L−1 | g 100 g−1 | g L−1 | g 100 g−1 | g L−1 | g 100 g−1 | g L−1 | g 100 g−1 | g L−1 | g 100 g−1 |
| a Data taken from ref. 8. b g of water/g of wheat straw XOS – xylooligosaccharides; GlcOS – gluco-oligosaccharides; AcO – acetyl groups linked to oligosaccharides; n.a. – not available. | |||||||||||||||
| XOS | 9.5 | 10.0 | 55.1 | 5.5 | 29.6 | 11.4 | 62.6 | 11.8 | 51.2 | 10.6 | 57.7 | 12.9 | 70.3 | 15.7 | 70.6 |
| GlcOS | 0.5 | 4.3 | 11.8 | 3.5 | 9.6 | 3.4 | 11.2 | 3.2 | 8.8 | 5.2 | 12.6 | 5.09 | 12.6 | 4.1 | 9.8 |
| AcO | 0.2 | 0.7 | — | 1.5 | — | 1.2 | — | 1.1 | — | 1.8 | — | 1.3 | — | 1.2 | — |
| Xylose | 1.7 | 3.4 | 16.4 | 2.0 | 9.7 | 2.4 | 11.8 | 4.0 | 19.6 | 0.5 | 2.3 | 0.5 | 2.4 | 3.3 | 16.1 |
| Arabinose | 1.2 | 0.9 | 17.8 | 1.2 | 13.2 | 1.3 | 15.4 | 2.0 | 25.5 | 0.4 | 3.7 | 0.5 | 4.2 | 2.1 | 20.7 |
| Glucose | 1.0 | 1.2 | 2.8 | 1.1 | 2.8 | 1.2 | 3.0 | 1.8 | 4.4 | 0.4 | 0.9 | 0.4 | 1.0 | 2.0 | 5.0 |
| Acetic acid | 2.1 | 2.4 | — | 0.6 | — | 1.0 | — | 3.0 | — | 1.1 | — | 1.6 | — | 2.7 | — |
| HMF | 0.0 | 0.1 | 0.5 | 0.0 | 0 | 0.1 | 0.3 | 0.2 | 0.7 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.5 |
| Furfural | 0.1 | 5.4 | 40.6 | 0.1 | 0.5 | 0.5 | 3.9 | 4.6 | 35.0 | 0.3 | 2.4 | 0.7 | 5.4 | 3.2 | 24.0 |
Xylooligosaccharides (XOS) were found to be major components present in liquors in all reactions. Considering all the biomass loading studied, the highest amount of XOS produced was determined to be at the lowest studied biomass loading (75/7.5) under the most severe conditions (log R0 = 3.48). On the other hand, at the highest biomass loading (250/25) under similar severity conditions (log R0 = 3.54), the concentration of XOS was as much as 36% lower than that presented before. The obtained concentration was comparable to the concentration (10.64 g L−1) obtained with the lowest biomass loading (75/7.5), but under less severe conditions (log R0 = 2.60). The remaining oligosaccharides (GlcOS and AcO) exhibited a significant concentration in the liquid fraction, which decreased with an increase of the severity of the reaction conditions. During the pre-treatment of wheat straw, pentoses are also co-produced from xylan and arabinan with xylose being the main monosaccharide present in all assays followed by arabinose revealing that pentose concentration enhances steadily with the conditions’ severity. Under the conditions leading to the maximal XOS recovery (biomass loading of 75/7.5 and log R0 = 3.48), the maximal arabinose concentration was obtained while the highest concentration of xylose (4.03 g L−1) was achieved at the biomass loading of 150/15 and under the severity conditions of log R0 = 3.44. The same trend was observed for monomers of glucose, acetic acid and for the degradation products. The sugar degradation products, HMF and furfural, were detected in low amounts in almost all reactions. The exceptions were reactions at the harshest conditions, for which an increase was more pronounced as the furfural concentration increased by 9 and 4.5 times in the case of the transition from 200 to 210 °C for 150/15 and 75/7.5 biomass loading, respectively.
The pH values of liquors from CO2-assisted autohydrolysis pre-treatments are also presented in Table 2. The pH of liquors decreases from 4.38 to 3.93 and from 4.55 to 4.03 with the increase of temperature for biomass loadings of 150/15 and 75/7.5, respectively.
The analysis of the influence of the CO2 amount shows that a larger amount of CO2 obtained by the relative reduction of the biomass amount by half in the reactor leads to an increase of XOS recovered by 1/3 (at 210 °C) and is counterbalanced by a reduction of xylose and furfural concentrations by 17% and 30%, correspondingly.
| Reaction | CO2-assisted | ||||||
|---|---|---|---|---|---|---|---|
| Biomass loadinga | 250/25 | 150/15 | 75/7.5 | ||||
| a g of water/g of wheat straw. | |||||||
| T (°C) | 210 | 180 | 200 | 210 | 180 | 200 | 210 |
| Log R0 | 3.54 | 2.58 | 3.16 | 3.44 | 2.60 | 3.08 | 3.48 |
| Solid yield | 56.27 | 77.84 | 60.54 | 55.98 | 70.54 | 62.89 | 54.56 |
| Glucan | 58.41 | 45.50 | 49.89 | 55.56 | 54.10 | 54.36 | 64.33 |
| Xylan | 8.90 | 15.56 | 6.72 | 5.00 | 9.16 | 5.89 | 2.23 |
| Arabinan | 0.04 | 0.53 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 |
| Acetyl groups | 1.35 | 2.63 | 0.46 | 0.00 | 2.67 | 1.21 | 0.00 |
| Klason lignin | 28.30 | 19.99 | 23.87 | 28.88 | 20.99 | 21.52 | 26.74 |
On the other hand, the amount of xylan in the processed solids decreases as the severity of the conditions increases and a complete removal of arabinan from the processed solids is verified except for the reaction at 180 °C. For this reaction a noticeable amount of arabinan (0.53 g L−1) was detected in the solid phase. Furthermore, harsher reaction conditions facilitate the complete dissolution of acetyl groups as they are absent in the solid phase. Moreover it can be observed that the applied treatment influences neither cellulose nor Klason lignin since their relative contents increase with the severity factor.
4. Discussion
4.1 Effect of temperature
The CO2-assisted autohydrolysis of wheat straw was carried out at three temperatures (180, 200 and 210 °C) selected according to the literature reports.8 Two different biomass loadings were used to study the influence of temperature (Table 2). In the case of both the examined ratios (150/15 and 75/7.5), the percentage of components present in liquor depends on the temperature of the process. In fact, the increase of the reaction severity is responsible for the decrease in the density and in the dielectric constant of water allowing the water dissociation which enables the disruption of the recalcitrant structure and, hence, leads to easier hydrolysis of xylan producing XOS-rich liquors.4The water dissociation caused by an increase of the temperature of the reaction can be proven by a decrease of medium pH for the same biomass loading reported elsewhere.8 The results presented in this work are in good agreement with these results; for higher temperature, decreases in the solubility of the gas is observed; therefore after depressurisation of the reactor, the equilibrium in the system for reactions at high temperatures can be achieved faster and the pH of the liquor is higher as is confirmed in this study.
The amount of solubilised xylan increases with increasing temperature to reach 37 to 85% of the initial amount at the maximal reaction temperature studied for a biomass loading of 150/15. The same trend was found for the biomass loading of 75/7.5 in which it was noticed that xylan dissolution depends strongly on temperature. The maximal yield of xylan solubilised as XOS was obtained under the severest conditions corresponding to 51% and 71% of the initial xylan amount for a biomass loading of 150/15 and 75/7.5, respectively.
The obtained data demonstrate that the CO2-assisted autohydrolysis pre-treatment results in XOS-rich liquor, which with the increase of severity conditions is randomly hydrolysed leading to smaller oligosaccharides or even to xylose monomers. This fact can be confirmed by the increase of xylose concentration accompanied by the increase of temperature.
Similar to xylose and XOS, the increase of arabinose concentration with an increase in temperature is observed. The arabinose release was achieved at lower temperature compared to xylose as arabinose shows a higher thermal sensitivity.31
During the pre-treatment the acetyl groups attached to the xylan backbone are released in the liquor. Thus, the content of acetic acid in liquors increases with temperature reaching a 3-fold higher value at 210 °C than at 200 °C. Yet the acetic acid content was still relatively lower even under maximal XOS concentration conditions. In addition, it is important to highlight that after pre-treatment, some acetyl groups remain bonded to oligosaccharides in the liquor solution, which explains why acetic acid concentration is augmented after post-hydrolysis of the liquors.
Furthermore, the achieved results permit us to draw a conclusion that increasing the temperature of the process leads to the formation of more sugar derived degradation products. For a biomass loading of 150/15 at 180 and 200 °C, insignificant concentrations of furfural and HMF were detected but at 210 °C, a furfural concentration equal to 4.60 g L−1 was observed. This is due to the fact that under the examined conditions, the increase of the severity factor results in the formation of degradation products, namely furfural that is a product derived from the arabinose present as a xylan side-chain. A similar increase of furfural concentration together with an increase of severity conditions correlates well with the results described for CO2-free autohydrolysis for different biomasses such as eucalyptus wood, wheat straw and brewery's spent grain.8,32,33 Nevertheless, under the conditions of maximal production of XOS, the furfural amounts were in the range of 0.33–3.19 g L−1 for 75/7.5 biomass loading.
It is worth to underline that under the highest furfural concentration conditions the concentration of HMF remains very low (0.20 g L−1) although 19.3% cellulose dissolution did occur. Furthermore, the concentration of glucose in the liquor is very low (1.76 g L−1) reaching a yield of 4.37% of glucan in feedstock. The severity of the pre-treatment seems to be insufficient to produce degradation products such as HMF in high concentration although enough to produce a relatively high concentration of gluco-oligosaccharides (3.20 g L−1).
The chemical composition of the processed solids (Table 3) shows an enrichment of lignin and cellulose contents accompanied by an increase of temperature. It is caused by complete hydrolysis of the hemicellulose fraction which additionally led to a continuous decrease in solid residue yield to reach values below 60% for the experiment with a final temperature of 210 °C. Furthermore, the increase of lignin content along with the increase of severity of pre-treatment conditions is related to condensation reactions between lignin and sugars and/or degradation products resulting from precipitation of the fibre inducing an apparent increase in Klason lignin content.34,35 Under the most severe conditions, a high recovery of lignin (91%) from the initial amount of lignin present in wheat straw (Table 1) was also attained and only 0.22 g of lignin was dissolved and found in the liquor. This strongly indicates that the CO2 presence does not lead to a significant dissolution of lignin. Regarding cellulose, 19.3% of glucan present in raw feedstock was dissolved in the liquid fraction. However, its percentage on solid residues increased steadily with the severity of treatments, chiefly due to the resistance of this polymer to hydrothermal treatments.32
The amount of xylan present in the processed solid decreases with an increase of temperature, showing a recovery of 14.6% at 210 °C. Therefore, to achieve complete removal more severe conditions are required although the results obtained in this work and available in the literature8 show that higher temperature promotes extensive degradation (Table 3).
4.2 Effect of CO2
The presence of GlcOS in the liquor was also found to be higher when CO2 was used (6-fold higher) in the reaction. The maximal GlcOS production was achieved at 180 °C and 75/7.5 biomass loading corresponding to a yield of GlcOS close to 13%. Zhao et al. achieved the same maximal yield of GlcOS produced from corn stalks and wheat straw at supercritical water hydrolysis (384 °C).22 This fact means that the presence of CO2 allowed obtaining approximately the same yield of GlcOS with a decrease by 204 °C allowing the use of subcritical conditions. Furthermore this also clearly indicates that the presence of CO2 leads to the minor dissolution of cellulose, even under less severe conditions than without CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 but further degradation of hexoses to HMF was negligible as HMF was detected in minimal concentration (0.14 g L−1).
8 but further degradation of hexoses to HMF was negligible as HMF was detected in minimal concentration (0.14 g L−1).
On the other hand, the presence of CO2 contributes to the formation of further degradation products from the hemicellulose fraction, e.g. furfural. This is caused by the easier degradation of pentoses to furfural while hexoses are less susceptible to the degradation to HMF.
Furthermore, CO2 plays an important role in the pH of the hydrolysate. It was found that the pH varies from 3.85 to 4.55. The decrease of the hydrolysate's pH can be explained by the fact that carbonic acid is formed in situ, especially that no additional acetic acid compared to the CO2-free autohydrolysis reaction8 was produced. Conversely, Walsum et al.13 revealed that CO2 addition leads to an increase of the final pH of the hydrolysate in comparison to the autohydrolysis without CO2. This inconsistency between the results presented by Walsum et al.13 and those in this work comes from the difference in the reaction conditions. The work of Walsum shows that the CO2/water ratio is equal to 0.04 while in this work the CO2/water ratio is at least 3-fold higher. Therefore, a relatively higher amount of CO2 leads to a considerably lower pH created in the course of the reaction; thus after the depressurisation, CO2 dissolved in the liquid phase acts as an acidifier of the medium.
Comparison of these results with those reported in the literature8 illustrates that the amount of XOS depends on the CO2 presence, and the same concentration of XOS can be achieved under less severe conditions. To produce 10 g L−1 of XOS a 5 °C higher temperature is needed that also corresponds to a 11% higher log R0.
The effect of CO2 is also observed in the composition of the processed solids. The main differences between treatments with and without CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 are perceptible in enrichment of the glucan and the Klason lignin content in the case of CO2-assisted reactions, as well as in complete removal of arabinan. In addition, lower xylan content is observed in the wheat straw CO2 pre-treatment. It indicates that CO2 enhances the dissolution of hemicellulose (xylan, arabinan and acetyl groups) and retains cellulose and lignin in the solid phase.
8 are perceptible in enrichment of the glucan and the Klason lignin content in the case of CO2-assisted reactions, as well as in complete removal of arabinan. In addition, lower xylan content is observed in the wheat straw CO2 pre-treatment. It indicates that CO2 enhances the dissolution of hemicellulose (xylan, arabinan and acetyl groups) and retains cellulose and lignin in the solid phase.
The results of CO2-assisted hydrothermal hydrolysis showed that the lignocellulosic materials can be partially solubilised and hydrolysed at temperatures significantly below the supercritical point.
where a = acα;
| κ = 0.37464 + 1.54226ω − 0.26993ω2. |
The constants used are: Tc (CO2) = 304.2 K; Pc (CO2) = 73.8 bar; ω (acentric factor) = 0.228;36R (gas constant) = 8.314 × 10−2 L bar K−1 mol−1.
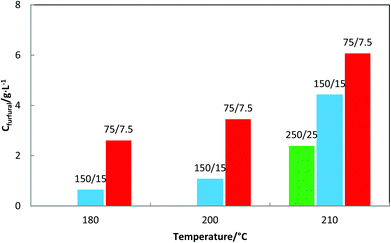
The increase of CO2 concentration makes liquors richer in hemicelluloses-derived products. Fig. 3 shows that at 210 °C, an increase of 17% and 57% of the XOS concentration is attained with the reduction of water/wheat straw loading from 250/25 to 150/15 and to 75 g of H2O/7.5 g of wheat straw, respectively.
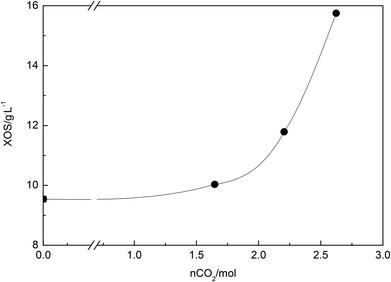 | ||
| Fig. 3 The XOS concentration (g L−1) as a function of the number of moles of CO2 at 210 °C. The data for the CO2-free reaction (■) taken from the literature.8 | ||
A biomass loading reduction by half (from 150/15 to 75/7.5) led to an increase of the number of moles of CO2 by more than 20% (Table 4). Therefore, as is expected the XOS concentration increases with an increase of the CO2 concentration in the system; however, the pH of the solution became less acidic. This controversial result can be explained by the fact that after the CO2 decompression, a prolonged time is needed to achieve the equilibrium in the system. In other words, the pH measurement is done immediately after the experiment is performed under non-equilibrium conditions. Furthermore, the time needed to achieve the equilibrium is strongly dependent on the amount of water present in the system due to the diffusion limitation of CO2 in the liquid phase. The complementary research on the pH of solutions 20 days after reactions was shown to be very similar to those reported elsewhere.8 Solutions obtained from the reaction produced at 180 and 210 °C for 75/7.5 biomass loading after 20 days get similar pH, that is, 5.79 and 5.92, respectively. The observed increase of the solutions’ pH proves again the fact that CO2 is released slowly from water and even more the obtained values are very similar to that reported by Carvalheiro et al. for which at the same severity factor (log R0 = 2.60) the pH of the solution was 5.61.
4.3 Volatile products
The volatile compound formed from the hemicellulose fraction was found to be in the gas phase. The obtained data depicted in Fig. 2 show that the biomass loading and reaction temperature play an important role in the amount of furfural recovered. The increase in furfural formation can be considered to be dependent upon the process temperature and the CO2 present. The temperature effect on furfural formation has already been discussed in this work. Another important aspect influencing the furfural volatility is the presence of acetic acid. To examine the acid–base interaction between furfural and acetic acid, the effect of different contents of acetic acid on the distribution behaviour of furfural and of the solvent properties of the weak acid on carbon dioxide has to be taken into account. The literature results show that up to a concentration of 5 wt%, acetic acid has modifier properties and enhances furfural extraction.37Another interesting aspect is that, besides the acetic acid present in the liquor, acetic acid was not detected in the gas phase entrapped after the reaction. The estimation of the VLE data for the system of CO2 + water + acetic acid provides information on the negligible solubility of acetic acid under the reaction conditions.38 Additionally, in the presence of CO2 there is an equilibrium between CO2, H2O and acetic acid; thus acetic acid is dissolved in water not only in molecular but also in ionic form39 inhibiting its volatility.
5. Conclusion
The CO2-assisted autohydrolysis treatment of wheat straw was investigated, in order to selectively dissolve the hemicellulose fraction. The autohydrolysis with CO2 allowed producing a liquid fraction rich in hemicellulose (mainly in oligomer form) and a solid containing mainly glucan together with lignin. These results prove the high selectivity of the pre-treatment towards hemicellulose fraction. The in situ formation of carbonic acid resulted in an increase of both xylose monomers and an increase of XOS concentration in comparison to the CO2-free pre-treatment of the wheat straw under analogous conditions (temperature and LSR). The effect of temperature on pre-treatments with CO2 addition was also examined. It was noticed that higher temperature (more severe conditions) led to an increase of xylose and XOS concentrations.Furthermore, a decrease of the final pH of the hydrolysate was observed. Therefore, the acidic character originating from the formation of carbonic acid enables disruption of the chemical bonds between hemicellulose and lignocellulose. Thus, it can be concluded that carbonic acid contributes to autohydrolysis. Nevertheless, further studies are required in order to determine the optimal conditions under which the consensus between temperature/initial pressure and hemicellulose dissolution is attained without extensive formation of degradation products.
The CO2-assisted autohydrolysis towards XOS formed at elevated amounts under much less severe conditions compared to the CO2-free process is proposed. Therefore, the valorisation of agriculture residues towards high value added products is more economically and environmentally favourable, especially when green compounds such as CO2 and water are used in the proposed processes. The major products of CO2-assisted autohydrolysis are XOS that can be later obtained in a pure form after the membrane separation process, for direct end-uses as prebiotic ingredients, or, alternatively, can be subjected to a post-hydrolysis followed by biofuel production through C5 fermentation.8
Acknowledgements
This work was supported by the European Commission financing the PROETHANOL2G project (FP7-ENERGY-2009-BRAZIL; grant agreement: 251151). Authors would like to acknowledge support of Dr Francisco Gírio, Dr Florbela Carvalheiro and Céu Penedo from Laboratório Nacional de Energia e Geologia in the execution of this work.References
- J. Van Spronsen, M. A. T. Cardoso, G. Witkamp, W. Jong and M. C. Kroon, Chem. Eng. Process., 2011, 50, 196–199 CAS.
- M. Galbe and G. Zacchi, Biomass. Bioenerg., 2012, 46, 70–78 CrossRef CAS PubMed.
- P. Alvira, E. Tomás-Pejó, M. Ballesteros and M. Negro, Bioresour. Technol., 2010, 101, 4851–4861 CrossRef CAS PubMed.
- F. M. Girio, C. Fonseca, F. Carvalheiro, L. C. Duarte, S. Marques and R. Bogel-Lukasik, Bioresour. Technol., 2010, 101, 4775–4800 CrossRef CAS PubMed.
- C. Cara, E. Ruiz, M. Ballesteros, P. Manzanares, M. J. Negro and E. Castro, Fuel, 2008, 87, 692–700 CrossRef CAS PubMed.
- H. B. Klinke, B. K. Ahring, A. S. Schmidt and A. B. Thomsen, Bioresour. Technol., 2002, 82, 15–26 CrossRef CAS.
- M. Palm and G. Zacchi, Biomacromolecules, 2003, 4, 617–623 CrossRef CAS PubMed.
- F. Carvalheiro, T. Silva-Fernandes, L. C. Duarte and F. M. Gírio, Appl. Biochem. Biotechnol., 2009, 153, 84–93 CrossRef CAS PubMed.
- A. M. da Costa Lopes, K. João, A. R. C. Morais, E. Bogel-Lukasik and R. Bogel-Lukasik, Sustain. Chem. Process., 2013, 1, 3 CrossRef.
- A. M. da Costa Lopes, K. João, D. Rubik, E. Bogel-Lukasik, L. C. Duarte, J. Andreaus and R. Bogel-Lukasik, Bioresour. Technol., 2013, 142, 198–208 CrossRef CAS PubMed.
- K. H. Kim and J. Hong, Bioresour. Technol., 2001, 77, 139–144 CrossRef CAS.
- T. Rogalinski, T. Ingram and G. Brunner, J. Supercrit. Fluids, 2008, 47, 54–63 CrossRef CAS PubMed.
- G. P. van Walsum and H. Shi, Bioresour. Technol., 2004, 93, 217–226 CrossRef CAS PubMed.
- F. M. Girio, F. Carvalheiro, L. C. Duarte and R. Bogel-Lukasik, in D-Xylitol Fermentative Production, Application and Commercialization, ed. S. Silverio da Silva and A. K. Chandel, Springer, 2012, ch. 1, pp. 3–37 Search PubMed.
- G. Y. S. Mtui, Afr. J. Biotechnol., 2009, 8, 1398–1415 CAS.
- M. M. Kirchhoff, Resour. Conserv. Recy., 2005, 44, 237–243 CrossRef PubMed.
- E. Bogel-Lukasik, M. G. da Silva, I. D. Nogueira, R. Bogel-Lukasik and M. Nunes da Ponte, Green Chem., 2009, 11, 1847–1856 RSC.
- C. Schacht, C. Zetzl and G. Brunner, J. Supercrit. Fluids, 2008, 46, 299–321 CrossRef CAS PubMed.
- M. Sasaki, T. Adschiri and K. Arai, Bioresour. Technol., 2003, 86, 301–304 CrossRef.
- T. H. Kim and Y. Y. Lee, Bioresour. Technol., 2006, 97, 224–232 CrossRef CAS PubMed.
- K. L. Toews, R. M. Shroll, C. M. Wai and N. G. Smart, Anal. Chem., 1995, 67, 4040–4043 CrossRef CAS.
- Y. Zhao, W. J. Lu, H. T. Wang and J. L. Yang, Bioresour. Technol., 2009, 100, 5884–5889 CrossRef CAS PubMed.
- M. Goto, R. Obuchi, T. Hiroshi, T. Sakaki and M. Shibata, Bioresour. Technol., 2004, 93, 279–284 CrossRef CAS PubMed.
- D. C. Ritter and A. G. Campbell, Wood Fiber Sci., 1991, 23, 98–113 CAS.
- G. P. Walsum, in Twenty-Second Symposium on Biotechnology for Fuels and Chemicals, ed. B. Davison, J. McMillan and M. Finkelstein, Humana Press, 2001, ch. 27, pp. 317–329 Search PubMed.
- R. P. Overend, E. Chornet and J. A. Gascoigne, Philos. Trans. R. Soc. London, Ser. A, Mathematical and Physical Sciences, 1987, 321, 523–536 CrossRef CAS.
- D. Peng and D. B. Robinson, Ind. Eng. Chem. Fundam., 1976, 15, 59–64 CAS.
- B. L. Browning, in Methods in Wood Chemistry, ed. K. V. Sarkanen and C. H. Ludwig, John Wiley & Sons, New York, 1967, pp. 795–798 Search PubMed.
- AOAC, Official Methods of Analysis, Washington, DC, 11th edn, 1975 Search PubMed.
- L. C. Duarte, F. Carvalheiro, S. Lopes, S. Marques, J. C. Parajo and F. M. Girio, Appl. Biochem. Biotechnol., 2004, 113, 1041–1058 CrossRef.
- D. Fengel and G. Wegener, Wood: chemistry, ultrastructure, reactions, de Gruyter, 1983 Search PubMed.
- A. Romani, G. Garrote, J. L. Alonso and J. C. Parajo, Bioresour. Technol., 2010, 101, 8706–8712 CrossRef CAS PubMed.
- F. Carvalheiro, M. P. Esteves, J. C. Parajo, H. Pereira and F. M. Girio, Bioresour. Technol., 2004, 91, 93–100 CrossRef CAS.
- L. Canilha, V. T. O. Santos, G. J. M. Rocha, J. B. A. E. Silva, M. Giulietti, S. S. Silva, M. G. A. Felipe, A. Ferraz, A. M. F. Milagres and W. Carvalho, J. Ind. Microbiol. Biotechnol., 2011, 38, 1467–1475 CrossRef CAS PubMed.
- M. J. Negro, P. Manzanares, J. M. Oliva, I. Ballesteros and M. Ballesteros, Biomass. Bioenerg., 2003, 25, 301–308 CrossRef CAS.
- J. R. Elliott and C. T. Lira, Introductory chemical engineering thermodynamics, Prentice Hall PTR Upper Saddle River, NJ, 1999 Search PubMed.
- T. Gamse, R. Marr, F. Froschl and M. Siebenhofer, Sep. Sci. Technol., 1997, 32, 355–371 CrossRef CAS.
- C. A. Perakis, E. C. Voutsas, K. G. Magoulas and D. P. Tassios, Ind. Eng. Chem. Res., 2007, 46, 932–938 CrossRef CAS.
- B. Rumpf, J. Z. Xia and G. Maurer, Ind. Eng. Chem. Res., 1998, 37, 2012–2019 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |