Superparamagnetic imposed diatom frustules for the effective removal of phosphates†
Jeremiah
Toster
a,
Izella
Kusumawardani
a,
Ela
Eroglu
ab,
K. Swaminathan
Iyer
a,
Federico
Rosei
c and
Colin L.
Raston
*d
aCentre for Strategic Nano-fabrication, School of Chemistry and Biochemistry, University of Western Australia, Crawley, WA 6009, Australia. E-mail: swaminatha.iyer@uwa.edu.au; Fax: +61 08 6488 1005; Tel: +61 08 6488 4470
bARC Centre of Excellence in Plant Energy Biology, The University of Western Australia M313, 35 Stirling Highway, Crawley, WA 6009, Australia
cCentre Energie, Materiaux et Telecommunications, Institut National de la Recherche Scientifique, 1650 Boul. Lionel Boulet, J3X1S2, Varennes, QC, Canada. E-mail: rosei@emt.inrs.ca; Fax: +1 (450) 929 8102; Tel: +1 (514) 228 8246
dSchool of Chemical and Physical Sciences, Flinders University, Bedford Park, SA 5042, Australia. E-mail: colin.raston@flinders.edu.au; Tel: +61 08 8021 7958
First published on 22nd October 2013
Abstract
Diatom frustules coated with magnetite nanoparticles 21 nm in size were synthesised without the requirement of stabilisers, or prior functionalisation of the frustule surface, with the resulting material exhibiting superparamagnetic behaviour, and with a high capacity for phosphate ion adsorption.
Nanomaterials have captured the attention of the wider scientific community due to their novel properties which differ to those for the same material in the bulk phase.1,2 They have wide ranging applications including electronics, photonics, energy, sensors and biotechnology.3 Magnetic nanoparticles such as magnetite, maghemite, cobalt and nickel have found use in bearings, seals, lubricants, printing and recording.4,5 A rapidly developing area featuring magnetic nanoparticles is in the medical field where they find applications as contrast enhancement agents for magnetic resonance imaging (MRI) and in targeted drug delivery, biological separation and magnetic hyperthermia therapy.5–9 Such applications usually require the nanoparticles to be superparamagnetic, less than 20 nm in diameter, and with a narrow size distribution so that uniform physical and chemical properties are attained.10 At this dimension, the size is comparable to that of viruses (20–450 nm), proteins (5–50 nm) and genes (2 nm wide and 10–100 nm long), which effectively allows the particles to travel freely through the body and get into close proximity of the target area.11 Iron oxide compounds in general are also well suited for water treatment and are highly effective at removing arsenate (AsO43−) and phosphate ions (PO43−).12 In addition it was established that iron oxides greatly reduce solubilised and particulate metal concentrations when added to standard sand filters.13
Superparamagnetic iron oxide compounds are most commonly prepared via wet chemical routes which are simpler, more energy efficient and allow greater control over size, composition and sometimes even shape, relative to other methods such as gas phase deposition and electron beam lithography.14 The control is achieved by adjusting the type of salt used (sulphates, chlorides, nitrates or perchlorates), Fe2+ and Fe3+ ratios and pH.14 Control can also be imposed for the synthesis of magnetite nanoparticles in water, using continuous flow processing involving a spinning disc and using ammonia gas as the source of base.15 Agglomeration of superparamagnetic nanoparticles can be a problem, since it results in a loss of magnetic properties and decrease of surface activity.11 This can be overcome by incorporating them in polymers or the use of charged surfactants.16,17
Attaching dispersed magnetite nanoparticles to larger structures circumvents agglomeration and the use of polymer stabilisers or surfactants. To this end, we developed a process for attaching superparamagnetic nanoparticles of magnetite to the hierarchical porous structure of diatom frustules. Diatoms are photosynthetic unicellular algae that possess a 3-dimensional porous exoskeleton comprised of silica, known as a frustule.18 These frustules provide an inexpensive avenue for access to complex 3-dimensional structures with a high level of precision at the nanoscale.18 Diatoms frustules have found use as abrasives, filters and polishes, but for their more advanced applications, functionalisation with more technological relevant materials is required. Previous work has reported the frustules coated with gold, titania and manganese oxides.18–21 Magnetite has been coated with silica, usually for imparting stability; at neutral pH magnetite has an isoelectric point which results in agglomeration, but on coating with silica the isoelectric point moves to ca. pH 3.22 We have avoided agglomeration of magnetite nanoparticles by immobilising them onto the silica surface of the frustule. The frustules of the diatoms vary in size from 1 μm to 1 mm with pore sizes ranging from less than 50 nm to 1 μm. There are many thousands of different diatom species, and the size and shape of the frustules is species specific.23 Losic et al. modified magnetite nanoparticles with dopamine to enhance their binding affinity to the frustule surface of diatoms for the purpose of drug delivery and Xiong and Peng functionalised the surface with a ferrihydrate material for phosphorous removal.24,25 Herein we have functionalised the surface of diatoms with pristine magnetite nanoparticles, thereby imparting a new property to an already highly effective filtration material.
Diatom frustules were cleaned by washing with water and filtering, followed by plasma treatment. This effectively removes any organic residues that may be present on the silica surface, without apparent damage to the frustule structure.18 The cleaned frustules (80 mg) were then dispersed in water and purged with argon to remove any dissolved oxygen, which minimises the likelihood of oxidation of Fe2+ in solution. The magnetite nanoparticles were synthesised according to eqn (1), using stock solution of Fe3+ and Fe2+ (via dissolution of FeCl3·6H2O (87.5 mg) and FeCl2·4H2O (35 mg)) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in 200 mL Milli-Q water. A portion (40 mL) of this solution was added to the diatom suspension (see ESI† for experimental details). Passing argon through an aqueous ammonia solution (5 mL of 30% ammonia added to 95 mL of DI water) then into the reaction mixture resulted in the controlled growth of magnetite nanoparticles on the surface of the diatom frustules. Ammonia rapidly dissolves in the reaction mixture in generating a basic solution. This relates to the use of ammonia gas for the controlled growth of magnetite in dynamic thin films on the surface of a rapidly rotating spinning disc.15 The overall synthesis is well streamlined, using iron chloride precursors and aqueous ammonia as the base, which is preferable to the use of monoethanolamine.26 No other materials are used to control the oxidation state of the iron compounds such as hydrazine or hydrogen peroxide and the synthesis is conducted at room temperature, making it a relatively green and facile method for the synthesis of superparamagnetic nanomaterials.27
1 ratio in 200 mL Milli-Q water. A portion (40 mL) of this solution was added to the diatom suspension (see ESI† for experimental details). Passing argon through an aqueous ammonia solution (5 mL of 30% ammonia added to 95 mL of DI water) then into the reaction mixture resulted in the controlled growth of magnetite nanoparticles on the surface of the diatom frustules. Ammonia rapidly dissolves in the reaction mixture in generating a basic solution. This relates to the use of ammonia gas for the controlled growth of magnetite in dynamic thin films on the surface of a rapidly rotating spinning disc.15 The overall synthesis is well streamlined, using iron chloride precursors and aqueous ammonia as the base, which is preferable to the use of monoethanolamine.26 No other materials are used to control the oxidation state of the iron compounds such as hydrazine or hydrogen peroxide and the synthesis is conducted at room temperature, making it a relatively green and facile method for the synthesis of superparamagnetic nanomaterials.27
| Fe2+ + 2Fe3+ + 8OH− → Fe3O4 + 4H2O | (1) |
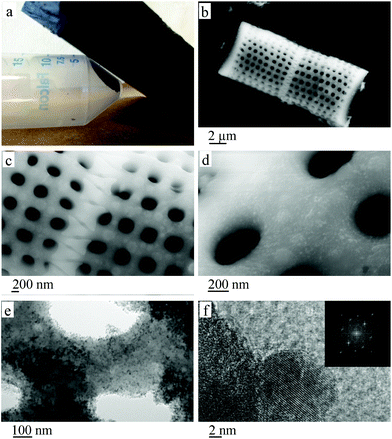
Powder X-ray diffraction (XRD), established the material to be magnetite, with the Scherrer equation used to calculate an average particle size of 21 nm (see ESI†) for the magnetite, which is slightly larger than the size of the particles derived from electron microscopy size distribution data (see ESI†) which showed an average particle size of 14 nm. This difference can be attributed to the proportion of observable particles using transmission electron microscopy (TEM), with the thickness of the diatom frustule blocking the electron beam image, except for the particles on the edges and around the pores. Very large particles would strongly affect the average results acquired from the Scherrer equation. TEM and scanning electron microscopy (SEM) show a dense covering of the surface of the frustule with nanoparticles ca. ∼20 nm in diameter. These were confirmed as magnetite nanoparticles using high resolution TEM (Fig. 1(f)), as established using the XRD pattern (see ESI†). The major peaks present in the XRD pattern as presented in Table 1, correspond to the planes of magnetite.28 Moreover the diffraction pattern acquired from a fast Fourier transform (FFT) of the HR-TEM image (Fig. 1(f)) matches the d-spacings estimated from the XRD, as well as closely matching literature values.29
| 2θ (°) | hkl | Calculated d-spacing | JCPDS data d-spacing (no. 19-0629) |
|---|---|---|---|
| 29.99° | (220) | 2.98 | 2.97 |
| 35.51° | (311) | 2.53 | 2.53 |
| 43.01° | (400) | 2.10 | 2.10 |
| 53.77° | (422) | 1.70 | 1.71 |
| 57.02° | (511) | 1.62 | 1.62 |
| 62.79° | (440) | 1.48 | 1.48 |
Composites of silica and magnetite have a number of potential interactions, which relate to covalent Si–O–Fe linkages, electrostatic interactions between negatively charged Si–O terminal ligands and positively charged surface groups on the particles, or hydrogen bonding interactions between the hydration layers of silanol groups and the particle surface.30 In the present study covalent bonding is not evident, with no FT-IR bands (ESI†) characteristic of Si–O–Fe moieties at 900 or 680 cm−1.30 Rather a strong oxide–oxide interaction is likely on supporting magnetite nanoparticles on silica, as reported by Lund and Dumesic.31 The interaction between silica and magnetite is described as a core shell model. Here Si4+ substitutes for Fe3+ in the tetrahedral sites on the surface of the interface,31 while the core region of the particle is undisturbed magnetite. This results in the displacement of Fe3+ into neighbouring octahedral sites with electron hoping throughout the particle.31 Attaching magnetite nanoparticles to the silica frustule results in the frustules being drawn towards a magnetic field, as shown in Fig. 1(a). While the magnetite coating is dense enough to imbue the whole material with magnetic properties, the physical properties of the frustules such as pore structure remain intact, as seen in Fig. 1(d) and (e). The approximately 20 nm diameter particles have very little effect on the often micron sized pores of the diatom. However if the magnetite layer were to be increased, the affect on pore structure could become an issue. The magnetite nanoparticles on the surface are pristine, being devoid of any polymer or other stabiliser coatings. Such coatings are often required to increase blood half-life times for biological applications, but in the present context, this becomes optional rather than required to avoid agglomeration.32
SQUID results establish that the magnetite nanoparticles on the surface of the silica frustules are readily magnetised by an external magnetic field. Fig. 2(a) is consistent with (a) superparamagnetic behaviour with the zero field curve and the field curve converging, and (b) that there is a small particle size distribution with the two curves having a consistent separation until converging. The hysteresis curve in Fig. 2(b) is consistent with superparamagnetic behaviour, with a maximum saturation magnetisation of 36.4 emu g−1.
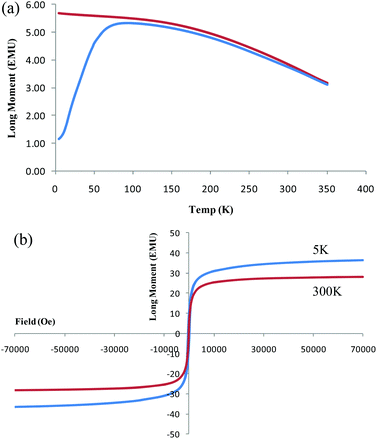 | ||
| Fig. 2 (a) Zero field cooled curve and (b) hysteresis loop at 5 K and 300 K for magnetite coated frustules acquired from superconducting quantum interference device (SQUID). | ||
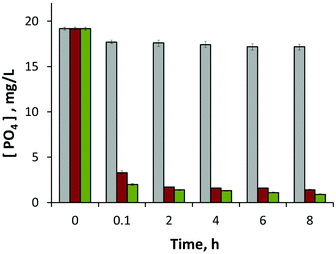
Phosphate removal from aqueous media by magnetite coated diatom frustules, as well as magnetite nanoparticles and diatom (frustules) devoid of such particles, are presented in Fig. 3. Although the latter are capable of adsorbing some phosphate ions, the presence of magnetite dramatically improves removal of phosphate ions, both for magnetite particles on the surface, and for suspensions of magnetite nanoparticles only, almost with the same efficacy. The samples are normalised by using an equal amount of magnetite (magnetite only sample) or plasma cleaned frustules (frustule devoid of magnetite sample) relative to the magnetite coated frustule material. Starting from the beginning of the experiment (ca. within first 5 minutes), magnetite containing samples had high affinity to remove phosphate ions from their liquid media. After 8 hours of treatment, the magnetite coated frustules had an overall phosphate removal rate of 95.3% as opposed to 10.4% for the diatom frustules only sample. This shows more than 9 fold increase in phosphate removal by incorporating magnetite on the surface of the frustules. In addition to the adsorption of 18.3 mg L−1 phosphate at the end of the first cycle, magnetite coated frustules yielded further adsorption upon consecutive recycling stages. This resulted in an overall adsorption value of 50.9 mg L−1 which corresponds to an adsorption capacity of 4.89 mg of phosphate per gram of magnetite coated frustules. As shown in Fig. 1, the 3D porous structure of the diatoms remains unaffected after coating with magnetite indicating that it can still be effectively used as a filtration material.
In summary, we have established a facile method of effectively coating diatom frustules with pristine magnetite nanoparticles of 21 nm in size, without the need for stabilisers or chemical surface modification of either the magnetite or the diatom frustules. We have shown this material to be superparamagnetic with a maximum saturation magnetisation of 36.4 emu g−1, and a highly effective additional property to diatomaceous earth filters, with a significant increase of phosphate removal relative to diatom frustules, from 10.4% to 95.3%. This process offers a benign and cost effective process for waste water treatment, to remove deleterious phosphate ions, with the ability to separate the composite active material using a magnet. In addition, the material has potential for removal of other species from aqueous systems, including isoelectronic arsenate ions, solubilised metals and metal particulates.
Support of this work by the Australian Research Council and The University of Western Australia is greatly appreciated. F.R. is grateful to the Institute of Advanced Studies of UWA for a Professor at Large award (2010–2012). F.R acknowledges partial salary support from the Canada Research Chairs program.
Notes and references
- Y.-H. Deng, C.-C. Wang, J.-H. Hu, W.-L. Yang and S.-K. Fu, Colloids Surf., A, 2005, 262, 87–93 CrossRef CAS PubMed.
- F. Rosei, J. Phys.: Condens. Matter, 2004, 16, S1373–S1436 CrossRef CAS.
- C. R. Martin, Science, 1994, 266, 1961–1966 CAS.
- D. Weller and M. F. Doerner, Annu. Rev. Mater. Sci., 2000, 30, 611–644 CrossRef CAS.
- T. J. Yoon, K. N. Yu, E. Kim, J. S. Kim, B. G. Kim, S. H. Yun, B. H. Sohn, M. H. Cho, J. K. Lee and S. B. Park, Small, 2006, 2, 209–215 CrossRef CAS PubMed.
- I. Willner and E. Katz, Angew. Chem., Int. Ed., 2003, 42, 4576–4588 CrossRef CAS PubMed.
- K. A. Hinds, J. M. Hill, E. M. Shapiro, M. O. Laukkanen, A. C. Silva, C. A. Combs, T. R. Varney, R. S. Balaban, A. P. Koretsky and C. E. Dunbar, Blood, 2003, 102, 867–872 CrossRef CAS PubMed.
- P. S. Doyle, J. Bibette, A. Bancaud and J. L. Viovy, Science, 2002, 295, 2237–2237 CrossRef CAS PubMed.
- O. Veiseh, J. W. Gunn and M. Zhang, Adv. Drug Delivery Rev., 2010, 62, 284–304 CrossRef CAS PubMed.
- S. H. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204–8205 CrossRef CAS PubMed.
- J. L. Zhang, R. S. Srivastava and R. D. K. Misra, Langmuir, 2007, 23, 6342–6351 CrossRef CAS PubMed.
- H. Zeng, B. Fisher and D. E. Giammar, Environ. Sci. Technol., 2008, 42, 147–152 CrossRef CAS.
- M. M. Benjamin, R. S. Sletten, R. P. Bailey and T. Bennett, Water Res., 1996, 30, 2609–2620 CrossRef CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef CAS PubMed.
- S. F. Chin, K. S. Iyer, C. L. Raston and M. Saunders, Adv. Funct. Mater., 2008, 18, 922–927 CrossRef CAS.
- G. Marcelo, A. Munoz-Bonilla, J. Rodriguez-Hernandez and M. Fernandez-Garcia, Polym. Chem., 2013, 4, 558–567 RSC.
- R. Massart, IEEE Trans. Magn., 1981, 17, 1247–1248 CrossRef.
- J. Toster, C. Harnagea, K. S. Iyer, F. Rosei and C. L. Raston, CrystEngComm, 2012, 14, 3446–3450 RSC.
- J. Toster, K. S. Iyer, R. Burtovyy, S. S. O. Burgess, I. A. Luzinov and C. L. Raston, J. Am. Chem. Soc., 2009, 131, 8356 CrossRef CAS PubMed.
- M. A. M. Khraisheh, Y. S. Al-Degs and W. A. M. McMinn, Chem. Eng. J., 2004, 99, 177–184 CrossRef CAS PubMed.
- J. Toster, K. S. Iyer, W. Xiang, F. Rosei, L. Spiccia and C. L. Raston, Nanoscale, 2013, 5, 873–876 RSC.
- R. A. Caruso and M. Antonietti, Chem. Mater., 2001, 13, 3272–3282 CrossRef CAS.
- M. Sumper, Science, 2002, 295, 2430–2433 CrossRef CAS PubMed.
- D. Losic, Y. Yu, M. S. Aw, S. Simovic, B. Thierry and J. Addai-Mensah, Chem. Commun., 2010, 46, 6323–6325 RSC.
- W. Xiong and J. Peng, Water Res., 2008, 42, 4869–4877 CrossRef CAS PubMed.
- B. Wang, Q. Wei and S. Qu, Int. J. Electrochem. Sci., 2013, 8, 3786–3793 CAS.
- R. S. Sapieszko and E. Matijević, J. Colloid Interface Sci., 1980, 74, 405–422 CrossRef CAS.
- T. Prozorov, S. K. Mallapragada, B. Narasimhan, L. Wang, P. Palo, M. Nilsen-Hamilton, T. J. Williams, D. A. Bazylinski, R. Prozorov and P. C. Canfield, Adv. Funct. Mater., 2007, 17, 951–957 CrossRef CAS.
- Z. Li, L. Wei, M. Y. Gao and H. Lei, Adv. Mater., 2005, 17, 1001–1005 CrossRef CAS.
- C. Chaneac, E. Tronc and J. P. Jolivet, J. Mater. Chem., 1996, 6, 1905–1911 RSC.
- C. R. F. Lund and J. A. Dumesic, J. Phys. Chem., 1981, 85, 3175–3180 CrossRef CAS.
- Y. Zhang, N. Kohler and M. Q. Zhang, Biomaterials, 2002, 23, 1553–1561 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, XRD data and FT-IR data. See DOI: 10.1039/c3gc41608a |
| This journal is © The Royal Society of Chemistry 2014 |