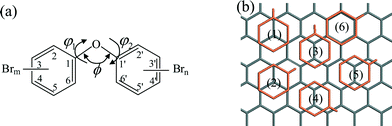Interactions between polybrominated diphenyl ethers and graphene surface: a DFT and MD investigation†
Ning
Ding
a,
Xiangfeng
Chen
*b and
Chi-Man Lawrence
Wu
*ab
aDepartment of Physics and Materials Science, City University of Hong Kong, Hong Kong SAR, PR China. E-mail: lawrence.wu@cityu.edu.hk
bKey Laboratory for Applied Technology of Sophisticated Analytical Instruments, Shandong Academy of Sciences, Jinan, PR China. E-mail: xiangfchen@aliyun.com
First published on 20th December 2013
Abstract
Understanding the adsorption behavior of chemical pollutant molecules on graphene surfaces is of both fundamental and practical importance for the application of graphene in environmental analysis. In this work, the mechanism and thermodynamics of adsorption of polybrominated diphenyl ethers (PBDEs) on graphene surfaces were studied by density functional theory and molecular dynamics methods. Nine types of PBDE molecules with different degrees of bromination and diphenyl ether (DE) molecules were selected as the adsorbates. It was found that the interaction strengths between the PBDE congeners (without 6 and 6′-substitution) and graphene increased with the degree of bromination. The adsorption energies of these systems also exhibited a positive linear correlation with the hydrophobicity of PBDE molecules, while for the other three PBDEs with 6 or 6′-substitution, the steric-hindrance effect leads to the molecules forming single π–π stacking interactions with the graphene surface and exhibiting a different adsorption behavior. The electronic density of states, charge transfer analysis and thermodynamic analysis indicated that the adsorption process of PBDEs on graphene is physisorption. The MD simulation indicates that graphene is sensitive to PBDE molecules and the adsorption process of PBDE on graphene is very fast. These findings will contribute to the understanding of the adsorption chemistry of aromatic pollutants on graphene-like nanomaterials.
Nano impactUnderstanding the adsorption behavior of chemical pollutant molecules on graphene surfaces is of both fundamental and practical importance for the application of graphene in environmental analysis. In this work, the mechanism and thermodynamics of adsorption of polybrominated diphenyl ethers on graphene surfaces were first and systematically studied by theoretical methods. The calculation results obtained and analyzed in this paper provided fundamental information which is difficult to obtain in experiments. The findings will contribute to the understanding of the adsorption chemistry of aromatic pollutants on graphene-like nanomaterials. |
1. Introduction
Polybrominated diphenyl ethers (PBDEs) have been widely used as brominated flame retardants for more than 30 years. They have high lipophilicity and high resistance to degradation processes. As PBDEs are non-covalently bonded additives, it has been found that they could migrate out of products like furniture and electronic products and wind up in house dust.1,2 PBDEs have been commonly found accumulating in blood, breast milk, and fat tissues.3–8 The detection and measurement of PBDEs in different matrices have drawn special attention in recent years. To date, many analytical methods have been developed for the detection of the level of PBDEs in water, milk, fish and soil in the environment.9–17In recent years, as a type of low-dimensional nanomaterial, graphene and its derivatives have drawn special interest in the areas of analytical and environmental chemistry.18–26 Due to its large surface area, unique chemical and thermal stability and high adsorption capacity, graphene has been explored as an adsorbent material for extraction,27 removal,28 sensing,29 and degradation of pollution molecules.30 For example, Lee and coworkers have used graphene coated fibers for solid-phase microextraction (SPME) of PBDEs from environmental samples.13 It has been found that the unique structure of graphene would enhance its interaction with the aromatic PBDEs. Jiang et al. explored the application of graphene assisted matrix solid-phase dispersion (GA-MSPD) for extraction of PBDEs and their methoxylated and hydroxylated analogs. A better extraction performance was obtained with GA-MSPD than with other adsorbents (C18 silica, Florisil and carbon nanotubes).14 The same research group also reported the use of graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction.27 Li and coworkers have developed a graphene-coated SPME fiber, which exhibited higher enrichment factors (EFs) for PBDEs as compared to commercial polydimethylsiloxane (PDMS) fibers.15
Despite the emergence of various applications of graphene in the detection and removal processes of the organic pollutants, the detailed adsorption mechanism of PBDEs on graphene surfaces is not well understood. Due to the limitation of experiments to investigate the interactions between various molecules and graphene, computational simulations have emerged as an efficient way to study the adsorption process.31,32 To the best of our knowledge, no theoretical investigation of the interaction mechanism between PBDEs and graphene was reported. In this study, the adsorption mechanism of PBDEs on a graphene surface was studied using density functional theory (DFT) and molecular dynamics (MD) methods. Nine PBDEs, including BDE 15, BDE 28, BDE 47, BDE 99, BDE 153, BDE 154, BDE 183, BDE 194 and BDE 209, as well as a diphenyl ether (DE) molecule were selected as the probe molecules. The objectives of this investigation are to elucidate the nature of interaction between the PBDEs and graphene and to determine the effect of bromination on the adsorption reaction.
2. Computational details
2.1. Details of the DFT simulation
The adsorption properties of PBDEs on graphene surfaces were explored by DFT methods. As mentioned in previous studies,33–35 both the local density approximation (LDA) and generalized gradient approximation (GGA) have been selected to describe the adsorption behavior of biomolecules on inert surfaces (for example, a graphene surface), in which the van der Waals interaction plays an important role. Actually, it is difficult for both LDA and GGA to describe the van der Waals interaction because of the lack of description of dispersive forces; thus, the van der Waals interaction correction is necessary.36,37 In this work, we employed both the LDA and GGA methods with the DFT-D correction method (DFT-D method of Grimme) to describe the adsorption systems. Compared with some ultrahigh-accuracy computing methods (for example, the MP2 method), the DFT method with dispersion correction is a relatively cheap and acceptable choice for calculating weak interactions in our systems. As mentioned in many reports, this method is now widely used to deal with adsorption systems with weak interactions. The calculations were based on the double-numeric quality with polarization functions (DNP) basis set. A 6 × 6 graphene unit with periodic boundary conditions was constructed as the adsorbent, which contains 72 carbon atoms. A distance of 20 Å was provided between adjacent graphene layers to avoid the interactions between them. The calculations were carried out in an aqueous environment. This was realized by setting the dielectric constant of the simulation box as 78.54, which is the value of the dielectric constant of water. In the calculation process, the Brillouin zone was separated by a 6 × 6 × 1 k-point mesh. The DFT semicore pseudopotential (DSPP) core treatment is adopted for the relativistic effects of bromine atoms. All the calculations were performed using the DMol3 module of Materials Studio 5.5 (Accelrys Inc.).For the adsorption systems, the interaction energy is defined to evaluate the interaction between PBDE congeners and graphene as
| ΔE = Etotal − (Egraphene + EPBDE) | (1) |
2.2. Details of the MD simulation
To obtain the information about the dynamic adsorption behavior of PBDEs on graphene surfaces in an aqueous environment, a simulation based on molecular mechanics (MM) and MD methods was carried out to supplement the DFT calculations. The COMPASS force field38 was chosen to describe the adsorption system, which has been used to study a considerable body of biomolecule- and graphene-based material containing systems.39,40 For the non-bonding interactions, the Ewald method41 was used to calculate the electrostatic interactions, while the atom-based method was chosen to calculate the van der Waals energy with a cut-off of 12.5 Å. All the calculations were performed using the Discover module.3. Results and discussion
In this study, nine types of PBDE congeners, (i.e. BDE 15, BDE 28, BDE 47, BDE 99, BDE 153, BDE 154, BDE 183, BDE 194 and BDE 209), which represent the PBDEs with bromination degree from di- to deca-bromination, were selected as the adsorbates to explore the adsorption behavior of PBDEs on graphene surfaces. As a reference, the adsorption of diphenyl ether (DE) molecules on graphene was also studied. The structures of the selected molecules were optimized first. The key structural parameters of selected PBDEs, including the bond angle C1–O–C1′ (ϕ) and the torsion angles C1′–O–C1–C6 (φ1) and C2′–C1′–O–C1 (φ2), are shown in Fig. 1(a) and Table 1. The chemical structures of these ten molecules are shown in the ESI.†| Molecules | Compound/substituent | Bond angle (°) | Torsion angle (°) | Dihedral angle between the phenyl plane and graphene surface (°) | HOMO–LUMO gap (kcal mol−1) | |
|---|---|---|---|---|---|---|
| C1–O–C1′ (ϕ) | C1′–O–C1–C6 (φ1) | C2′–C1′–O–C1 (φ2) | ||||
| DE | NA | 120/122 | 37/32 | 146/150 | 10/40 | 93.63 |
| BDE 15 | 4,4′ | 121/128 | 36/20 | 150/170 | 8/15 | 86.76 |
| BDE 28 | 2,4,4′ | 119/128 | 41/18 | 141/170 | 7/15 | 85.37 |
| BDE 47 | 2,2′,4,4′ | 119/131 | 55/11 | 144/177 | 1/5 | 84.36 |
| BDE 99 | 2,2′,4,4′,5 | 120/131 | 27/10 | 134/176 | 0/4 | 83.69 |
| BDE 153 | 2,2′,4,4′,5,5′ | 121/132 | −31/10 | −141/−170 | 3/1 | 82.51 |
| BDE 194 | 2,2′,3,3′,4,4′,5,5′ | 120/132 | 32/9 | 135/−170 | 1/4 | 77.62 |
| BDE 154 | 2,2′,4,4′,5,6′ | 120/117 | 0/42 | −108/−70 | 10/89 | 84.70 |
| BDE 183 | 2,2′,3,4,4′,5′,6 | 118/117 | −69/95 | −158/−178 | 2/87 | 78.96 |
| BDE 209 | 2,2′,3,3′,4,4′,5,5′,6,6′ | 126/121 | −47/−55 | −134/−145 | 8/75 | 71.92 |
There are many different conformers of the PBDE molecules, which are flexible and could transform to each other via low energy barriers.42–44 In this work, the lowest energy conformers of the selected PBDEs obtained from previous results were optimized first. It was found that the lowest energy conformations of the PBDEs were all in the “twist” form with different torsion angles, which are basically consistent with previous results.
3.1. Geometries of the adsorption systems
The adsorption systems were constructed to investigate the adsorption properties of PBDEs on graphene. Previously, it was found that the aromatic ring favors adsorbing on graphene with a parallel configuration towards the graphene surface.33 Herein, we constructed the initial adsorption configurations by putting each phenyl ring of the PBDE molecule parallel to the graphene surface with different orientations. For each phenyl ring, six types of locations towards graphene were considered. As shown in Fig. 1(b), two ‘top sites’ with the center of the phenyl ring on top of a carbon atom, three ‘bridge sites’ with the center of the phenyl ring at the bridge site of a C–C bond and one ‘hollow site’ with the phenyl ring of PBDE located on top of and coincident with a hexagonal ring of graphene were considered. The initial distance between the parallel phenyl ring and the graphene surface was set as about 4 Å.The most stable adsorption configurations for the ten PBDE adsorption systems are shown in Fig. 2. The geometric angles of the target PBDE (or DE) congeners after adsorption are also given in Table 1. For the DE molecule, the dihedral angle between the phenyl ring and graphene is about 40°. The torsion angles (φ1, φ2) as well as the bond angle (ϕ) inside the DE molecule were slightly changed (by <5°) after the adsorption. The adsorption distance of DE on the graphene surface is about 3.56 Å. The PBDE–graphene systems structurally fall into two groups. The first group includes the BDE 15, BDE 28, BDE 47, BDE 99, BDE 153 and BDE 194 adsorption systems. For these adsorption systems, the two phenyl rings of the PBDE congeners were nearly parallel to the graphene surface. Each dihedral angle between the phenyl ring and graphene surface is less than 15° (Table 1). As shown in Table 2, the distances between the center of mass of the adsorbate molecules and graphene surface ranged from 3.31 to 3.41 Å. The torsion angles inside the congeners changed significantly after adsorption on the graphene surface. Taking BDE 47 as an example, the two torsion angles inside the BDE 47 molecule changed from 55° to 11°, and 144° to 177°, respectively. In addition, after the adsorption, the C1–O–C1′ (ϕ) bond angle for each PBDE molecule increased by about 10°, making the two phenyl rings of these PBDE molecules parallel to the graphene surface.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values of the PBDE congeners
Kow values of the PBDE congeners
| Systems | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow Kow |
Adsorption distance (Å) | Interaction energy (kcal mol-1) | Charge transfer (e) | |
|---|---|---|---|---|---|
| LDA | GGA+DFT-D | ||||
| a Experimental data in ref. 49. b Experimental data in ref. 50. c Extrapolated value by linear regression in ref. 51. | |||||
| DE-G | 4.21a | 3.56 | −11.54 | −17.70 | 0.065 |
| BDE 15-G | 5.51b | 3.39 | −15.90 | −22.03 | 0.069 |
| BDE 28-G | 5.94b | 3.41 | −16.55 | −23.89 | 0.071 |
| BDE 47-G | 6.81b | 3.31 | −20.67 | −25.43 | 0.065 |
| BDE 99-G | 7.32b | 3.36 | −20.98 | −30.24 | 0.066 |
| BDE 153-G | 7.90b | 3.36 | −21.70 | −31.69 | 0.064 |
| BDE 194-G | 8.48c | 3.37 | −24.60 | −36.74 | 0.058 |
| BDE 154-G | 7.82b | 4.76 | −14.20 | −20.28 | 0.048 |
| BDE 183-G | 8.27b | 4.76 | −18.97 | −27.61 | 0.044 |
| BDE 209-G | 9.98c | 4.91 | −17.84 | −26.85 | 0.038 |
The adsorption configuration of the BDE 154, BDE 183 and BDE 209 congeners on the graphene surface is obviously different from that of the first group. Rather than being coplanar with the graphene, one of the phenyl rings in the PBDE molecule is nearly parallel to the graphene surface (dihedral angle <10°) (see Table 1), while the other one forms an almost right dihedral angle with graphene, i.e. 89°, 87° and 75° for BDE 154, BDE 183 and BDE 209, respectively. That is, one of the phenyl rings in these three PBDE congeners is nearly vertical to the graphene surface, which is like a ‘T-shape’. The parallel-stacked structure and T-shape structure are two types of preferred configurations for aromatic ring dimers.45 However, the T-shape configuration is not as stable as the parallel-stacked configuration. The adsorption distances of these three systems are about 40% larger than that of the first group. The changes of the C1–O–C1′ (ϕ) bond angle are less than 5° for the three molecules.
3.2. Energetics of the adsorption systems
The calculated interaction energies between the PBDEs (or DE) and graphene using LDA and GGA+DFT-D are summarized in Table 2. For the first group of PBDEs (BDE 15, BDE 28, BDE 47, BDE 99, BDE 153 and BDE 194) and DE, both the LDA and GGA+DFT-D calculation results show a similar trend. That is, the interaction energy increases with the increase of bromination degree in the molecule. For example, the interaction energy between BDE 28 (triBDE) and graphene is −23.89 kcal mol−1, and that between BDE 99 (pentaBDE) and graphene increases to −30.24 kcal mol−1. Among these seven adsorbates, DE has the lowest interaction energy with graphene, i.e. −11.54 kcal mol−1 by LDA and −17.70 kcal mol−1 by GGA+DFT-D, which suggests the relatively weak interaction strength between DE and graphene. Thus, as mentioned above, the conformation of the DE molecule almost does not change after the adsorption. It is important to note that the GGA+DFT-D results give higher interaction energies than LDA. Similar correlations between LDA energies and corresponding GGA+DFT-D energies for van der Waals interactions were also observed in many biomolecular adsorption systems.46–48For the BDE 183 (heptaBDE) and BDE 209 (decaBDE) congeners, the number of bromine atoms in these two molecules is greater than that in BDE 153 (hexaBDE); however, their interaction energies with graphene were lower than those of BDE 153. As mentioned in previous studies,33 π–π stacking plays the main role in the interactions between the phenyl ring and graphene. Structurally, only one phenyl ring in BDE 183 (or BDE 209) forms the π–π stacking with graphene, which significantly lowers the interactions between the PBDE congeners and graphene as compared to those in BDE 153. In addition, it is believed that the ortho-position substitution of bromine (here it is 6 or 6′-position substitution) in the structure of BDE 183 and BDE 209 leads to the steric-hindrance effect for these two molecules, which blocks the coplanar orientation of these two molecules on the graphene surface.
For further comparison, the adsorption behavior of BDE 154, which has the same number of bromine atoms as BDE 153, was investigated. The only difference between BDE 154 and BDE 153 is the position of bromine substitution. As expected, the adsorption configuration of BDE 154 on the graphene surface is similar to that of BDE 183 and BDE 209. Accordingly, the interaction energy between BDE 154 and graphene is 7.5 and 11.41 kcal mol−1 lower than that between BDE 153 and graphene using LDA and GGA+DET-D calculations, respectively.
Thus, it is obvious that bromine atoms could affect the adsorption of PBDEs on graphene surfaces. However, the interaction energies between the PBDEs and graphene surfaces were not only affected by the increase of bromine atoms. First, the substitution positions of bromine atoms in PBDEs affect the adsorption configurations of PBDEs on graphene surfaces (with or without steric-hindrance effect). For the seven PBDE adsorption systems without steric-hindrance effect, the increase of bromine atoms enhanced the interaction energies between the PBDEs and graphene due to the relatively strong interactions between the bromine atoms and graphene.
3.3. Relationship between the adsorption energies and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Kow
Kow
As mentioned in previous studies,32 the adsorption strength of some organic pollutants (for example, dichlorobenzene and trichlorobenzene) on the surface of CNTs is strongly correlated with the hydrophobicity of the adsorbates. Here, to confirm the effect of hydrophobicity of PBDEs on the adsorption strength, the relationship between the interaction energies of PBDEs with graphene and the logarithm of the octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) was obtained and is shown in Fig. 3. log
Kow) was obtained and is shown in Fig. 3. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow reflects the ratio of concentrations of the compound between the two solutions (octanol and water), thus the larger value of log
Kow reflects the ratio of concentrations of the compound between the two solutions (octanol and water), thus the larger value of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow indicates the stronger hydrophobicity of the compound. According to a considerable body of experimental works, the log
Kow indicates the stronger hydrophobicity of the compound. According to a considerable body of experimental works, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values of PBDE congeners basically increase with the increasing number of the substituent bromine atoms.49–51
Kow values of PBDE congeners basically increase with the increasing number of the substituent bromine atoms.49–51
As shown in Fig. 3, the interaction energies for DE, BDE 15, BDE 28, BDE 47, BDE 99, BDE 153 and BDE 194 show obvious linear correlations with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow. The relationship can be expressed by the following equation
Kow. The relationship can be expressed by the following equation
| ΔE = 1.38 − 4.28 log Kow |
| (n = 7, r = 0.97, p < 0.01) | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow, which indicates that hydrophobic interaction plays an important role in the adsorption of PBDEs on the graphene surface.
Kow, which indicates that hydrophobic interaction plays an important role in the adsorption of PBDEs on the graphene surface.
However, eqn (2) could not be used to illustrate the relationship between interaction energies and hydrophobicity for BDE 154, BDE 183 and BDE 209. For example, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow value of BDE 154 is 7.82, which is similar to that of BDE 153 (7.90). However, the interaction energy of BDE 154 with graphene is much lower than that of BDE 153. In addition, BDE 209 has the highest log
Kow value of BDE 154 is 7.82, which is similar to that of BDE 153 (7.90). However, the interaction energy of BDE 154 with graphene is much lower than that of BDE 153. In addition, BDE 209 has the highest log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow value among these ten molecules, but the interaction energy between BDE 209 and graphene is even lower than that between BDE 99 and graphene. This discrepancy between the interaction energies for these three PBDE congeners and eqn (2) indicates that the adsorption strength of PBDEs on graphene is not simply controlled by the hydrophobicity of the adsorbates. Actually, π–π interactions play a more important role in the PBDEs and graphene adsorption systems. As analyzed above, the lower interaction energies between these three PBDE congeners and graphene are caused by the coordination mode of the molecules, in which only one of the phenyl rings forms π–π stacking with graphene. The torsional motion (or polarization) of these three PBDE molecules on the graphene surface required the conformations to consume a large amount of energy, which is an unfavorable process as compared with the other congeners. In addition, compared with BDE 183, the interaction energies of BDE 209 and BDE 154 deviated much more from the correlation line. From the geometry of the BDE 183 adsorption system in Fig. 3, the so called ‘co-planar’ phenyl ring with the graphene surface is nearly strictly parallel to the graphene surface (the dihedral angle between them is 2°, Table 1). However, the dihedral angle between the ‘co-planar’ phenyl ring in BDE 209 (BDE 154) and the graphene surface is 8° (10°), which reduced the interaction energies between the PBDEs and graphene, thus making these two cases farther from the correlation line than BDE 183. This phenomenon may be caused by the perpendicular phenyl rings in these three BDE congeners, in which the substitution positions of bromine atoms are different.
Kow value among these ten molecules, but the interaction energy between BDE 209 and graphene is even lower than that between BDE 99 and graphene. This discrepancy between the interaction energies for these three PBDE congeners and eqn (2) indicates that the adsorption strength of PBDEs on graphene is not simply controlled by the hydrophobicity of the adsorbates. Actually, π–π interactions play a more important role in the PBDEs and graphene adsorption systems. As analyzed above, the lower interaction energies between these three PBDE congeners and graphene are caused by the coordination mode of the molecules, in which only one of the phenyl rings forms π–π stacking with graphene. The torsional motion (or polarization) of these three PBDE molecules on the graphene surface required the conformations to consume a large amount of energy, which is an unfavorable process as compared with the other congeners. In addition, compared with BDE 183, the interaction energies of BDE 209 and BDE 154 deviated much more from the correlation line. From the geometry of the BDE 183 adsorption system in Fig. 3, the so called ‘co-planar’ phenyl ring with the graphene surface is nearly strictly parallel to the graphene surface (the dihedral angle between them is 2°, Table 1). However, the dihedral angle between the ‘co-planar’ phenyl ring in BDE 209 (BDE 154) and the graphene surface is 8° (10°), which reduced the interaction energies between the PBDEs and graphene, thus making these two cases farther from the correlation line than BDE 183. This phenomenon may be caused by the perpendicular phenyl rings in these three BDE congeners, in which the substitution positions of bromine atoms are different.
3.4. Electronic properties of the adsorption systems
The electronic properties of the PBDE–graphene adsorption systems were also analyzed. Fig. 4 shows the electronic density of states (DOS) for the BDE 47 and BDE 209 adsorption systems. For both adsorption systems, the total DOS around the Fermi level was nearly a direct superposition of DOS for the PBDE molecule and graphene. No obvious difference was observed for the DOS of graphene after the PBDE adsorption. In addition, Mulliken analysis results showed that only a little charge transfer (<0.08 e) occurred between the PBDEs and graphene surface (shown in Table 2). Fig. 5 shows the electron density difference between the adsorption system and the isolated adsorbate and adsorbent in the system, which reflects the effect of adsorption on the electron density redistribution. For the BDE 47 adsorption system, there is a little charge loss in the intra-space between the main plane of the phenyl ring and graphene, which is caused by the typical π electron repulsion. It is worthy to note that there is a small amount of electron accumulation between the bromine atom and graphene, which is believed to have enhanced the interaction strength between the BDE 47 molecule and graphene to a certain extent. For the BDE 209 adsorption system, it is obvious that electron accumulation occurs between graphene and the nearest bromine atom just like a covalent bond; however, the value of the electron accumulation is minor (<0.004 e A−3). Taking into account the above analysis, the adsorption process of PBDEs on graphene surfaces is physisorption.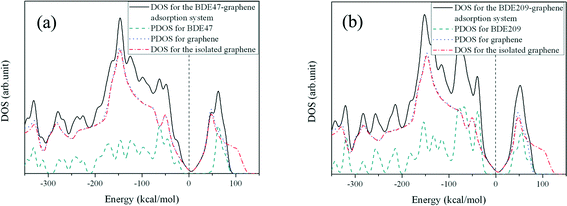 | ||
| Fig. 4 DOS for (a) the BDE47 adsorption system and (b) the BDE209 adsorption system. The Fermi level was set as zero. | ||
The HOMO–LUMO gaps of isolated PBDE congeners were calculated in an aqueous environment. As shown in Table 1, in general, with an increasing degree of bromination, the HOMO–LUMO gap of PBDE congeners decreases accordingly. It is believed that the PBDE congeners with larger HOMO-LUMO gaps would give a relatively low molecular polarizability and vice versa. As graphene is a non-polar material with homogeneous atoms, the interaction strength between the adsorbate and graphene is positively correlated with the molecular polarizability of the adsorbates in a remarkable way.44,52,53 Therefore, except those of BDE 154, 183 and 209, the interaction energies of PBDEs with graphene increase with an increasing degree of bromination.
3.5. Thermodynamic parameters
The thermodynamic parameters including free energy, enthalpy and entropy of the BDE 47 and BDE 209 adsorption systems were calculated to compare the differences between these two types of adsorption configurations. The changes of the free energy (ΔG), enthalpy (ΔH) and entropy (ΔS), which are defined as the difference between the relevant thermodynamic parameters of the entire adsorption system and the sum of the isolated reactants, are shown in Table 3. For both the BDE 47 and BDE 209 systems, the ΔG values are negative, which implies that the adsorption process of the two systems is spontaneous. In addition, the adsorption processes in these two systems are exothermic, which can be judged from the negative values of ΔH for the two systems. However, the changes of entropy for the two systems were not coincident. ΔS for the BDE 47 adsorption system is negative. Taking into account the ΔH value of the BDE 47 system (ΔH < 0, ΔS < 0), the dominant driving force in this system is van der Waals interactions.54 It confirms the previous analysis that van der Waals interaction is the dominating factor that contributes to the π–π stacking.45 On the other hand, the changes of enthalpy and entropy for the BDE 209 system (ΔH < 0, ΔS > 0) indicate that electrostatic interaction is the relatively obvious driving force in this adsorption system.54 It may be caused by the nearly vertical phenyl ring of BDE 209 molecule on graphene because attractive electrostatics is the most important factor affecting the T-shape configuration.45 Although the driving forces of the BDE 47 and BDE 209 systems are different, they are both classified into the domain of non-covalent interactions, which are very weak interactions. This can also be reflected by the relatively low absolute values of ΔH in these two adsorption systems (<40 kcal mol−1), which indicate that the heat releases are very low and further confirm that the adsorption process for both the two types of adsorption systems is physisorption.| Systems | Thermodynamic properties (298.15 K) | ||
|---|---|---|---|
| ΔG (kcal mol−1) | ΔH (kcal mol−1) | ΔS (cal (mol K)−1) | |
| BDE 47-G | −22.51 | −18.00 | −5.55 |
| BDE 209-G | −22.31 | −16.14 | 2.85 |
3.6. Dynamic behaviors of PBDEs adsorbing on graphene surfaces
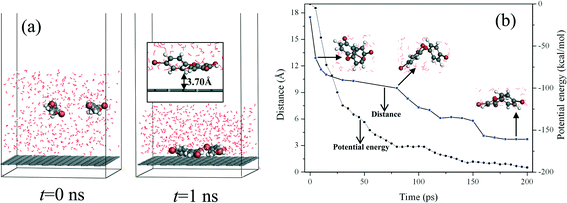
In this section, an additional MD simulation was carried out to study the dynamic adsorption behavior of PBDEs on the graphene surface in water. BDE 28 was selected as an example. By considering the balance between the calculation efficiency and computational resources, a simple simulation model containing two BDE 28 molecules, 500 water molecules, and a graphene surface was constructed. As shown in Fig. 6(a), in the initial simulation box, the two BDE 28 molecules were randomly put in the water box with a distance of approximately 18 Å to the graphene surface. An energy minimization process was performed, then 1 ns constant temperature and constant volume (NVT) simulation was carried out at a temperature of 298 K to obtain the equilibration adsorption system. Fig. 6(a) shows the snapshots of the initial and final configurations of the BDE 28 adsorption system. Both the two BDE 28 molecules were adsorbed on the graphene surface after MD simulation. As shown in the inset of Fig. 6(a), the adsorption distance between the center of mass of BDE 28 and graphene is about 3.70 Å, which is close to our DFT calculation result (3.41 Å). Fig. 6(b) shows the relationship between the adsorption distance of one BDE 28 molecule and the simulation time. It can be found that the conformation of the BDE 28 molecule changes ceaselessly during the adsorption process. After about 200 ps, the center of mass of the BDE 28 molecule has reached the equilibrium position (that is about 3.70 Å from the graphene surface) with a relatively parallel conformation to the graphene surface. Within this duration of 200 ps, the potential energy of the entire system decreased by about 200 kcal mol−1 (Fig. 6(b)). This process reflected a fast adsorption process of BDE 28, which indicated that graphene was sensitive to PBDE congeners and confirmed the remarkable performance of graphene in the application of PBDE extraction.4. Conclusions
In this work, the adsorption properties of PBDEs on graphene surfaces were studied. The calculation results showed that the interaction strength of the first group of PBDEs (BDE 15, BDE 28, BDE 47, BDE 99, BDE 153 and BDE 194) and DE exhibited a positive linear correlation with the hydrophobicity of PBDEs. The DOS and charge transfer analysis indicate that the adsorption process of PBDEs on graphene is physisorption. The π–π stacking plays an important role in the PBDE adsorption systems. The MD simulation showed that graphene was sensitive to PBDE and the adsorption process of PBDE on graphene was very fast, which confirmed the remarkable performance of graphene in the application of PBDE extraction. This work will contribute to the understanding of the adsorption chemistry of aromatic pollutants on graphene-like materials.Acknowledgements
This work was supported by the Centre for Applied Computing and Interactive Media at City University of Hong Kong. It was also supported by a grant from the Shandong Province Special Grant for High-Level Overseas Talents (grant no. tshw20120745), the National Natural Science Foundation of China (grant no. 21205071) and the Natural Science Foundation of Shandong Province, China (grant no. R2012BQ009).References
- T. P. Whitehead, F. R. Brown and C. Metayer, et al., Polybrominated diphenyl ethers in residential dust: sources of variability, Environ. Int., 2013, 57, 11–24 CrossRef PubMed.
- Y. X. Yu, Y. P. Pang, C. Li and X. Y. Zhang, et al., Concentrations and seasonal variations of polybrominated diphenyl ethers (PBDEs) in in- and out-house dust and human daily intake via dust ingestion corrected with bioaccessibility of PBDEs, Environ. Int., 2012, 42, 124–131 CrossRef CAS PubMed.
- M. Meneses, H. Wingfors, M. Schuhmacher, J. L. Domingo, G. Lindströ and B. V. Bavel, Polybrominated diphenyl ethers detected in human adipose tissue from Spain, Chemosphere, 1999, 39, 2271–2278 CrossRef CAS.
- K. Hooper and T. A. McDonald, The PBDEs: an emerging environmental challenge and another reason for breast-milk monitoring programs, Environ. Health Perspect., 2000, 108, 387–392 CrossRef CAS.
- J. X. Wang, Z. K. Lin, K. F. Lin, C. Y. Wang, W. Zhang, C. Y. Cui, J. D. Lin, Q. X. Dong and C. J. Huang, Polybrominated diphenyl ethers in water, sediment, soil, and biological samples from different industrial areas in Zhejiang, China, J. Hazard. Mater., 2011, 197, 211–219 CrossRef CAS PubMed.
- A. Sjödin, D. G. Patterson Jr. and Å. Bergman, A review on human exposure to brominated flame retardants—particularly polybrominated diphenyl ethers, Environ. Int., 2003, 29, 829–839 CrossRef.
- S. Lunder, L. Hovander and Å. Bergman, Polybrominated diphenyl ethers in serum from Californian mother–child pairs, Organohalogen Compd., 2009, 71, 002726–002730 Search PubMed.
- H. A. Chao, S. C. C. Chen, C. M. Chang, T. W. Koh and G. P. C. Chien, et al., Concentrations of polybrominated diphenyl ethers in breast milk correlated to maternal age education level, and occupational exposure, J. Hazard. Mater., 2010, 175, 492–500 CrossRef CAS PubMed.
- S. J. Chen, Y. J. Ma, J. Wang, M. Tian, X. J. Luo, D. Chen and B. X. Mai, Measurement and human exposure assessment of brominated flame retardants in household products from South China, J. Hazard. Mater., 2010, 176, 979–984 CrossRef CAS PubMed.
- J. T. Sun, J. Y. Liu, Q. Liu, G. B. Qu, T. Ruan and G. B. Jiang, Sample preparation method for the speciation of polybrominated diphenyl ethers and their methoxylated and hydroxylated analogues in diverse environmental matrices, Talanta, 2012, 88, 669–676 CrossRef CAS PubMed.
- X. F. Chen, C. G. Cheng, X. Wang and R. S. Zhao, Sensitive determination of polybrominated diphenyl ethers in environmental water samples with etched stainless steel wire based on solid-phase microextraction prior to gas chromatography–mass spectrometry, Anal. Methods, 2012, 4, 2908–2913 RSC.
- C. Moeckel, B. Gasic, M. MacLeod, M. Scheringer, K. C. Jones and K. Hungerbühler, Estimation of the Source Strength of Polybrominated Diphenyl Ethers Based on Their Diel Variability in Air in Zurich, Switzerland, Environ. Sci. Technol., 2010, 44, 4225–4231 CrossRef CAS PubMed.
- H. Zhang and H. K. Lee, Plunger-in-needle solid-phase microextraction with graphene-based sol–gel coating as sorbent for determination of polybrominated diphenyl ethers, J. Chromatogr., A, 2011, 1218, 4509–4516 CrossRef CAS PubMed.
- Q. Liu, J. B. Shi, J. T. Sun and T. Wang, et al., Graphene-assisted matrix solid-phase dispersion for extraction of polybrominated diphenyl ethers and their methoxylated and hydroxylated analogs from environmental samples, Anal. Chim. Acta, 2011, 708, 61–68 CrossRef CAS PubMed.
- S. L. Zhang, Z. Du and G. K. Li, layer-by-layer fabrication of chemical-bonded graphene coating for solid-phase microextraction, Anal. Chem., 2011, 83, 7531–7541 CrossRef CAS PubMed.
- K. Gustafsson, M. Bjork, S. Burreau and M. Gilek, Bioaccumulation kinetics of brominated flame retardants in blue mussels, Environ. Toxicol. Chem., 1999, 18, 1218–1224 CAS.
- J. Nacher-Mestre, R. Serrano, F. Hernandez and L. Benedito-Palos, Gas chromatography–mass spectrometric determination of polybrominated diphenyl ethers in complex fatty matrices from aquaculture activities, Anal. Chim. Acta, 2010, 664, 190–198 CrossRef CAS PubMed.
- M. S. Mauter and M. Elimelech, Environmental applications of carbon-based nanomaterials, Environ. Sci. Technol., 2008, 42, 5843–5859 CrossRef CAS.
- B. Pérez-López and A. Merkoçi, Carbon nanotubes and graphene in analytical sciences, Microchim. Acta, 2012, 179, 1–16 CrossRef.
- Q. Liu, J. B. Shi and G. B. Jiang, Application of graphene in analytical sample preparation, TrAC, Trends Anal. Chem., 2012, 37, 1–11 CrossRef CAS PubMed.
- H. Wang, X. Z. Yuan, Y. Wu and H. J. Huang, et al., Graphene-based materials: Fabrication, characterization and application for the decontamination of wastewater and wastegas and hydrogen storage/generation, Adv. Colloid Interface Sci., 2013, 195–196, 19–40 CrossRef CAS PubMed.
- P. Lazar, F. Karlický, P. Jurečka, M. Kocman, E. Otyepková, K. Šafářová and M. Otyepka, Adsorption of Small Organic Molecules on Graphene, J. Am. Chem. Soc., 2013, 135, 6372–6377 CrossRef CAS PubMed.
- X. Yang, J. X. Li, T. Wen, X. M. Ren, Y. S. Huang and X. K. Wang, Adsorption of naphthalene and its derivatives on magnetic graphene composites and the mechanism investigation, Colloids Surf., A, 2013, 422, 118–125 CrossRef CAS PubMed.
- Q. Wang, J. X. Li, Y. Song and X. K. Wang, Facile synthesis of high-quality plasma-reduced graphene oxide with ultrahigh 4,4′-dichlorobiphenyl adsorption capacity, Chem.–Asian J., 2013, 8, 225–231 CrossRef CAS PubMed.
- S. B. Wang, H. Q. Sun, H. M. Ang and M. O. Tadé, Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials, Chem. Eng. J., 2013, 226, 336–347 CrossRef CAS PubMed.
- H. Q. Sun, S. Z. Liu, G. L. Zhou, H. M. Ang, M. O. Tadé and S. B. Wang, Reduced graphene oxide for catalytic oxidation of aqueous organic pollutants, ACS Appl. Mater. Interfaces, 2012, 4, 5466–5471 CAS.
- Q. Liu, J. B. Shi, J. T. Sun, T. Wang, L. X. Zeng and G. B. Jiang, Graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction, Angew. Chem., 2011, 123, 6035–6039 CrossRef.
- G. X. Zhao, L. Jiang, Y. D. He, J. X. Li, H. L. Dong, X. K. Wang and W. P. Hu, Sulfonated graphene for persistent aromatic pollutant management, Adv. Mater., 2011, 23, 3959–3963 CrossRef CAS PubMed.
- S. Z. Deng, V. Tjoa and H. M. Fan, Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor, J. Am. Chem. Soc., 2012, 134, 4905–4917 CrossRef CAS PubMed.
- Y. H. Zhang, Z. R. Tang, X. Z. Fu and Y. J. Xu, TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: is TiO2-graphene truly different from other TiO2-carbon composite materials, ACS Nano, 2010, 4, 7303–7314 CrossRef CAS PubMed.
- Z. Wang, J. W. Chen, Q. Sun and W. J. G. M. Peijnenburg, C60-DOM interactions and effects on C60 apparent solubility: a molecular mechanics and density functional theory study, Environ. Int., 2011, 37, 1078–1082 CrossRef CAS PubMed.
- M. Y. Zou, J. D. Zhang, J. W. Chen and X. H. Li, Simulating adsorption of organic pollutants on finite (8,0) single-walled carbon nanotubes in water, Environ. Sci. Technol., 2010, 44, 4225–4231 CrossRef PubMed.
- C. Rajesh, C. Majumder, H. Mizuseki and Y. Kawazoe, A theoretical study on the interaction of aromatic amino acids with graphene and single walled carbon nanotube, J. Chem. Phys., 2009, 130, 124911 CrossRef PubMed.
- Y. V. Shtogun, L. M. Woods and G. I. Dovbeshko, Adsorption of adenine and thymine and their radicals on single-wall carbon nanotubes, J. Phys. Chem. C, 2007, 111, 18174–18181 CAS.
- Q. Lin, X. L. Zou, G. Zhou, R. Liu, J. Wu, J. Li and W. H. Duan, Adsorption of DNA/RNA nucleobases on hexagonal boron nitride sheet: an ab initio study, Phys. Chem. Chem. Phys., 2011, 13, 12225–12230 RSC.
- N. Ding, X. F. Chen, C. M. L. Wu and H. Li, Adsorption of nucleobase pairs on hexagonal boron nitride sheet: hydrogen bonding versus stacking, Phys. Chem. Chem. Phys., 2013, 15, 10767–10776 RSC.
- J. Antony and S. Grimme, Structures and interaction energies of stacked graphene-nucleobase complexes, Phys. Chem. Chem. Phys., 2008, 10, 2722–2729 RSC.
- H. Sun, P. Ren and J. R. Fried, The COMPASS force field: parameterization and validation for phosphazenes, Comput. Theor. Polym. Sci., 1998, 8, 229–246 CrossRef CAS.
- Z. Xu, S. L. Yuan, H. Yan and C. B. Liu, Adsorption of histidine and histidine-containing peptides on Au (111): a molecular dynamics study, Colloids Surf., A, 2011, 380, 135–142 CrossRef CAS PubMed.
- K. Y. Yan, Q. Z. Xue, D. Xia, H. J. Chen, J. Xie and M. D. Dong, The core/shell composite nanowires produced by self-scrolling carbon nanotubes onto copper nanowires, ACS Nano, 2009, 3, 2235–2240 CrossRef CAS PubMed.
- P. P. Ewald, Evaluation of optical and electrostatic lattice potentials, Ann. Phys., 1921, 64, 253–287 CrossRef.
- Y. Y. Zhao, F. M. Tao and E. Y. Zeng, Theoretical study on the chemical properties of polybrominated diphenyl ethers, Chemosphere, 2008, 70, 901–907 CrossRef CAS PubMed.
- J. W. Hu, L. Eriksson, Å. Bergman and E. Jakobsson, et al., Molecular orbital studies on brominated diphenyl ethers. Part I— conformational properties, Chemosphere, 2005, 59, 1033–1041 CrossRef CAS PubMed.
- J. W. Hu, L. Eriksson, Å. Bergman and E. Jakobsson, et al., Molecular orbital studies on brominated diphenyl ethers. Part II— reactivity and quantitative structure-activity (property) relationships, Chemosphere, 2005, 59, 1043–1057 CrossRef CAS PubMed.
- G. B. McGaughey, M. Gagné and A. K. Rappé, Π-stacking interactions, J. Biol. Chem., 1998, 273, 15458–15463 CrossRef CAS PubMed.
- Y. D. Ma, Y. Dai, M. Guo and B. B. Huang, Graphene-diamond interface: Gap opening and electronic spin injection, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 235448 CrossRef.
- I. Hamada and M. Otani, Comparative van der Waals density-functional study of graphene on metal surface, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 153412 CrossRef.
- J. H. Lee, Y. K. Choi, H. J. Kim, R. H. Scheicher and J. H. Cho, Physisorption of DNA nucleobases on h-BN and graphene: vdW-corrected DFT calculations, J. Phys. Chem. C, 2013, 117, 13435–13441 CAS.
- A. Palm, The environmental fate of polybrominated diphenyl ethers in the centre of Stockholm - assessment using a multimedia fugacity model, 2001, http://www.ivl.se/download/18.7df4c4e812d2da6a416800071601/b1400.pdf Search PubMed.
- L. N. Li, S. D. Xie, H. Cai, X. T. Bai and Z. Xue, Quantitative structure–property relationships for octanol-water partition coefficients of polybrominated diphenyl ethers, Chemosphere, 2008, 72, 1602–1606 CrossRef CAS PubMed.
- M. Bläuenstein, Modeling the environmental fate of polybrominated diphenyl ethers in lake thun, 2007, http://www.sust-chem.ethz.ch/docs/PBDEs_in_LakeThun.pdf Search PubMed.
- M. Karelson and V. S. Lobanov, Quantum-chemical descriptors in QSAR/QSPR studies, Chem. Rev., 1996, 96, 1027–1043 CrossRef CAS PubMed.
- A. Gowtham, R. H. Scheicher, R. Ahuja, R. Pandey and S. P. Karna, Physisorption of nucleobases on graphene: density-functional calculations, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 033401 CrossRef.
- P. D. Ross and S. Subramanian, Thermodynamics of protein association reactions: forces contributing to stability, Biochemistry, 1981, 20, 3096–3102 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3en00037k |
| This journal is © The Royal Society of Chemistry 2014 |