 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Precious metal carborane polymer nanoparticles: characterisation of micellar formulations and anticancer activity
Nicolas P. E.
Barry
*a,
Anaïs
Pitto-Barry
a,
Isolda
Romero-Canelón
a,
Johanna
Tran
a,
Joan J.
Soldevila-Barreda
a,
Ian
Hands-Portman
b,
Corinne J.
Smith
b,
Nigel
Kirby
c,
Andrew P.
Dove
a,
Rachel K.
O'Reilly
a and
Peter J.
Sadler
*a
aDepartment of Chemistry, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK. E-mail: N.Barry@warwick.ac.uk; P.J.Sadler@warwick.ac.uk
bSchool of Life Sciences, University of Warwick, Gibbet Hill Road, Coventry CV4 7AL, UK
cAustralian Synchrotron, 800 Blackburn Road, Clayton, Victoria 3168, Australia
First published on 1st October 2014
Abstract
We report the encapsulation of highly hydrophobic 16-electron organometallic ruthenium and osmium carborane complexes [Ru/Os(p-cymene)(1,2-dicarba-closo-dodecarborane-1,2-dithiolate)] (1 and 2) in Pluronic® triblock copolymer P123 core–shell micelles. The spherical nanoparticles RuMs and OsMs, dispersed in water, were characterized by dynamic light scattering (DLS), cryogenic transmission electron microscopy (cryo-TEM), and synchrotron small-angle X-ray scattering (SAXS; diameter ca. 15 and 19 nm, respectively). Complexes 1 and 2 were highly active towards A2780 human ovarian cancer cells (IC50 0.17 and 2.50 μM, respectively) and the encapsulated complexes, as RuMs and OsMs nanoparticles, were less potent (IC50 6.69 μM and 117.5 μM, respectively), but more selective towards cancer cells compared to normal cells.
1 Introduction
Dicarba-closo-dodecarboranes are a class of boron-rich compounds with globular structures and diameters of ca. 1 nm (diameter of a rotating phenyl) that possess unusual properties, including high symmetry and remarkable stability.1 These characteristics have given rise to numerous applications, and carboranes have been used as building blocks in various systems, such as dendrimers,2 polymers3 and nanoparticles.4,5 They also have been extensively studied as potential boron neutron capture (BNCT) therapeutic agents,6,7 and as bioisosters of phenyl groups and pharmacophores for targeted drug development.8 Nevertheless, selective and effective delivery of boron agents is still a key issue that hinders their further clinical development.9 On the other hand, half-sandwich complexes of ruthenium and osmium are a versatile class of organometallic compounds. Their biological properties have raised considerable expectations for their use in the treatment of cancer since the early 2000s, and they are considered a promising alternative to platinum-based chemotherapeutics.10,11 We have recently discussed how the combination of arene ruthenium(II)/osmium(II) complexes and carboranes has unexplored potential in medicine.12 Furthermore, such complexes exhibit unusual chemistry: coordination of the bulky, electron-deficient carborane ligand 1,2-dicarba-closo-dodecarborane-1,2-dithiolato to an arene-Ru or Os metal centre leads to the isolation of rare stable 16-electron complexes, such as [Ru/Os(p-cymene)(1,2-dicarba-closo-dodecarborane-1,2-dithiolate)] (1 and 2). However, these complexes are highly hydrophobic, and their biological applications are impaired by the lack of solubility in water. We have recently discussed how nanotechnology may help to overcome such challenges in medicinal inorganic chemistry.13 Here, we report the encapsulation of these hydrophobic complexes in water-soluble polymer micelles.The class of ABA triblock copolymers, where A = hydrophilic block poly(ethylene oxide) (PEO) and B = hydrophobic block poly(propylene oxide) (PPO), are commercially available as Pluronic® (non-proprietary name “poloxamers”) and offer a pool of more than 50 amphiphilic,14 water-soluble and polymorphic materials. The physical and chemical properties of Pluronic® copolymers can be finely tuned by modifying the molar mass ratio between the PEO and PPO blocks (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 8
9 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), which directly modifies their in vivo properties and interactions with cells and cell membranes, thus providing high potential for the design of innovative nanomedicines and new biomaterials.15 To exploit the chemistry of carborane-containing arene ruthenium complexes in aqueous solution, and to take advantage of their unique properties, we have encapsulated the 16-electron complexes 1 and 2 in Pluronic® triblock copolymer P123 micelles (Fig. 1). The resulting polymeric micelles RuMs and OsMs, dispersed in water, have been characterized by UV-visible spectroscopy, DLS, cryo-TEM, and SAXS studies.
2), which directly modifies their in vivo properties and interactions with cells and cell membranes, thus providing high potential for the design of innovative nanomedicines and new biomaterials.15 To exploit the chemistry of carborane-containing arene ruthenium complexes in aqueous solution, and to take advantage of their unique properties, we have encapsulated the 16-electron complexes 1 and 2 in Pluronic® triblock copolymer P123 micelles (Fig. 1). The resulting polymeric micelles RuMs and OsMs, dispersed in water, have been characterized by UV-visible spectroscopy, DLS, cryo-TEM, and SAXS studies.
 | ||
| Fig. 1 Self-assembly formation of RuMs and OsMs (the red dots in 1 and 2 are boron–hydrogen vertices). | ||
Complexes 1 and 2 and micelles RuMs and OsMs were tested against the A2780 human ovarian cancer cell line. The ruthenium-based complex and micelles were also tested against MRC5 fibroblasts, as an example of non-cancerous, but fast dividing, cells to determine their selectivity and potential therapeutic window. Such a nanotechnology-based strategy not only allows the utilisation of precious metal complexes containing carborane ligands in aqueous solution, but also offers the possibility of formulating and delivering a large number of these complexes (ca. 60 per particle) to cancer cells, thus generating a high intracellular boron concentration (ca. 600 per particle). There is currently much interest in the therapeutic use of boron-based pharmaceuticals, owing to their chemico-biological properties, to expand potential medical applications in prevention, diagnosis and therapy.16
2 Characterisation of the micellar formulations RuMs and OsMs
The organometallic half-sandwich RuII and OsII arene complexes [Ru/Os(p-cymene)(1,2-dicarba-closo-dodecarborane-1,2-dithiolate)] (1/2) were synthesized as previously reported.17 These complexes have a pseudo-octahedral structure, with a π-bonded arene occupying 3 coordination sites, a S-bound chelated dithiolato dicarba-closo-dodecarborane ligand, and a vacant 6th site (Fig. 1). They are 16-electron complexes and therefore electron-deficient at the metal.18 Complexes 1 and 2 are highly hydrophobic and insoluble in water.8 To achieve dispersion in water,13 we encapsulated 1 and 2 in the water-soluble amphiphilic triblock copolymer P123 (poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol)) (PEO-PPO-PEO), by mixing a tetrahydrofuran (THF) solution of 1 or 2 with an aqueous solution of polymer P123 (THF–H2O: 1/10 v/v; [complex] = 5 mg mL−1), at ambient temperature for 4 h (Fig. 1). The encapsulation of the metal complexes in P123 micelles (P123Ms) was accompanied by a dramatic colour change from transparent to blue and purple for RuMs and OsMs, respectively, indicative of the presence of the 16-electron complexes 1 and 2 in the micelles. We have recently reported the full characterisation of OsMs micelles,19 and now present the characterisation of micellar formulation RuMs, in comparison with previous data obtained on OsMs micelles.Dynamic light scattering (DLS) experiments (Fig. 2) unambiguously demonstrated that polymer P123 and complex 1 and 2 self-assemble in aqueous solution. Encapsulation decreased the micellar size (hydrodynamic diameter) of P123 from 19.6 ± 1.8 nm to 11.9 ± 4.1 nm for RuMs and 11.5 ± 2.3 nm for OsMs with dispersities (Đ) of 0.04 and 0.03, respectively. Although micellar size usually increases after encapsulation of organic molecules, incorporation of hydrophobic molecules can result in expulsion of water from micelles, causing a contraction.20 The hydrophobicity of 1 and 2 probably results in a stronger folding of the PPO chains around the complexes through hydrophobic interactions, with concomitant expulsion of water from the core. A small-in-number (<0.01% in number) second population of RuMs and OsMs particles is found at Dh ∼ 220 nm, which exhibits a strong intensity in DLS and is due to aggregation of some particles.
Cryo-TEM analysis without staining was performed on Quantifoil® carbon-coated grids, in order to observe the morphology of the nanoparticles in solution. The high contrast provided by the presence of the heavy ruthenium and osmium centres allowed facile imaging without staining (Fig. 3). From these analyses, it was clear that spherical micellar morphologies are formed when polymer P123 encapsulates complexes 1 or 2. The observed diameters of these nano-spheres are 10.45 ± 3.43 nm for RuMs and 7.85 ± 1.97 nm for OsMs with very low dispersities (<1.12). These data are in accordance with the hydrodynamic diameters determined by DLS, within experimental error.
To gain further insight into their structures in aqueous solution, and to confirm cryo-TEM and DLS results, RuMs, OsMs and P123Ms were analyzed by SAXS (Fig. 4). The experimental profiles were fitted using IgorPro software21 to three model functions for spherical micelles: SphereForm,22 CoreShellSphere,22 and PolyCoreShellRatio23 (PCR). The PCR model fitted excellently for all micelles with very low dispersity parameters (0.124 for RuMs, 0.161 for OsMs and 0.146 for P123Ms, 0 being an ideal mono-disperse system, Table 1). These analyses demonstrated that RuMs and OsMs self-assembly leads to core–shell micelles with a core diameter of 9.88 ± 0.25 nm and 9.06 ± 0.12 nm, and a shell diameter of 9.09 ± 0.29 nm and 6.50 ± 0.15 nm, respectively (Table 1). The diameters of RuMs and OsMs micelles determined by DLS and cryo-TEM are in accordance with the core diameter determined by SAXS within experimental error. From these data (scattering length density calculations, degrees of polymerization of Pluronic® P123, and the molecular formulae of the polymer and of complex 1/2), aggregation numbers for RuMs, OsMs and P123Ms micelles were determined as 20 ± 2 monomer chains per P123Ms micelle, 66 ± 4 monomer chains per RuMs micelles and 52 ± 6 monomer chains per OsMs micelles. Determinations of ruthenium and osmium by inductively coupled plasma mass spectrometry (ICP-MS) gave a polymer/complex 1 ratio of 1/0.9 ± 0.11 for RuMs and 1/1 ± 0.09 for OsMs showing that the 66 polymer chains self-assembled with 59 ± 14 complexes 1 in RuMs and 52 monomer chains self-assembled with 52 ± 11 complexes 2 (see Table 1).
 | ||
| Fig. 4 Small-angle X-ray scattering (SAXS) experimental profiles and fitting with the PolyCoreShellRatio model of micelles (A) P123Ms, (B) RuMs, (C) OsMs: 1 mg mL−1 aqueous solutions. | ||
| Parameter | Micelles | ||
|---|---|---|---|
| RuMs | OsMs | P123Ms | |
| a Data from ref. 19. b Not determined. | |||
| Aggregation number | 66 ± 4 | 52 ± 6 | 20 ± 2 |
| Ru/Os complexes per micelle | 59 ± 14 | 52 ± 11 | 0 |
| DLS diameter (nm) | 11.9 ± 4.1 | 11.5 ± 2.3 | 19.6 ± 1.8 |
| DLS dispersity | 0.04 | 0.03 | 0.03 |
| Cryo-TEM diameter (nm) | 10.45 ± 3.43 | 7.85 ± 1.97 | ndb |
| Cryo-TEM dispersity | 1.11 | 1.06 | ndb |
| SAXS total diameter (nm) | 18.97 ± 0.54 | 15.56 ± 0.27 | 18.96 ± 0.23 |
| SAXS core diameter (nm) | 9.88 ± 0.25 | 9.06 ± 0.12 | 6.74 ± 0.06 |
| SAXS shell diameter (nm) | 9.09 ± 0.29 | 6.50 ± 0.15 | 12.22 ± 0.17 |
| SAXS dispersity | 0.124 | 0.161 | 0.146 |
3 Anticancer activity of complexes 1 and 2 and micellar formulations RuMs and OsMs
Synthetic polymer therapeutics are of particular interest in medicine, due to their synthetic versatility, as well as their tunable properties.24 A number of biologically active polymer–drug conjugates and polymeric formulations, such as micelles, hydrogels and polymer-coated nanoparticles, are currently in clinical development.25 Among the most commonly used polymers for applications in medicine are the ABA Pluronic® triblock block copolymers, which are particularly suitable for the design of bio-inspired, bioengineered and biomimetic polymer nanoparticles. The utilisation of Pluronic® block copolymers as drug delivery systems,26–31 biological response modifiers,32–36 pharmaceutical ingredients,27,37,38 and steric stabilizers to lyotropic liquid crystalline particles39–41 has led to recent clinical advances.15Inorganic compounds offer different mechanisms of drug action depending on the metal used, their structures and their redox properties.11,42–85 They can this be utilized for the design of novel drugs in the treatment of a broad range of diseases,86 and their combination with nanotechnology tools, such as polymer nanoparticles, may provide opportunities for tackling medical challenges in the near future.13 Surprisingly, only a few studies have been devoted to the encapsulation of inorganic compounds (mainly cisplatin) in Pluronic® block copolymer-based nanostructures.87,88
We studied the antiproliferative activity of complexes 1, 2 and micelles RuMs and OsMs in A2780 human ovarian cancer cells (Table 2). Cells were exposed for 24 h to the complexes (dissolved in 5% dimethyl sulfoxide (DMSO)/95% saline and further diluted in cell culture medium until working concentrations in the range of 100–0.01 μM were achieved with a maximum DMSO concentration of 1%) or micelles (100% saline, further diluted with cell culture medium to a range of concentration 200–0.10 μM). After this, drugs were removed and cells were washed and placed in fresh growth medium for a further 72 h as a recovery period. Cell viability was then assessed using the sulforhodamine B (SRB) colorimetric assay. Complex 1 is highly potent (IC50 170 nM), about 7× more potent than the clinical drug cisplatin. Complex 1 is also 39× more potent than RuMs micelles, which still exhibit good (micromolar) activity towards cancer cells and are as potent as the clinical drug carboplatin. Interestingly, RuMs micelles are 4× more selective than 1, showing only moderate toxicity towards MRC5-fibroblast cells. Finally, the formulation of complexes 1 and 2 in polymer micelles has allowed dispersal of hydrophobic ruthenium and osmium arene complexes in water in a manner suitable for administration to cancer cells (administration to cancer cells in water, without the need to add DMSO).
4 Conclusion
We have encapsulated highly hydrophobic carborane-containing precious metal complexes in triblock copolymer micelles. This has allowed dispersal of hydrophobic ruthenium and osmium arene complexes in water in a manner suitable for administration to cancer cells. Although entrapment of the 16-electron complexes 1 and 2 in Pluronic® micelles leads to a reduction in their anticancer potency, the micelles exhibit enhanced selectivity towards cancer cells compared to normal cells. Polymer encapsulation of metal carborane complexes also provides the potential for delivering high amounts of boron to cells, which is of interest for boron neutron capture therapy (BNCT). The neutron capture ability of such micelles is currently under investigation.5 Experimental section
Materials
The preparation of the complexes [Ru/Os(p-cym)(1,2-dicarba-closo-dodecaborane-1,2-dithiolato)] (1 and 2) was based on a previous report.17 The preparation of the OsMs micelles was based on a previous report.19 The triblock copolymer P123 [poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol)] was purchased from Sigma-Aldrich and used as received. Anhydrous tetrahydrofuran (Aldrich) was used. 18.2 mega-ohm purity water was collected from a Purelab® UHQ USF Elga system. Holey carbon grids with 200 mesh and lacey carbon grids were purchased from Quantifoil Micro Tools Gmbh and Elektron Technology UK Ltd, respectively, and used as received.Synthesis of RuMs
A tetrahydrofuran (THF) solution (1 mL) of complex 1 (5 mg mL−1) was added to an aqueous solution (10 mL) of polymer P123 (5 mg mL−1) and the resultant mixture was stirred at ambient temperature for 4 h. The solution was then dialyzed to remove the THF (MWCO = 1000 Da), for 48 h, and then freeze-dried to give RuMs. A similar procedure was used for synthesizing OsMs with 1 mol equiv. of the Os analogue and 1 mol equiv. of polymer P123.Instrumentation
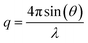 | (1) |
The scattering from a blank (H2O) was measured in the same location as sample collection and was subtracted for each measurement. Data were normalized for total transmitted flux using a quantitative beamstop detector and absolute scaled using water as an absolute intensity standard. The two-dimensional SAXS images were converted in one-dimensional SAXS profiles (I(q) versus q) by circular averaging, where I(q) is the scattering intensity. Functions were used from the NCNR package. Scattering length densities were calculated using the “Scattering Length Density Calculator” provided by NIST Center for Neutron Research.
Acknowledgements
We thank the Leverhulme Trust (Early Career Fellowship no. ECF-2013-414 to NPEB), the University of Warwick (Grant no. RDF 2013-14 to NPEB), the Swiss National Science Foundation (Grant no. PA00P2_145308 to NPEB and PBNEP2_142949 to APB), the ERC (Grant no. 247450 to PJS), EPSRC (EP/G004897/1 to APB, and EP/F034210/1 to PJS), Institute of Advanced Study (IAS) – University of Warwick (Fellowship to JJSB), and Science City (AWM/ERDF) for support. We thank the Wellcome Trust (055663/Z/98/Z) for funding to the Electron Microscopy Facility, School of Life Sciences, University of Warwick. We also thank COST Action CM1105 for stimulating discussions, Thomas Wilks for supplying the micelle image for Fig. 1, Dr Magdalena Moss for technical asssistance with the cell culture, the Australian Synchrotron and the University of Monash for allocation of beamtime on the SAXS/WAXS beamline and funding.Notes and references
- Z. J. Lesnikowski, Collect. Czech. Chem. Commun., 2007, 72, 1646–1658 CrossRef CAS.
- R. Djeda, J. Ruiz, D. Astruc, R. Satapathy, B. P. Dash and N. S. Hosmane, Inorg. Chem., 2010, 49, 10702–10709 CrossRef CAS PubMed.
- K. Kokado, M. Tominaga and Y. Chujo, Macromol. Rapid Commun., 2010, 31, 1389–1394 CrossRef CAS PubMed.
- D. C. Kennedy, D. R. Duguay, L.-L. Tay, D. S. Richeson and J. P. Pezacki, Chem. Commun., 2009, 6750–6752 RSC.
- J. F. Valliant, K. J. Guenther, A. S. King, P. Morel, P. Schaffer, O. O. Sogbein and K. A. Stephenson, Coord. Chem. Rev., 2002, 232, 173–230 CrossRef CAS.
- W. Tjarks, R. Tiwari, Y. Byun, S. Narayanasamy and R. F. Barth, Chem. Commun., 2007, 4978–4991 RSC.
- I. B. Sivaev and V. V. Bregadze, Eur. J. Inorg. Chem., 2009, 2009, 1433–1450 CrossRef.
- M. Scholz and E. Hey-Hawkins, Chem. Rev., 2011, 111, 7035–7062 CrossRef CAS PubMed.
- S. Korbe, P. J. Schreiber and J. Michl, Chem. Rev., 2006, 106, 5208–5249 CrossRef PubMed.
- G. Süss-Fink, Dalton Trans., 2010, 39, 1673–1688 RSC.
- A. M. Pizarro, N. P. E. Barry and P. J. Sadler, in Comprehensive Inorganic Chemistry II, ed. J. Reedijk, Elsevier, 2013, vol. 3, pp. 752–784 Search PubMed.
- N. P. E. Barry and P. J. Sadler, Chem. Soc. Rev., 2012, 41, 3264–3279 RSC.
- N. P. E. Barry and P. J. Sadler, ACS Nano, 2013, 7, 5654–5659 CrossRef CAS PubMed.
- http://worldaccount.basf.com/wa/NAFTA/Catalog/ChemicalsNAFTA/pi/BASF/Brand/pluronic .
- A. Pitto-Barry and N. P. E. Barry, Polym. Chem., 2014, 5, 3291–3297 RSC.
- M. A. Soriano-Ursúa, B. C. Das and J. G. Trujillo-Ferrara, Expert Opin. Ther. Pat., 2014, 24, 485–500 CrossRef PubMed.
- M. Herberhold, H. Yan and W. Milius, J. Organomet. Chem., 2000, 598, 142–149 CrossRef CAS.
- N. P. E. Barry, T. F. Kemp, P. J. Sadler and J. V. Hanna, Dalton Trans., 2014, 43, 4945–4949 RSC.
- N. P. E. Barry, A. Pitto-Barry, A. M. Sanchez, A. P. Dove, R. J. Procter, J. J. Soldevila-Barreda, N. Kirby, I. Hands-Portman, C. J. Smith, R. K. O’Reilly, R. Beanland and P. J. Sadler, Nat. Commun., 2014, 5, 3851 Search PubMed.
- A. Parmar, V. K. Aswal and P. Bahadur, Spectrochim. Acta, Part A, 2012, 97, 137–143 CrossRef CAS PubMed.
- S. Kline, J. Appl. Crystallogr., 2006, 39, 895–900 CrossRef CAS.
- A. Guinier and G. Fournet, Small-angle scattering of X-rays, Wiley, New York, 1955 Search PubMed.
- J. B. Hayter, in Physics of Amphiphiles–Micelles, Vesicles, and Microemulsions, ed. V. DeGiorgio and M. Corti, North-Holland Publishing Company, 1983, pp. 59–93 Search PubMed.
- A. R. Kirtane and J. Panyam, Nat. Nanotechnol., 2013, 8, 805–806 CrossRef CAS PubMed.
- O. C. Steinbach, Ther. Delivery, 2014, 5, 113–118 CrossRef CAS.
- D. Y. Alakhova, Y. Zhao, S. Li and A. V. Kabanov, PLoS One, 2013, 8, e72238 CAS.
- E. V. Batrakova, S. Li, A. M. Brynskikh, A. K. Sharma, Y. Li, M. Boska, N. Gong, R. L. Mosley, V. Y. Alakhov, H. E. Gendelman and A. V. Kabanov, J. Controlled Release, 2010, 143, 290–301 CrossRef CAS PubMed.
- E. V. Batrakova and A. V. Kabanov, J. Controlled Release, 2008, 130, 98–106 CrossRef CAS PubMed.
- T. Minko, E. V. Batrakova, S. Li, Y. Li, R. I. Pakunlu, V. Y. Alakhov and A. V. Kabanov, J. Controlled Release, 2005, 105, 269–278 CrossRef CAS PubMed.
- A. V. Kabanov, E. V. Batrakova and V. Y. Alakhov, J. Controlled Release, 2002, 82, 189–212 CrossRef CAS.
- E. V. Batrakova, S. Li, W. F. Elmquist, D. W. Miller, V. Y. Alakhov and A. V. Kabanov, Br. J. Cancer, 2001, 85, 1987–1997 CrossRef CAS PubMed.
- D. Y. Alakhova, N. Y. Rapoport, E. V. Batrakova, A. A. Timoshin, S. Li, D. Nicholls, V. Y. Alakhov and A. V. Kabanov, J. Controlled Release, 2010, 142, 89–100 CrossRef CAS PubMed.
- E. V. Batrakova, S. Li, V. Y. Alakhov, W. F. Elmquist, D. W. Miller and A. V. Kabanov, Pharm. Res., 2003, 20, 1581–1590 CrossRef CAS.
- E. Batrakova, S. Li, V. Alakhov, D. Miller and A. Kabanov, J. Pharmacol. Exp. Ther., 2003, 304, 845–854 CrossRef CAS PubMed.
- A. Kabanov, E. Batrakova and V. Alakhov, Adv. Drug Delivery Rev., 2002, 54, 759–779 CrossRef CAS.
- A. Venne, S. Li, R. Mandeville, A. Kabanov and V. Alakhov, Cancer Res., 1996, 56, 3626–3629 CAS.
- E. R. Gariepy and J. C. Leroux, Eur. J. Pharm. Biopharm., 2004, 58, 409–426 CrossRef PubMed.
- I. R. Schmolka, Poloxamers in the Pharmaceutical Industry, CRC Press, Boca Ratin FL, 1991 Search PubMed.
- J. Y. T. Chong, X. Mulet, L. J. Waddington, B. J. Boyd and C. J. Drummond, Soft Matter, 2011, 7, 4768–4777 RSC.
- K. W. Lee, T. H. Nguyen, T. Hanley and B. J. Boyd, Int. J. Pharm., 2009, 365, 190–199 CrossRef CAS PubMed.
- B. J. Boyd, Y. D. Dong and T. Rades, J. Liposome Res., 2009, 19, 12–28 CrossRef CAS PubMed.
- Z. Liu and P. J. Sadler, Acc. Chem. Res., 2014, 47, 1174–1185 CrossRef CAS PubMed.
- Y. Zhao, J. A. Woods, N. J. Farrer, K. S. Robinson, J. Pracharova, J. Kasparkova, O. Novakova, H. Li, L. Salassa, A. M. Pizarro, G. J. Clarkson, L. Song, V. Brabec and P. J. Sadler, Chem.–Eur. J., 2013, 19, 9578–9591 CrossRef CAS PubMed.
- N. A. Smith and P. J. Sadler, Philos. Trans. R. Soc., A, 2013, 371, 1995 CrossRef PubMed.
- I. Romero-Canelón, L. Salassa and P. J. Sadler, J. Med. Chem., 2013, 56, 1291–1300 CrossRef PubMed.
- I. Romero-Canelón and P. J. Sadler, Inorg. Chem., 2013 Search PubMed.
- Z. Liu, I. Romero-Canelón, B. Qamar, J. M. Hearn, A. Habtemariam, N. P. E. Barry, A. M. Pizarro, G. J. Clarkson and P. J. Sadler, Angew. Chem., Int. Ed., 2014, 53, 3941–3946 CrossRef CAS PubMed.
- Y. Fu, R. Soni, M. J. Romero, A. M. Pizarro, L. Salassa, G. J. Clarkson, J. M. Hearn, A. Habtemariam, M. Wills and P. J. Sadler, Chem.–Eur. J., 2013, 19, 15199–15209 CrossRef CAS PubMed.
- J. Schulz, J. Tauchman, I. Císařová, T. Riedel, P. J. Dyson and P. Štěpnička, J. Organomet. Chem., 2014, 751, 604–609 CrossRef CAS PubMed.
- A. A. Nazarov, C. G. Hartinger and P. J. Dyson, J. Organomet. Chem., 2014, 751, 251–260 CrossRef CAS PubMed.
- K. J. Kilpin, S. Crot, T. Riedel, J. A. Kitchen and P. J. Dyson, Dalton Trans., 2014, 43, 1443–1448 RSC.
- C. M. Clavel, E. Paunescu, P. Nowak-Sliwinska and P. J. Dyson, Chem. Sci., 2014, 5, 1097–1101 RSC.
- K. J. Kilpin and P. J. Dyson, Chem. Sci., 2013, 4, 1410–1419 RSC.
- G. Gupta, A. Garci, B. S. Murray, P. J. Dyson, G. Fabre, P. Trouillas, F. Giannini, J. Furrer, G. Suss-Fink and B. Therrien, Dalton Trans., 2013, 42, 15457–15463 RSC.
- F. Giannini, J. Furrer, G. Süss-Fink, C. M. Clavel and P. J. Dyson, J. Organomet. Chem., 2013, 744, 41–48 CrossRef CAS PubMed.
- A. Leonidova, V. Pierroz, R. Rubbiani, J. Heier, S. Ferrari and G. Gasser, Dalton Trans., 2014, 43, 4287–4294 RSC.
- T. Joshi, V. Pierroz, C. Mari, L. Gemperle, S. Ferrari and G. Gasser, Angew. Chem., Int. Ed., 2014, 53, 2960–2963 CrossRef CAS PubMed.
- M. Patra, T. Joshi, V. Pierroz, K. Ingram, M. Kaiser, S. Ferrari, B. Spingler, J. Keiser and G. Gasser, Chem.–Eur. J., 2013, 19, 14768–14772 CrossRef CAS PubMed.
- M. Patra, K. Ingram, A. Leonidova, V. Pierroz, S. Ferrari, M. N. Robertson, M. H. Todd, J. Keiser and G. Gasser, J. Med. Chem., 2013, 56, 9192–9198 CrossRef CAS PubMed.
- M. Patra, J. Hess, S. Konatschnig, B. Spingler and G. Gasser, Organometallics, 2013, 32, 6098–6105 CrossRef CAS.
- P. K. Sasmal, C. N. Streu and E. Meggers, Chem. Commun., 2013, 49, 1581–1587 RSC.
- Y. Xiang, C. Fu, T. Breiding, P. K. Sasmal, H. Liu, Q. Shen, K. Harms, L. Zhang and E. Meggers, Chem. Commun., 2012, 48, 7131–7133 RSC.
- A. L. Merkel, E. Meggers and M. Ocker, Expert Opin. Ther. Pat., 2012, 21, 425–436 CAS.
- A. Kastl, A. Wilbuer, A. L. Merkel, L. Feng, P. Di Fazio, M. Ocker and E. Meggers, Chem. Commun., 2012, 48, 1863–1865 RSC.
- E. Meggers, Angew. Chem., Int. Ed., 2011, 50, 2442–2448 CrossRef CAS PubMed.
- K. D. Mjos and C. Orvig, Chem. Rev., 2014, 114, 4540–4563 CrossRef CAS PubMed.
- M. A. Telpoukhovskaia and C. Orvig, Chem. Soc. Rev., 2013, 42, 1836–1846 RSC.
- Y. L. K. Tan, P. Pigeon, S. Top, E. Labbe, O. Buriez, E. A. Hillard, A. Vessieres, C. Amatore, W. K. Leong and G. Jaouen, Dalton Trans., 2012, 41, 7537–7549 RSC.
- G. Sava, G. Jaouen, E. A. Hillard and A. Bergamo, Dalton Trans., 2012, 41, 8226–8234 RSC.
- A. Vessières, C. Corbet, J. M. Heldt, N. Lories, N. Jouy, I. Laïos, G. Leclercq, G. Jaouen and R.-A. Toillon, J. Inorg. Biochem., 2010, 104, 503–511 CrossRef PubMed.
- J. W. Yi, N. P. E. Barry, M. A. Furrer, O. Zava, P. J. Dyson, B. Therrien and B. H. Kim, Bioconjugate Chem., 2012, 23, 461–471 CrossRef CAS PubMed.
- A. Pitto-Barry, O. Zava, P. J. Dyson, R. Deschenaux and B. Therrien, Inorg. Chem., 2012, 51, 7119–7124 CrossRef CAS PubMed.
- N. P. E. Barry, O. Zava, W. Wu, J. Zhao and B. Therrien, Inorg. Chem. Commun., 2012, 18, 25–28 CrossRef CAS PubMed.
- N. P. E. Barry, O. Zava, P. J. Dyson and B. Therrien, J. Organomet. Chem., 2012, 705, 1–6 CrossRef CAS PubMed.
- G. S. Smith and B. Therrien, Dalton Trans., 2011, 40, 10793–10800 RSC.
- A. Pitto-Barry, N. P. E. Barry, O. Zava, R. Deschenaux and B. Therrien, Chem.–Asian J., 2011, 6, 1595–1603 CrossRef CAS PubMed.
- A. Pitto-Barry, N. P. E. Barry, O. Zava, R. Deschenaux, P. J. Dyson and B. Therrien, Chem.–Eur. J., 2011, 17, 1966–1971 CrossRef CAS PubMed.
- L. E. H. Paul, B. Therrien and J. Furrer, Inorg. Chem., 2011, 51, 1057–1067 CrossRef PubMed.
- N. P. E. Barry, O. Zava, J. Furrer, P. J. Dyson and B. Therrien, Dalton Trans., 2010, 39, 5272–5277 RSC.
- N. P. E. Barry, O. Zava, P. J. Dyson and B. Therrien, Aust. J. Chem., 2010, 63, 1529–1537 CrossRef CAS.
- N. P. E. Barry, F. Edafe, P. J. Dyson and B. Therrien, Dalton Trans., 2010, 39, 2816–2820 RSC.
- G. Gupta, J. Mahesh Kumar, A. Garci, N. Rangaraj, N. Nagesh and B. Therrien, ChemPlusChem, 2014, 79, 610–618 CrossRef CAS.
- M. Pernot, N. P. E. Barry, T. Bastogne, C. Frochot, M. Barberi-Heyob and B. Therrien, Inorg. Chim. Acta, 2014, 414, 134–140 CrossRef CAS PubMed.
- G. Gupta, B. Murray, P. Dyson and B. Therrien, Materials, 2013, 6, 5352–5366 CrossRef CAS PubMed.
- P. Chellan, K. M. Land, A. Shokar, A. Au, S. H. An, D. Taylor, P. J. Smith, T. Riedel, P. J. Dyson, K. Chibale and G. S. Smith, Dalton Trans., 2014, 43, 513–526 RSC.
- N. P. E. Barry and P. J. Sadler, Chem. Commun., 2013, 49, 5106–5131 RSC.
- A. Sonoda, N. Nitta, S. Ohta, A. Nitta-Seko, S. Morikawa, Y. Tabata, M. Takahashi and K. Murata, CardioVasc. Interventional Radiol., 2010, 33, 135–142 CrossRef PubMed.
- J.-Y. Fang, S.-H. Hsu, Y.-L. Leu and J.-W. Hu, J. Biomater. Sci., Polym. Ed., 2009, 20, 1031–1047 CrossRef CAS PubMed.
- S. Dhar and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 22199–22204 CrossRef CAS PubMed.
- R. E. Aird, J. Cummings, A. A. Ritchie, M. Muir, R. E. Morris, H. Chen, P. J. Sadler and D. I. Jodrell, Br. J. Cancer, 2002, 86, 1652–1657 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |


