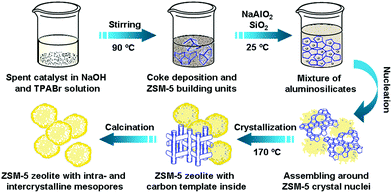
Facile fabrication of mesopore-containing ZSM-5 zeolite from spent zeolite catalyst for methanol to propylene reaction†
Qing
Zhang
,
Si
Hu
,
Lanlan
Zhang
,
Zhijie
Wu
,
Yanjun
Gong
* and
Tao
Dou
*
State Key Laboratory of Heavy Oil Processing, The Key Laboratory of Catalysis of CNPC, China University of Petroleum, Beijing 102249, China. E-mail: gongyj@cup.edu.cn; doutao@cup.edu.cn; Fax: +86 10 8973 4979; Tel: +86 10 8973 3066
First published on 26th September 2013
Abstract
A facile and eco-friendly approach for the synthesis of mesopore-containing ZSM-5 zeolite with small crystal size was proposed by subtly making use of a coke-deposited spent zeolite catalyst. When used in the methanol to propylene reaction again, the refabricated ZSM-5 catalyst exhibited a much higher propylene selectivity and a longer catalytic lifetime.
Zeolites have been widely applied in the petrochemical industry, adsorption and separation fields. In particular, ZSM-5 zeolite as an important shape-selective catalyst is extensively used in various catalytic processes, such as naphtha catalytic cracking, isomerization, alkylation, and methanol to hydrocarbons reaction, etc.1–3 Beyond all doubt, with the rapid development of the chemical industry, large amounts of spent zeolite catalysts are produced every year worldwide. However, these waste catalysts are mostly disposed of in landfills or are used as additives in the production of construction materials, which could lead to a serious environmental problem and a large waste of valuable materials.4,5 Considering its crystalline phase and the main chemical composition, this spent catalyst could be potentially used for the synthesis of high-value silicon/aluminum products, such as zeolite. Thus, the cost of the zeolite catalyst will be reduced greatly, and the related environmental pollution would be alleviated effectively, rendering the zeolite-based catalytic reaction an environmentally-friendly process.
The methanol to propylene (MTP) process, originally developed by Lurgi (the Lurgi MTP® process), is an important technology for the specific production of propylene based on the zeolite catalyst. Since the first industrial MTP unit was put into commercial operation at the Shenhua Group in China,6 much attention has been paid to the process from the scientific and application viewpoints, including enhancement of the MTP process and the development of a high-efficiency catalyst.7–10 Currently, the high-silica ZSM-5 zeolite is a well-known catalyst and it plays a critical role in the high yield of propylene and long catalytic lifetime of the MTP process. Extensive researches have indicated that the MTP reaction is susceptible to diffusion limitation, and precise matching of acidity, crystal size and the pore properties of the ZSM-5 zeolite is the key to optimizing its catalytic performance.10–14 Since the acidity of a zeolite can be easily adjusted by varying the silica/alumina ratio in the synthesis process, creation of mesopores and control of the crystal size over ZSM-5 zeolites by a one-step method has aroused much interest.15,16 The synthesis of mesopore-modified ZSM-5 zeolite is crucially completed by using both a structure-directing agent (i.e., tetrapropylammonium bromide, TPABr) and a mesopore-generating template. So far, various carbonaceous materials have been well-worked as meso-scale templates, e.g., carbon black particles,17 carbon aerogels,18 mesoporous carbon,19etc. However, the preparation of carbon templates is mostly complicated, energy-intensive and high cost, which limits the application of mesopore-containing ZSM-5 zeolites on the large scale.
It is well known that methanol conversion over ZSM-5 zeolite is a typical solid-acid catalytic reaction, and the deactivation of the MTP catalyst is dominated by coke deposition.20 The coke species are mainly excessively dehydrogenated hydrocarbons, such as polycyclic aromatics, high molecular weight polymeric carbon, amorphous carbon and so on.20,21 As the coke species are formed gradually in the channels or on the surface of the zeolite during reaction, their dimension size is limited by spatial constraints in the meso-scale. Besides, these carbonaceous materials are inert at low temperature and could easily be burned off at calcination temperatures. This inspired us to utilize the coke deposition in the spent catalyst as a hard template for the synthesis of ZSM-5 zeolite with developed intracrystalline mesopores. Meanwhile, as the spent zeolite catalyst is rich in silica and alumina, it can provide the partial raw material for the synthesis of the zeolite. Based on the above concept, a facile and green strategy for the fabrication of mesopore-containing ZSM-5 zeolite is proposed, as shown in Scheme 1. Herein, the spent catalyst is a fully deactivated MTP catalyst, whose parent is a conventional, high-silica ZSM-5 zeolite which has been repeatedly regenerated until the initial methanol conversion was lower than 50 wt%. The final coke content in the spent catalyst is 11.30 wt% (by thermogravimetric analysis, TG, Fig. S1 in the ESI†), and the mass fraction of SiO2 and Al2O3 is 88.15% and 0.448%, respectively (by X-ray fluorescence analysis, XRF, Table S1 in the ESI†). X-ray diffraction (XRD) results show that the ZSM-5 crystal phase is partially retained in the spent catalyst, and the crystallinity is 64.1% relative to its fresh counterpart (Table S2 in the ESI†).
In a typical synthesis, the spent catalyst was pretreated firstly under stirring in an aqueous solution of sodium hydroxide (NaOH) and TPABr at 90 °C for 6 h to obtain a well dispersed black gel. Then, additional deionized water, along with a quantitative amount of sodium aluminate (NaAlO2) and silica gel (SiO2) were added successively amounting to the desired SiO2/Al2O3 and C/SiO2. Finally, the mixture gel was crystallized at 170 °C for 28–48 h. The resulting zeolite product was obtained by centrifugation, washing and drying. A reasonable amount of intercrystalline and intracrystalline pores would be created after removal of the coke deposition together with TPABr by calcination. As the silica and alumina in the spent catalyst are supposed to be involved in the process of zeolite crystallization, the composition (e.g., SiO2/Al2O3 ratio) and mesoporosity of the final zeolite product could be modulated easily through adjusting the additive amount of spent catalyst during the synthesis. In this work, two ZSM-5 samples with the same SiO2/Al2O3 ratio but different C/SiO2 proportions were synthesized by using different amounts of spent catalyst (25 wt% and 50 wt% of total SiO2 in the gel), which are denoted as MZ-1 and MZ-2, respectively. To gain a better understanding of how the spent catalyst and its “coke” species worked in the crystallization of the ZSM-5 zeolite, two control samples were synthesized without and with coke-free spent catalyst, respectively. The former was denoted as CZ-1, and the latter was denoted as CZ-2. For the synthesis of CZ-2, the same amount of spent catalyst as MZ-2 was used, but its coke deposition was removed beforehand. Thus, the final mixture gel of all the samples has the same molar composition of 350SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3
Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52Na2O
52Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25TPABr
25TPABr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5400H2O, but with the differing coke content.
5400H2O, but with the differing coke content.
The crystallization process for synthesis of the ZSM-5 zeolite was monitored by the XRD technique. As seen in Fig. 1a, all four samples exhibit sharp reflections for the ZSM-5 zeolite, indicating that the resulting zeolites are well crystallized and of pure phase. The synthesis parameters and physicochemical properties of the as-synthesized ZSM-5 zeolites are listed in Table 1. Compared to the CZ-1 sample, the optimal crystallization time for CZ-2, MZ-1 and MZ-2 samples is greatly shortened from the conventional 48 h to 28 h. This is because the remaining zeolite crystals in the spent catalyst could provide plenty of secondary building units for the ZSM-5 zeolite after pretreatment in NaOH and TPABr solution (Fig. S2 in the ESI†), which would serve as crystal nuclei to “direct” the crystallization of the amorphous aluminosilicate into the zeolite phase. Thus, using the spent catalyst, the growth of the ZSM-5 crystals was promoted to a large extent. This is in good agreement with previous research.22,23 Reding et al. have made a detailed study on the synthesis of nano-crystalline ZSM-5 zeolite by using seed crystals,23 and showed that with the increase in seed concentration, the growth of the ZSM-5 zeolite was undoubtedly accelerated. Besides, from Table 1, it can be also found that the product yield of CZ-2, MZ-1 and MZ-2 samples is noticeably higher than that of the sample CZ-1 (ca. 90% vs. 75.2%). However, the SiO2/Al2O3 ratios of the four samples are rather similar, close to the theoretical value in the precursor mixture. This indicates that the spent catalyst could participate well in the refabrication of the ZSM-5 zeolite, and that the SiO2/Al2O3 ratio of the ZSM-5 product could be well controlled using our approach.
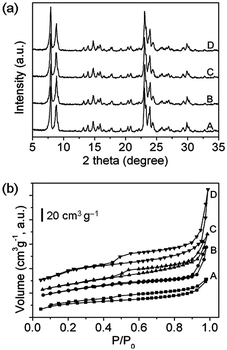 | ||
| Fig. 1 XRD patterns (a) and N2 adsorption–desorption isotherms (b) of the control samples CZ-1 (A), CZ-2 (B) and the mesopore-containing ZSM-5 zeolites MZ-1 (C), MZ-2 (D). | ||
| Sample | Spent catalysta (wt% of SiO2) | C/SiO2 ratiob | Crystallization time/h | Yieldc (%) | SiO2/Al2O3 ratiod | S BET/m2 g−1 | V total/cm3 g−1 | V meso/cm3 g−1 |
|---|---|---|---|---|---|---|---|---|
| a The mass ratio of SiO2 offered by the spent catalyst to the total SiO2 in the synthesis gel. b The molar ratio of coke to the total SiO2 in the synthesis gel, and the molecular weight of the coke deposition is based on a C/H ratio of 1.25.14 c Product yield calculated by the mass ratio of the calcined solid product to SiO2 and Al2O3 in the synthesis gel. d SiO2/Al2O3 molar ratio of the synthesized ZSM-5 zeolite determined by XRF. | ||||||||
| CZ-1 | 0 | 0 | 48 | 75.2 | 330 | 332 | 0.22 | 0.07 |
| CZ-2 | 50 | 0 | 28 | 91.4 | 337 | 387 | 0.28 | 0.12 |
| MZ-1 | 25 | 0.12 | 32 | 86.5 | 334 | 366 | 0.29 | 0.14 |
| MZ-2 | 50 | 0.24 | 28 | 89.8 | 336 | 371 | 0.38 | 0.24 |
Fig. 1b shows the N2 adsorption–desorption isotherms of the four samples. As seen, the MZ-1 and MZ-2 samples synthesized with the involvement of the spent catalyst give clear hysteresis loops as well as a steep increase in their adsorption curves at the relative pressure of 0.4–1.0, indicating the presence of an appreciable amount of mesopores in the resulting zeolites.24 Generally speaking, the hysteresis loop in the range of P/P0 = 0.4–0.8 is attributed to the capillary condensation of mesopores with narrow distribution, corresponding to the most probable distribution at the pore diameter of ca. 2 nm (Fig. S3 in the ESI†), whereas at higher pressure (P/P0 > 0.8), it is usually associated with the existence of larger mesopores, corresponding to the broad distribution of pore sizes at 20–40 nm (Fig. S3 in the ESI†).25 The two kinds of pore models will be further clarified by transmission electron microscopy (TEM) images in the following section. Quantitatively, from the textural parameters summarized in Table 1, distinctly higher specific surface areas (366–387 m2 g−1) and pore volumes (0.28–0.38 cm3 g−1) are found for the MZ-1, MZ-2 and CZ-2 samples as compared to the control sample CZ-1 (332 m2 g−1 and 0.22 cm3 g−1, respectively). And the total pore volume depends on the amount of carbonaceous material added to the precursor gel during the synthesis. In particular, the MZ-2 sample synthesized with a higher C/SiO2 ratio of 0.24 exhibits an extremely high BET surface area (371 m2 g−1), as well as a markedly large pore volume (0.38 cm3 g−1). Considering that the micropore volume of all samples is around 0.15 cm3 g−1, the increase of total pore volume should be attributed to the presence of mesopores (0.24 cm3 g−1). Meanwhile, the significantly increased mesopores in the MZ-1 and MZ-2 samples are conducive to improving their diffusion properties, which would offer an additional benefit in catalysis.
Fig. 2 displays the scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the four ZSM-5 zeolites. All of the samples exhibit almost spherical-like bodies with a uniform distribution but different crystal sizes. Compared to the sample CZ-1, the other three samples synthesized using the spent catalyst show a rather smaller crystal size, and the crystal surface appears to be clear and smooth. Particularly for the samples CZ-2 and MZ-2 obtained with higher additive amounts of spent catalyst, their average crystal size is as small as about 150 nm (Fig. 2c and 2g). This could be attributed to the addition of spent catalyst, by which numerous secondary building units or micro crystal nuclei of the ZSM-5 zeolite were produced after the pretreatment process. Thus, the nucleation of ZSM-5 crystals was greatly promoted, giving rise to an obvious decrease in the crystal size of the synthesized zeolites.26 More interestingly, many light spots with diameters ranging from two to tens of nanometers could be observed clearly in the interior of the MZ-1 and MZ-2 crystals (Fig. 2f and 2h). These bright spots are randomly distributed throughout the whole crystal, and their diameters are in line with the results of the pore size distribution determined from the N2 adsorption analysis. However, the samples synthesized without the spent catalyst (CZ-1) or with spent catalyst that is free of carbon deposits (CZ-2) show the conventional morphology of the ZSM-5 zeolite without visible voids (Fig. 2b and 2d). Thus, those bright areas in the MZ-1 and MZ-2 crystals could be recognized as the intracrystalline mesopores. It is important to point out that the templating efficiency for casting mesopores will work best only if the released coke deposition is well dispersed within the zeolite precursor during the synthesis process, which could be achieved through controlling the pretreatment conditions. By comparing the results of the MZ-2 and CZ-2 samples (Fig. 2h and 2d), it suggests that the carbonaceous material in the spent catalyst has a significant templating effect on creating the intracrystalline pores within the zeolite crystal. Taken together, the above results further confirmed that the abundant mesoporosity in the MZ-1 and MZ-2 samples is largely related to the intracrystalline voids imprinted by the coke species from the spent catalyst and the secondary porosity contributed by nanoparticles of the ZSM-5 zeolite.
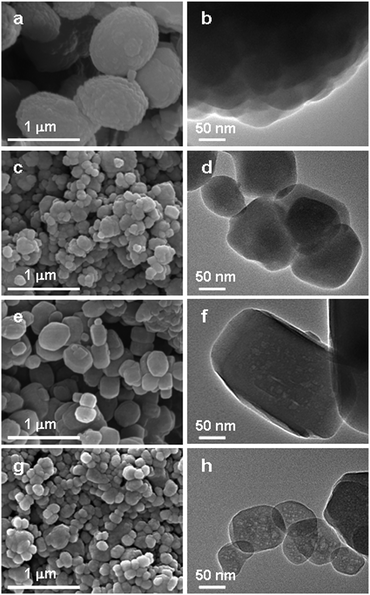 | ||
| Fig. 2 SEM images (left) and the corresponding TEM images (right) of the control samples CZ-1 (a,b), CZ-2 (c,d) and mesopore-containing ZSM-5 zeolites MZ-1 (e,f), MZ-2 (g,h). | ||
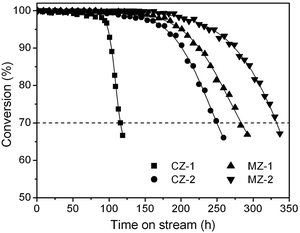
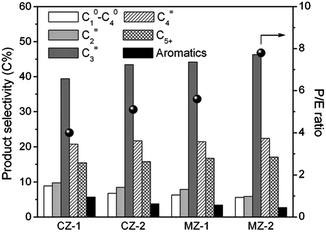
The catalytic performance of the as-synthesized mesopore-containing ZSM-5 zeolites for the MTP reaction was investigated under atmospheric pressure at 470 °C and weight hourly space velocity (WHSV) of 2 h−1. All the samples present almost 100% methanol conversion in the initial stages, but the mesopore-containing ZSM-5 zeolites MZ-1 and MZ-2 are prominent for long catalytic lifetime (Fig. 3) and excellent propylene selectivity (Fig. 4 and Fig. S4 in the ESI†). Especially for the MZ-2 sample synthesized with 50 wt% added amount of spent catalyst, its catalytic lifetime is nearly three times longer than that of the CZ-1 sample, and its average selectivity toward propylene reaches as high as 46.3%, seven percentage points higher than that of the CZ-1 catalyst. Also, its aromatic selectivity is only 2.7%, less than half that of the CZ-1 catalyst, which is conducive to inhibiting the generation of coke deposition in the zeolite. More evidently, the MZ-2 catalyst gives a markedly higher propylene to ethylene (P/E) ratio (7.8 on average), which is a very important index parameter in the MTP process.9,10 Since the four samples are of similar acidity (Fig. S5 in the ESI†), the outstanding MTP catalytic performance of the MZ-2 catalyst, in terms of propylene selectivity and catalytic lifetime, could be ascribed to its smaller particle size and incorporated mesopores. Owing to its larger mesopore volume than that of the conventional CZ-1 sample, the product molecules can more easily escape from the zeolite channels, and thus the formation of heavy aromatics, which will eventually lead to coking, is greatly reduced. Hence, the enhanced diffusion properties of the mesopore-containing ZSM-5 zeolites would have a great effect upon optimizing its product distribution in the MTP reaction.
It should be illustrated here that the parent catalyst employed in this study gives a similar performance to the CZ-1 sample, in terms of lifetime and product distribution in the MTP reaction (Fig. S6 and Table S3 in the ESI†). Hence, the refabricated ZSM-5 zeolite exhibits a more excellent catalytic performance than its parent counterpart. Additionally, if a higher proportion of spent catalyst is used as a raw material, high-crystallinity ZSM-5 zeolite can be obtained by optimizing the synthesis conditions, and its SiO2/Al2O3 ratio can also be extended over a large range to meet the various reactions. Further studies will be contributed in the near future.
In summary, ZSM-5 zeolites with controllable mesoporosity were successfully synthesized by using a spent MTP catalyst as part of the silica/alumina raw material. The attached coke species in the spent catalyst play a key role in creating intracrystalline mesopores for the resulting zeolite. The remaining zeolite crystals in the spent catalyst could act as seeds to promote crystallization of the ZSM-5 zeolite, and bring about an obvious increase in product yield and decrease in the crystal size. More importantly, compared to the parent sample, the refabricated ZSM-5 catalyst exhibited a 3-fold increase in catalytic lifetime and a seven percentage point rise in propylene selectivity in the MTP reaction. From the green chemistry perspective, the synthesis approach we report here turns out to be simple and cost-effective, and thus is easy to be applied on the large scale. In general, this work makes an attractive opportunity for the production of a high-value zeolite product from spent zeolite catalyst, and especially sheds light on recycling of the MTP catalyst in the future.
Acknowledgements
This work was supported by the State Key Development Program for Basic Research of China (2012CB215002), and the National Natural Science Foundation of China (21276278 and 21176255).Notes and references
- A. Corma, Chem. Rev., 1997, 97, 2373–2419 CrossRef CAS PubMed.
- W. Vermeiren and J. P. Gilson, Top. Catal., 2009, 52, 1131–1161 CrossRef CAS.
- T. Li, H. Liu, Y. Fan, P. Yuan, G. Shi, X. T. Bi and X. Bao, Green Chem., 2012, 14, 3255–3259 RSC.
- E. Furimsky, Catal. Today, 1996, 30, 223–286 CrossRef CAS.
- X. Liu, L. Li, T. Yang and Z. Yan, J. Porous Mater., 2012, 19, 133–139 CrossRef CAS.
- http://www.nxmy.com/mtjj/4208.htm .
- W. Wu, W. Guo, W. Xiao and M. Luo, Chem. Eng. Sci., 2011, 66, 4722–4732 CrossRef CAS PubMed.
- U. Olsbye, S. Svelle, M. Bjorgen, P. Beato, T. V. W. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810–5831 CrossRef CAS PubMed.
- S. Hu, Y. Gong, Q. Xu, X. Liu, Q. Zhang, L. Zhang and T. Dou, Catal. Commun., 2012, 28, 95–99 CrossRef CAS PubMed.
- C. Mei, P. Wen, Z. Liu, H. Liu, Y. Wang, W. Yang, Z. Xie, W. Hua and Z. Gao, J. Catal., 2008, 258, 243–249 CrossRef CAS PubMed.
- S. Hu, J. Shan, Q. Zhang, Y. Wang, Y. Liu, Y. Gong, Z. Wu and T. Dou, Appl. Catal., A, 2012, 445–446, 215–220 CrossRef CAS PubMed.
- M. Firoozi, M. Baghalha and M. Asadi, Catal. Commun., 2009, 10, 1582–1585 CrossRef CAS PubMed.
- C. Sun, J. Du, J. Liu, Y. Yang, N. Ren, W. Shen, H. Xu and Y. Tang, Chem. Commun., 2010, 46, 2671–2673 RSC.
- J. Kim, M. Choi and R. Ryoo, J. Catal., 2010, 269, 219–228 CrossRef CAS PubMed.
- Y. S. Tao, H. Kanoh, L. Abrams and K. Kaneko, Chem. Rev., 2006, 106, 896–910 CrossRef CAS PubMed.
- K. Na, M. Choi and R. Ryoo, Microporous Mesoporous Mater., 2013, 166, 3–19 CrossRef CAS PubMed.
- J.-B. Koo, N. Jiang, S. Saravanamurugan, M. Bejblová, Z. Musilová, J. Čejka and S.-E. Park, J. Catal., 2010, 276, 327–334 CrossRef CAS PubMed.
- Y. S. Tao, H. Kanoh and K. Kaneko, J. Am. Chem. Soc., 2003, 125, 6044–6045 CrossRef CAS PubMed.
- W. Fan, M. A. Snyder, S. Kumar, P.-S. Lee, W. C. Yoo, A. V. McCormick, R. L. Penn, A. Stein and M. Tsapatsis, Nat. Mater., 2008, 7, 984–991 CrossRef CAS PubMed.
- H. Schulz, Catal. Today, 2010, 154, 183–194 CrossRef CAS PubMed.
- S. He, C. Sun, X. Yang, B. Wang, X. Dai and Z. Bai, Chem. Eng. J., 2010, 163, 389–394 CrossRef CAS PubMed.
- Q. Xu, Y. Gong, W. Xu, J. Xu, F. Deng and T. Dou, J. Colloid Interface Sci., 2011, 358, 252–260 CrossRef CAS PubMed.
- G. Reding, T. Maurer and B. Kraushaar-Czarnetzki, Microporous Mesoporous Mater., 2003, 57, 83–92 CrossRef CAS.
- S.-T. Tsai, C.-H. Chen and T.-C. Tsai, Green Chem., 2009, 11, 1349–1356 RSC.
- H. Jin, M. B. Ansari and S.-E. Park, Chem. Commun., 2011, 47, 7482–7484 RSC.
- G. Majano, A. Darwiche, S. Mintova and V. Valtchev, Ind. Eng. Chem. Res., 2009, 48, 7084–7091 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details on the synthesis and characterization of zeolite, physicochemical properties of the spent catalyst, pore size distribution curves and NH3-TPD profiles of the as-synthesized ZSM-5 zeolites. See DOI: 10.1039/c3gc41327f |
| This journal is © The Royal Society of Chemistry 2014 |