Surface functional groups and defects on carbon nanotubes affect adsorption–desorption hysteresis of metal cations and oxoanions in water†
Jie
Li
ab,
Changlun
Chen
*a,
Shouwei
Zhang
a and
Xiangke
Wang
*ab
aKey Laboratory of Novel Thin Film Solar Cells, Institute of Plasma Physics, Chinese Academy of Sciences, P.O. Box 1126, Hefei, 230031, PR China
bSchool for Radiological and Interdisciplinary Sciences (RAD-X), Soochow University, Suzhou 215123, PR China. E-mail: clchen@ipp.ac.cn; xkwang@ipp.ac.cn; Tel: +86 551 65592788
First published on 10th July 2014
Abstract
This study investigated the influence of the structure characteristics of carbon nanotubes (CNTs), such as surface oxygen-containing functional groups, specific surface area (SSA) and concentration of defects, on the adsorption–desorption hysteresis of a metal cation (Cu(II)) and two oxoanions (As(V) and Cr(VI)), from single, double and multi-walled CNTs (SWCNTs, DWCNTs and MWCNTs), and two oxidized MWCNTs with different oxygen concentrations (MWCNTs-O1, 2.51 wt% O and MWCNTs-O2, 3.5 wt% O). Oxygen-containing functional groups contributed to an increase in the adsorption capacity for Cu(II) from aqueous solutions, but a decrease in adsorption capacity for Cr(VI) and As(V). The order of adsorption capacities based on CNT SSA was MWCNTs-O2 > MWCNTs-O1 > MWCNTs > DWCNTs > SWCNTs, which was consistent with the order of CNT defect contents. Desorption hysteresis index (HI) values for Cu(II) increased as the number of functional groups increased. For Cr(VI) and As(V), however, HI values decreased as the number of functional groups increased. HI values decreased with an increase in metal ion surface coverage on CNTs. There may be a shift in the mechanisms of metal ion adsorption by CNTs, from more irreversible to more reversible processes, with an increase in adsorbed metal ions. An understanding of the desorption hysteresis of heavy metal ions is important and useful for the application and risk assessment of CNTs in the natural environment.
Nano impactAn understanding of the adsorption–desorption hysteresis of metal cations and anions from CNTs is important and useful for the application and risk assessment of CNTs. This work probes the influence of the structural properties of CNTs, such as surface functional groups, specific surface area and concentration of defects, on their adsorption–desorption hysteresis of metal ions. It compares the differences in adsorption behavior and possible hysteresis phenomena of metal cations and anions. |
Introduction
The potential applications of carbon nanotubes (CNTs) vary from power hand-held devices1 to drug delivery,2 flexible conductive thin films, cell regeneration and 3D scaffolds for tissue.3,4 Because of their large specific surface area (SSA) and hollow and layered structure, CNTs have already been investigated as promising sorbents for various organic pollutants, metal ions and radionuclides.5–12 The increase in the extensive applications of, and commercial interest in, CNTs will result in their subsequent mass production, thus creating greater possibilities for interactions with the environment and human beings.13 Because of their small size, they can enter into cells, causing damage to plants, animals and humans.14–16 The primary risk of CNTs originates from their toxicity, which results from the adsorbed harmful pollutants.17 To some extent, mobile CNTs may also carry adsorbates to ecological or biological receptors, and they can effectively act as a “Trojan Horse”,11 potentially transporting harmful pollutants to places that they cannot otherwise reach through adsorption and desorption. Metal ions represent a significant risk to ecosystems, but can also be public health hazards, inducing conditions such as low blood pressure, diarrhea, paralysis, lung irritation and bone defects.18 Therefore, it is necessary to consider the synergistic or antagonistic interactions of metal ions and CNTs, in order to understand the toxicology and environmental impacts of CNTs for potential applications.The study of the adsorption–desorption behavior of harmful pollutants from CNTs is motivated by the need to understand the toxicity of CNTs and to assess potential environmental risks. As the reverse process of adsorption, desorption of toxic pollutants from CNTs makes harmful pollutants mobile and bioavailable in aqueous environments, and thus the toxicity of both the pollutants and the CNTs remain a threat.19 Desorption hysteresis, which is generally shown with the desorption isotherm branch above the adsorption isotherm branch, includes reversible and irreversible hysteresis. Irreversible hysteresis indicates that a fraction of the adsorbed pollutants remain on the CNTs. Desorption hysteresis of organic pollutants from carbon nano-materials has been studied.20–26
Depending on their layers, there exist two distinct types of CNTs; single walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs). The outermost surface, inner cavities, interstitial channels, and grooves of CNTs constitute the four possible types of adsorption site.20 The adsorption capacity of CNTs will be affected by their physicochemical characteristics (such as the number of walls, surface functional groups, SSA and defects). CNTs are extremely hydrophobic and prone to aggregation into bundles. Aggregation results in a reduction in the SSA, which has a potential negative impact on adsorption.27 The interstitial channels and grooves on the periphery of the nanotube bundles have a potential positive impact, resulting in greater adsorption per mass.28
The surface functional groups on CNTs, such as carboxyl, phenol and lactone groups, affect their interactions with specific or polar adsorbates.29 Defective sites within CNTs could be responsible for the sensitivity of the nanotubes to adsorbates, thus affecting the adsorption ability of CNTs.30 Zhang et al.31 studied the adsorption behavior of aromatic compounds on CNTs , and found that the adsorption capacity depended upon the inner cavities and structure of the CNT bundles. Wu et al.20 proposed that the adsorption behavior of organic compounds was closely related to the oxygen-containing groups of CNTs. Feng et al.32 calculated that adsorbed ammonia was located on top of the tube and in the vicinity of defect sites, using the self-consistent charge density functional tight binding method. However, neither CNT surface area and surface functional groups, nor defects alone, can be used to fully explain CNT adsorption characteristics. In order to understand the adsorption of metal ions by CNTs, their structural characteristics must be systematically considered in the analysis of the adsorption data. Up to now, little information on such analyses exists in the literature.33 Comparison of the adsorption–desorption hysteresis of metal cations and anions from CNTs is scarce. Thus, in the context of both engineered water treatment and the risk assessment of CNTs in the environment, the understanding of the adsorption–desorption hysteresis of metal cations and anions from CNTs is important and useful.
The objectives of this work are to probe the influence of structural properties, such as surface functional groups, SSA and defect contents, on the adsorption–desorption hysteresis of all three adsorbates (one cationic species (Cu(II) and two anionic species (anionic species of As(IV) and Cr(IV)) on CNTs in aqueous solution, and to compare the differences in adsorption behavior and possible hysteresis phenomena of metal cations and anions. Cu(II) was chosen as a model of metal cations. Cr(VI) and As(V) were selected as metal anions because they are the most toxic heavy metals. The existence of the three metal ions in aquatic environments and their species distribution in solution are described in the ESI† SI-1. On the basis of the prominent differences in the aqueous chemistry of the three metal ions, it was proposed that their adsorption and desorption behaviors would also be significantly different. To better understand the influence of the structural properties of CNTs on the adsorption–desorption hysteresis of metal ions, we have combined our adsorption–desorption studies with surface characterization.
Experimental details
Chemicals
Cu(II), Cr(VI) and As(V) stock solutions at 0.1 mol L−1 were prepared using Cu(NO3)2, K2Cr2O7 and Na2HAsO4, which were purchased from Shanghai Chemical Reagent Co., Ltd. All received reagents were of analytical reagent grade and used without any further treatment. Milli-Q water (Millipore, Billerica, MA, USA) was used in all experiments.Carbon nanotubes
SWCNTs, DWCNTs, MWCNTs and two oxidized MWCNTs with different oxygen concentrations (MWCNTs-O1, 2.51 wt% O and MWCNTs-O2, 3.5 wt% O) were used in this study. The CNTs, purchased from Beijing DK nano technology Co., Ltd,. were prepared using the chemical vapor deposition (CVD) method and used as received. The ash contents of these five CNTs were determined via a thermogravimetric analysis method. The Boehm titration method was carried out to determine the surface acidic groups (lactonic, hydroxyl and carboxyl groups, which are associated with the pH values) of the CNTs (see SI-2 of the ESI† for details). Carbon atomic percentages and surface oxygen of the CNTs were calculated via X-ray photoelectron spectroscopy (see SI-3 of the ESI†). SSA values for the CNTs were determined by the Brunauer–Emmett–Teller method (see SI-4 of the ESI†). The surface chemistry of CNTs was further characterized by Raman spectroscopy, performed at a wavelength of 514.57 nm (Ar+) with an NR-1800 laser Raman spectrometer (JASCO, Japan). The as-determined properties of the CNTs are tabulated in Table 1.| Adsorbents | SSA (m2 g−1) | Purity (wt%) | I D/IG | Surface atom (wt%) | Surface acidic group content (mmol g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Hydroxyl | Carboxyl | Lactonic | Total | ||||
| SWCNTs | 380.1 | >95 | 0.39 | 98.9 | 1.1 | 0.00245 | 0.004301 | 0.01629 | 0.02304 |
| DWCNTs | 299.5 | >95 | 0.55 | 98.7 | 1.3 | 0.00237 | 0.003757 | 0.018893 | 0.02502 |
| MWCNTs | 131.8 | >95 | 0.67 | 99.2 | 0.8 | 0.00272 | 0.003632 | 0.015061 | 0.02141 |
| MWCNTs-O1 | 90.7 | >95 | 0.88 | 97.4 | 2.6 | 0.06311 | 0.003967 | 0.004144 | 0.07122 |
| MWCNTs-O2 | 78.6 | >95 | 0.94 | 96.5 | 3.5 | 0.02024 | 0.072151 | 0.011369 | 0.10376 |
Batch adsorption–desorption experiments
The adsorption of Cu(II), Cr(VI) and As(V) on the five types of CNT was carried out in a 50 mL polyethylene centrifuge tube by a batch technique. The system was adjusted to the desired concentrations of the different components by adding stock suspensions of the CNTs and metal ions. Sonication was used in an attempt to disperse the nanotubes in the suspensions. The desired pH was achieved by adding negligible volumes of 0.01 or 0.1 mol L−1 HNO3 or NaOH. The ionic strength (I) was fixed at 0.01 M using NaNO3 as background. Preliminary kinetic studies confirmed that the adsorption equilibrium with the five types of CNT was effectively reached for Cu(II), Cr(VI) and As(V) within 10 h (seen in Fig. S4†). The suspensions were shaken by end-over-end rotation for 24 h to ensure that the adsorption equilibrium of Cu(II), Cr(VI) and As(V) was fully achieved. The solid and liquid phases were separated by centrifugation at 18 000 rpm for 20 min. UV–vis absorption at 500 nm can be calibrated to the amount of CNTs and was used to confirm the full centrifugation of all CNTs in the supernatant.34 The detection limit was approximately 0.8 mg L−1.The Cu(II), Cr(VI) and As(V) concentrations were measured by atomic absorption spectrophotometry. After centrifugation, the adsorbed amounts of heavy metal ions were calculated from the difference between the initial (C0) and equilibrium (Ce) concentrations in the supernatant. Control experiments using metal ions in a solution without CNTs showed that the control centrifuge tubes adsorbed less than 2% of the metal ions. The adsorption percentage was calculated as: adsorption (%) = (C0 − Ce)/C0 × 100.
Desorption experiments were carried out by substituting the supernatant with an electrolyte solution without the metal ions of interest, in order to reduce the metal ion concentration of the solution phase. In detail, after the adsorption experiments, half of the supernatant was quickly removed, and an equal volume of background electrolyte solution (0.01 M NaNO3) with the same pH value was injected into each centrifuge tube. Under the same conditions as in the adsorption experiments, the suspension was shaken for 2 days. The desorption experimental procedures were repeated for a second cycle. All of the adsorption–desorption experiments were carried out at 298 K.
Results and discussion
Influence of pH
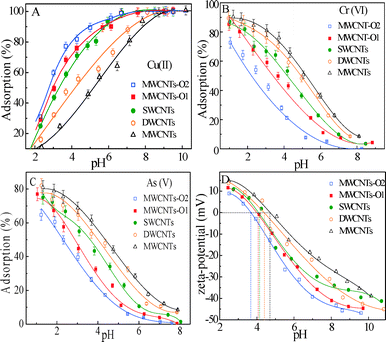
The influence of pH on Cu(II), Cr(VI) and As(V) removal by the CNTs is shown in Fig. 1. It is well known that the removal of metal ions by CNTs is highly dependent on pH.5–7,11Fig. 1A shows that Cu(II) removal by the CNTs increases gradually as the pH increases from 2.0 to 8.5, and finally stays constant with increasing pH. However, Cr(VI) or As(V) adsorption decreases as the pH increases from 1.0 to 8.0 (Fig. 1B and C). The ionization degree, speciation of the surface functional groups and surface charges of the CNTs are affected by the solution pH. The zeta-potential of CNTs at different pH values was measured and is shown in Fig. 1D. Under acidic pH conditions, surface functional groups (carboxyl, phenolic and lactonic) exist in the solution in protonated form. On the other hand, the functional groups on the adsorbent surface exist in deprotonated form under basic pH conditions. These results are consistent with prior research and with the Derjaguin–Landau–Verwey–Overbeek theory,35–37 as explained through basic electrostatic interactions between charged surfaces and charged species in solution.The mechanism of Cu(II) removal mainly involves ionic attraction between Cu2+, Cu2(OH)22+, Cu(OH)+, Cu(OH)2, Cu(OH)3− and Cu(OH)42− species (Fig. S1A†) and the surface functional groups of the adsorbents. The decrease in competition between Cu(II) ions and the protons for the functional groups of CNTs, and the increase in negative surface charge, result in an increase in Cu(II) adsorption as the pH increases. The Cu(II) concentration in solution is no longer controlled by adsorption at pH > 8.5, which is where precipitation occurs. However, Cr(VI) or As(V) adsorption decreases as the pH increases from 1.0 to 8.0, which could be related to Cr(VI) or As(V) speciation in the solution, and the ionic state and type of the functional groups on the surface of the CNTs. At a pH below the electrostatic point of the CNTs, electrostatic attraction between the positively charged adsorbent surface and the negatively charged HCrO4− or H2AsO4− (Fig. S1B and C†) results in higher adsorption. At a high pH, the reduction in Cr(VI) and As(V) adsorption contributes to electrostatic repulsion and competition for the effective adsorption sites of the metal anions with the OH−. At a pH of 4.0, the order of adsorption efficiency for HCrO4− or H2AsO4− was MWCNTs > DWCNTs > SWCNTs > MWCNTs-O1 > MWCNTs-O2.
Adsorption isotherms
The adsorption isotherms for Cu(II), As(V) and Cr(VI) by the CNTs are shown in Fig. 2. The Langmuir plots are also represented and the regression data are shown in Table S2.† From Fig. 2A and Table S2,† the adsorption capacities for Cu(II) on the basis of adsorbent mass follow the order of MWCNTs-O2 > MWCNTs-O1 > SWCNTs > DWCNTs > MWCNTs. Similar results were reported by Cho et al.,11 indicating that Zn(II) and Cd(II) adsorption on MWCNTs increased with an increase in the amount of oxygen-containing functional groups. From Fig. 2C, E and Table S2,† the trend of adsorption capacity for As(V) and Cr(VI) was observed to be MWCNTs-O2 < MWCNTs-O1 < SWCNTs < DWCNTs < MWCNTs, which clearly shows a systematic decrease in the extent of As(V) and Cr(VI) adsorption with an increase in the amount of total functional groups in the CNTs. The trend is exactly opposite to that of the cations. For metal anions, more functional groups (such as –COOH and –OH) are not conducive to As(V) and Cr(VI) adsorption, due to electrostatic repulsion between deprotonation oxygen-containing functional groups and negatively charged HCrO4− or H2AsO4−. Similar findings were also observed for the adsorption of Cr(VI) onto functionalized and non-functionalized MWCNTs.38 This phenomenon is in good agreement with Cr(VI) removal by oxidized activated carbon.39 Arslan and Pehlivan40 also showed that the adsorption of Cr(VI) onto a carbon surface decreased in the presence of oxygen surface complexes.From Table 1, the SSA values for the SWCNTs, DWCNTs, MWCNTs, MWCNTs-O1 and MWCNTs-O2 are 380.1, 299.5, 131.8, 90.7 and 78.6 m2 g−1, respectively. Both the SSA and surface defects have a positive influence on CNT adsorption capacity. It is useful to consider the influence of the SSA by comparing Qs after surface area normalization. For this purpose, the SSA-normalized adsorption capacity (Qs/SSA) was applied to evaluate the influence of CNT surface defects; results are shown in Fig. 2B, D and F. A consistent sequence of CNT Qs/SSA for Cu(II), As(V) and Cr(VI) is obtained: MWCNTs-O2 > MWCNTs-O1 > MWCNTs > DWCNTs > SWCNTs.
It has been hypothesized that the structural defects within CNTs could serve as nucleating sites, thus affecting the adsorption ability of the CNTs.41 Experimental results and theoretical studies showed that NH3 adsorption on SWCNT surfaces was sensitive to functionalities and defects.25 Raman spectroscopy is a sensitive method of probing the electronic structure in CNTs and the presence of defects.42 The D band in graphite at around 1300 cm−1 involves scattering from a defect which breaks the basic symmetry of the graphene sheet; it is observed in sp2 carbons containing pores, impurities or other symmetry-breaking defects. The changes in the D band of Raman spectra can be used for materials characterization, to probe and monitor structural modifications of the nanotube sidewalls that originate from the introduction of defects and the attachment of different chemical species. When estimating the defect concentration, the D mode intensity is usually normalized with respect to the intensity of the G mode at around 1600 cm−1.43 This approach relies on the assumption that the intensity of the G mode is independent of the defect concentration and originates from a single resonant Raman process. A comparison of the ratios of these two peak intensities gives a measure of the quality of the bulk samples. The relative relationship between the D band and G band is the key here. In general, the ratio of the D and G band intensities (ID/IG) is used to measure the structural defect concentrations of CNTs.44,45 If both bands have similar intensities, this indicates a high quantity of structural defects.46
In this study, Raman modes have been analyzed to testify the defective nature of the CNTs; the spectra are shown in the frequency range 1100–1800 cm−1 (Fig. 3). The different ID/IG values of SWCNTs, DWCNTs, and MWCNTs can be explained as follows: Defects such as vacancies and dangling bonds may be formed during the growth of CNTs, especially when using CVD due to the high temperatures involved. With an increase in the wall number of CNTs, the ID/IG value increases. MWCNTs exhibit a higher quantity of structural defects due to their multiple graphite layers and intershell structural defects, compared with SWCNTs and DWCNTs.47
In addition, the additional oxygen-containing groups in MWCNTs-O1 and MWCNTs-O2 will behave as covalent bonding-like defects. The observed ID/IG values are 0.39, 0.55, 0.67, 0.88 and 0.94 for SWCNTs, DWCNTs, MWCNTs, MWCNTs-O1 and MWCNTs-O2, respectively (Table 1). Therefore, the order of the CNT defect concentrations is as follows: MWCNTs-O2 > MWCNTs-O1 > MWCNTs > DWCNTs > SWCNTs. This is in good agreement with the adsorption capacities per unit SSA. It proves that lattice defects, which cause the so-called acceptor-like “localized states”,46 may be one of the factors which result in the adsorption ability of CNTs. CNT defects have a positive effect on Cr(VI) and As(V) adsorption, while the deprotonation oxygen-containing functional groups of CNTs have a negative effect on Cr(VI) and As(V) adsorption. The introduction of oxygen-containing functional groups can produce more surface defects, so it is difficult to separately determine the importance of charge and of surface defects . In our case, the order of the CNT defect concentrations is in good agreement with the adsorption capacities per unit SSA. The CNT surface defect contributions may overcome the negative effect of the deprotonation oxygen-containing functional groups for Cr(VI) and As(V) adsorption.
Adsorption–desorption hysteresis
Fig. 4 shows the adsorption–desorption hysteresis of heavy metal ions on or from CNTs, initiated by substituting the supernatant with an electrolyte solution without metal ions. The adsorption isotherms of Cu(II), Cr(VI) and As(V) are shown below the desorption isotherms, and the first desorption isotherms are below the second desorption isotherms. From Fig. S4 and S5,† the adsorption and desorption processes of Cu(II), Cr(VI) and As(V) occurred at a significant level in the initial rapid stage and reached equilibrium within 2 d. The observed phenomenon was unlikely to be completely due to a non-equilibrium state because the suspension in this study was shaken for 2 days. This phenomenon implies that irreversible reactions may exist in the adsorption processes. In some researchers’ reports,21,24,26 the deformation–rearrangement of CNT aggregates and adsorbate penetration into the closed interstitial spaces of CNT bundles was proposed to explain the adsorption hysteresis of polycyclic aromatic hydrocarbons. However, Yang and Xing21 reported that CNTs with long and cylindrical structures could not form closed interstitial spaces, and thus desorption hysteresis for organic compounds could not occur. Hummer et al.48 studied the adsorption of H2O by uncapped SMCNTs and found that water molecules could enter the central channels of the nanotubes by forming hydrogen-bonded chains. Opinions in the literature are different, and have not reached a uniform understanding. For our experiment, sonication was used in an attempt to disperse the nanotubes in the suspensions, and to reduce the effect of large aggregates on metal ion adsorption. In our earlier study of the desorption kinetics of Am(III) bound to MWCNTs using chelating resin, the results clearly proved the occurrence of strong chemical binding of Am(III) to the nanotubes.5 The experimental results indicated that Am(III) formed kinetically stable complexes with MWCNTs and did not rapidly desorb into the solution. Two types of adsorption complexes formed for Am(III) on MWCNTs, one of a strong complexation and the other of a weak complexation, which provided the ‘readily desorbed’ and ‘less readily desorbed’ fractions of Am(III), respectively.A adsorption–desorption hysteresis index (HI, eqn (1) and (2))20 was used to standardize the gap between the adsorption and desorption branches of the isotherms as follows:
| HI1 = (Qd1e − Qse)/Qse|T,Ce | (1) |
| HI2 = (Qd2e − Qse)/Qse|T,Ce | (2) |
| CNTs | pH | HI1 | HI2 | ||
|---|---|---|---|---|---|
| 0.005 mmol L−1 | 0.03 mmol L−1 | 0.005 mmol L−1 | 0.03 mmol L−1 | ||
| a HI1 and HI2 represent the hysteresis indices of the first and second desorption cycles, respectively. | |||||
| Cu(II) | |||||
| SWCNTs | 5.0 | 0.10 ± 0.01 | 0.05 ± 0.01 | 0.20 ± 0.01 | 0.09 ± 0.02 |
| DWCNTs | 5.0 | 0.12 ± 0.01 | 0.08 ± 0.01 | 0.24 ± 0.01 | 0.16 ± 0.01 |
| MWCNTs | 5.0 | 0.14 ± 0.02 | 0.10 ± 0.01 | 0.26 ± 0.02 | 0.18 ± 0.01 |
| MWCNTs-O1 | 5.0 | 0.16 ± 0.01 | 0.12 ± 0.01 | 0.27 ± 0.01 | 0.20 ± 0.01 |
| MWCNTs-O2 | 5.0 | 0.27 ± 0.04 | 0.14 ± 0.01 | 0.38 ± 0.02 | 0.22 ± 0.01 |
| Cr(VI) | |||||
| SWCNTs | 4.0 | 0.27 ± 0.02 | 0.25 ± 0.02 | 0.59 ± 0.02 | 0.54 ± 0.02 |
| DWCNTs | 4.0 | 0.22 ± 0.01 | 0.18 ± 0.02 | 0.53 ± 0.01 | 0.48 ± 0.01 |
| MWCNTs | 4.0 | 0.21 ± 0.01 | 0.17 ± 0.01 | 0.43 ± 0.02 | 0.36 ± 0.01 |
| MWCNTs-O1 | 4.0 | 0.16 ± 0.01 | 0.14 ± 0.02 | 0.37 ± 0.03 | 0.32 ± 0.03 |
| MWCNTs-O2 | 4.0 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.27 ± 0.01 | 0.24 ± 0.02 |
| As(V) | |||||
| SWCNTs | 4.0 | 0.17 ± 0.02 | 0.15 ± 0.02 | 0.54 ± 0.03 | 0.51 ± 0.02 |
| DWCNTs | 4.0 | 0.15 ± 0.02 | 0.14 ± 0.02 | 0.48 ± 0.02 | 0.46 ± 0.02 |
| MWCNTs | 4.0 | 0.14 ± 0.01 | 0.13 ± 0.02 | 0.35 ± 0.01 | 0.31 ± 0.02 |
| MWCNTs-O1 | 4.0 | 0.12 ± 0.01 | 0.11 ± 0.01 | 0.33 ± 0.01 | 0.28 ± 0.02 |
| MWCNTs-O2 | 4.0 | 0.07 ± 0.02 | 0.06 ± 0.01 | 0.27 ± 0.01 | 0.22 ± 0.01 |
Conclusions
Oxygen-containing functional groups contributed to an increase in adsorption capacity for Cu(II), but a decrease in adsorption capacity for Cr(VI) and As(V). The adsorption capacity based on the CNT SSA increased with an increase in CNT defect content. The HI values for cations (such as Cu(II)) increased as the amount of oxygen-containing functional groups increased. However, for anions (such as Cr(VI) and As(V)), HI values decreased as the amount of oxygen-containing functional groups increased. HI values decreased with increasing metal ion surface coverage on the CNTs. The mechanisms of metal ion adsorption by CNTs may shift from more irreversible to more reversible processes with an increase in adsorbed metal ions. The immobilization of metal ions may originate from the irreversible formation of surface complexes and nucleation. The desorption hysteresis and irreversible immobilization of metal ions on CNTs will influence their mobility and bioavailability, and consequently their toxicity will be altered in the environment. Therefore, the desorption hysteresis and irreversible immobilization of metal cations and anions on different CNTs and the changes that occur in toxicity should be studied, to allow detailed risk assessment of metal ions and CNTs in the natural environment.Acknowledgements
Financial support from NSFC (91126020, 41273134, 91326202, 21225730), the Chinese National Fusion Project for ITER (no. 2013GB110000), the Hefei Center for Physical Science and Technology (2012FXZY005), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions are acknowledged.Notes and references
- F. S. Gittleson, D. J. Kohn, X. Li and A. D. Taylor, ACS Nano, 2012, 6, 3703–3711 CrossRef CAS PubMed.
- Z. Liu, K. Chen, C. Davis, S. Sherlock, Q. Z. Cao, X. Y. Chen and H. J. Dai, Cancer Res., 2008, 68, 6652–6660 CrossRef CAS PubMed.
- D. Movia, A. Prina-Mello, D. Bazou, Y. Volkov and S. Giordani, ACS Nano, 2011, 5, 9278–9290 CrossRef CAS PubMed.
- M. A. Correa-Duarte, N. Wagner, J. Rojas-Chapana, C. Morsczeck, M. Thie and M. Giersig, Nano Lett., 2004, 4, 2233–2236 CrossRef CAS.
- X. K. Wang, C. L. Chen, W. P. Hu, A. P. Ding, D. Xu and X. Zhou, Environ. Sci. Technol., 2005, 39, 2856–2860 CrossRef CAS.
- C. L. Chen, J. Hu, D. Xu, X. L. Tan, Y. D. Meng and X. K. Wang, J. Colloid Interface Sci., 2008, 323, 33–41 CrossRef CAS PubMed.
- C. L. Chen and X. K. Wang, Ind. Eng. Chem. Res., 2006, 45, 9144–9149 CrossRef CAS.
- R. Q. Long and R. T. Yang, J. Am. Chem. Soc., 2001, 123, 2058–2059 CrossRef CAS.
- X. L. Wang, J. L. Lu and B. S. Xing, Environ. Sci. Technol., 2008, 42, 3207–3212 CrossRef CAS.
- K. Yang, L. Z. Zhu and B. S. Xing, Environ. Sci. Technol., 2006, 40, 1855–1861 CrossRef CAS.
- H. H. Cho, K. Wepasnick, B. A. Smith, F. K. Bangash, D. H. Fairbrother and W. P. Ball, Langmuir, 2010, 26, 967–981 CrossRef CAS PubMed.
- H. H. Cho, B. A. Smith, J. D. Wnuk, D. H. Fairbrother and W. P. Ball, Environ. Sci. Technol., 2008, 42, 2899–2905 CrossRef CAS.
- S. Kang, M. Herzberg and D. F. Rodrigues, Langmuir, 2008, 24, 6409–6413 CrossRef CAS PubMed.
- N. W. S. Kam, T. C. Jessop, P. A. Wender and H. J. Dai, J. Am. Chem. Soc., 2004, 126, 6850–6851 CrossRef CAS PubMed.
- Q. Lu, J. M. Moore, G. Huang, A. S. Mount, A. M. Rao, L. L. Larcom and P. C. Ke, Nano Lett., 2004, 4, 2473–2477 CrossRef CAS.
- S. Foley, C. Crowley, M. Smaihi, C. Bonfils, B. F. Erlanger, P. Seta and C. Larroque, Biochem. Biophys. Res. Commun., 2002, 294, 116–119 CrossRef CAS.
- Y. L. Zhao, G. M. Xing and Z. F. Chai, Nat. Nanotechnol., 2008, 3, 191–192 CrossRef CAS PubMed.
- D. C. Adriano, Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals, Springer, New York, 2001 Search PubMed.
- J. Zhao, Z. Y. Wang, H. Mashayekhi, P. Mayer, B. Chefetz and B. S. Xing, Environ. Sci. Technol., 2012, 46, 5369–5377 CrossRef CAS PubMed.
- W. H. Wu, W. Jiang, W. D. Zhang, D. H. Lin and K. Yang, Environ. Sci. Technol., 2013, 47, 8373–8382 CAS.
- K. Yang and B. S. Xing, Environ. Pollut., 2007, 145, 529–537 CrossRef CAS PubMed.
- X. K. Cheng, A. T. Kan and M. B. Tomson, J. Nanopart. Res., 2005, 7, 555–567 CrossRef CAS.
- X. B. Wang, Y. Q. Liu, W. F. Qiu and D. B. Zhu, J. Mater. Chem., 2002, 12, 1636–1639 RSC.
- B. Pan, D. H. Lin, H. Mashayekhi and B. S. Xing, Environ. Sci. Technol., 2008, 42, 5480–5485 CrossRef CAS.
- P. Oleszczuk, B. Pan and B. S. Xing, Environ. Sci. Technol., 2009, 43, 9167–9173 CrossRef CAS PubMed.
- Z. Y. Wang, X. D. Yu, B. Pan and B. S. Xing, Environ. Sci. Technol., 2010, 44, 978–984 CrossRef CAS PubMed.
- S. J. Zhang, T. Shao, S. S. K. Bekaroglu and T. J. Karanfil, Environ. Sci. Technol., 2009, 43, 5719–5725 CrossRef CAS.
- J. J. Zhao, A. Buldum, J. Han and J. P. Lu, Nanotechnology, 2002, 13, 195–200 CrossRef CAS.
- Y. H. Li, S. G. Wang, Z. K. Luan, J. Ding, C. L. Xu and D. H. Wu, Carbon, 2003, 41, 1057–1062 CrossRef CAS.
- L. Valentini, L. Lozzi, S. Picozzi, C. Cantalini, S. Santucci and J. M. Kenny, J. Vac. Sci. Technol. A, 2004, 22, 1450–1454 CAS.
- S. J. Zhang, T. Shao, H. S. Kose and T. Karanfil, Environ. Sci. Technol., 2010, 44, 6377–6383 CrossRef CAS PubMed.
- X. Feng, S. Irle, H. Witek, K. Morokuma, R. Vidic and E. Borguet, J. Am. Chem. Soc., 2005, 127, 10533–10538 CrossRef CAS PubMed.
- J. Li, C. L. Chen, S. W. Zhang, X. M. Ren, X. L. Tan and X. K. Wang, Chem. – Asian J., 2014, 9, 1144–1151 CrossRef PubMed.
- B. Smith, K. Wepasnick, K. E. Schrote, H. H. Cho, W. P. Ball and D. H. Fairbrother, Langmuir, 2009, 25, 9767–9776 CrossRef CAS PubMed.
- N. B. Saleh, L. D. Pfefferle and M. Elimelech, Environ. Sci. Technol., 2008, 42, 7963–7969 CrossRef CAS.
- B. Smith, K. Wepasnick, K. E. Schrote, A. R. Bertele, W. P. Ball, C. O'Melia and D. H. Fairbrother, Environ. Sci. Technol., 2009, 43, 819–825 CrossRef CAS.
- M. Sano, J. Okamura and S. Shinkai, Langmuir, 2001, 17, 7172–7173 CrossRef CAS.
- K. Pillay, E. M. Cukrowska and N. J. Coville, J. Hazard. Mater., 2009, 166, 1067–1075 CrossRef CAS PubMed.
- S. J. Park and Y. S. Jang, J. Colloid Interface Sci., 2002, 249, 458–463 CrossRef CAS PubMed.
- G. Arslan and E. Pehlivan, Bioresour. Technol., 2007, 98, 2836–2845 CrossRef CAS PubMed.
- B. C. Regan, S. Aloni, R. O. Ritchie, U. Dahmen and A. Zettl, Nature, 2004, 428, 924–928 CrossRef CAS PubMed.
- M. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. Cancado, A. Jorio and R. Saito, Phys. Chem. Chem. Phys., 2007, 9, 1276–1290 RSC.
- K. Jeet, V. K. Jindal, L. M. Bharadwaj, D. K. Avasthi and K. Dharamvir, J. Appl. Phys., 2010, 108, 034302 CrossRef PubMed.
- W. Z. Qian, T. Liu, F. Wei and H. Y. Yuan, Carbon, 2003, 41, 1851–1854 CrossRef CAS.
- D. K. Singh, P. K. Giri and P. K. Iyer, J. Phys. Chem. C, 2011, 115, 24067–24072 CAS.
- M. S. Dresselhaus, G. Dresselhaus, R. Saito and A. Jorio, Phys. Rep., 2005, 409, 47–99 CrossRef PubMed.
- X. M. Shi, B. B. Jiang, J. D. Wang and Y. R. Yang, Carbon, 2012, 50, 1005–1013 CrossRef CAS PubMed.
- G. Hummer, J. C. Rasaiah and J. P. Noworyta, Nature, 2001, 414, 188–190 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: More details on heavy metal ion species distribution, Boehm titration, XPS survey spectra of CNTs, BET data of CNTs, adsorption and desorption kinetics, and Langmuir model fitted results for adsorption–desorption. See DOI: 10.1039/c4en00044g |
| This journal is © The Royal Society of Chemistry 2014 |