Quantification of carbon nanomaterials in vivo: direct stable isotope labeling on the skeleton of fullerene C60
Xue-Ling
Chang
*a,
Longfei
Ruan
ac,
Sheng-Tao
Yang
*ab,
Baoyun
Sun
a,
Cuibin
Guo
a,
Liangjun
Zhou
a,
Jinquan
Dong
a,
Hui
Yuan
a,
Gengmei
Xing
a,
Yuliang
Zhao
*a and
Min
Yang
c
aCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, PR China. E-mail: changxl@ihep.ac.cn; zhaoyuliang@ihep.ac.cn; Tel: +86 10 88236709
bCollege of Chemistry and Environment Protection Engineering, Southwest University for Nationalities, Chengdu, 610041, PR China. E-mail: yangst@pku.edu.cn; Tel: +86 28 85522269
cHubei University of Chinese Medicine, Wuhan, 430065, PR China
First published on 24th December 2013
Abstract
Concerns over the biosafety of carbon nanomaterials have been raised, due to their unique structure, properties and applications. However, a lack of easily accessible quantification methods for in vivo carbon nanomaterials largely limits the evaluation of their biosafety. Here, for the first time we adopted 13C stable isotopic labeling for the quantification of a fullerene in vivo. 13C-enriched fullerene C60 was synthesized by arc discharge and purified by high performance liquid chromatography. The 13C-enriched C60 was dispersed in tween 80 aqueous solution for animal administration and was then monitored and quantified by isotope ratio mass spectrometry after intravenous injection (i.v.). Our results showed that C60 quickly cleared from the blood circulation with a half-life of 14 min, and selective accumulation in the liver, spleen and lungs was observed, with slight decreases seen within 24 h. The pharmacokinetics of C60 could be evaluated using the two-compartmental model, in which the fast clearance after i.v. from the blood circulation and slow clearance by the uptake in tissues were revealed. The present findings demonstrated the feasibility of using 13C stable isotopically labeled carbon nanomaterials such as fullerene to trace and quantitatively monitor their bio-behavior in vivo, and suggested that 13C-enriched carbon nanomaterials might bring about a new platform to study the environmental fate of carbon nanomaterials.
Nano impact |
Carbon nanomaterials are widely used, and their interactions with biological/environmental systems are inevitable. Their biosafety needs to be fully considered and investigated. However, a lack of easily accessible quantification methods of in vivo carbon nanomaterials largely limits evaluation of their biosafety. Here, highly stable fullerene C60 was prepared, labeled with 13C on the carbon skeleton. The biodistribution of 13C labeled C60 was quantified after intravenous injection into mice. Quick blood clearance and high uptake in the liver, spleen and lungs were observed. The present results unambiguously demonstrate for the first time that the 13C labeling technique is applicable for tracing the absorption, distribution, metabolism, and excretion (ADME) of fullerene, which could benefit biosafety studies of carbon nanomaterials.
Introduction
Since their discovery, carbon nanomaterials have attracted great attention for their unique structure and properties. Carbon nanomaterials have found applications in biomedicine, electronics, energy, etc. In particular, biomedical applications of carbon nanomaterials have been widely studied, with significant results. As an example, fullerene and its derivatives could be used for cancer treatment,1–5 antioxidation against oxidative stress and free radicals,1,6,7 bioimaging8 and desensitization of IgE-induced anaphylaxis.9 Commercial products containing fullerene are now available to the public. Obviously, before the large-scale production for such application, the biosafety of fullerene needs to be fully considered and investigated. Among various evaluations of biosafety, quantitative and non-destructive investigation of the pharmacokinetics and biodistribution of fullerene nanomaterials is fundamental and crucial.To date, there have been a number of reports of the in vivo quantification of unmodified fullerene nanomaterials. Generally, the in vivo quantification of fullerene can be performed by high-performance liquid chromatography (HPLC) coupled with mass spectrometry (MS). Since 1996, Moussa et al. have quantitatively analyzed C60 in blood and tissue by HPLC with photodiode-array and MS. They investigated the pharmacokinetics,10 biodistribution,10 potential liver metabolites,11 and the toxicity12 of fullerene in animals. Shinohara et al. reported the long-term tissue clearance kinetics of C60 from rat lung by HPLC with ultraviolet absorptiometry.13 Recently, Kubota et al. separated and quantified liposome encapsulated fullerene by HPLC-MS/MS and studied time-dependent variation in the biodistribution of C60 in rats.14,15 Beyond the development of liquid chromatography methods, other reliable methods are also in high demand for the in vivo quantification of fullerene.
Isotopic labeling is widely adopted in the quantification of nanomaterials in vivo. Many radioactive isotopes, such as 14C, 67Ga, 99mTc, 125I and 166Ho, have been used to label fullerene.16–21 However, radioactive labeling suffers several drawbacks. In general, the conditions for radioactive labeling and the operation are not available for most toxicologists and pharmacologists, and the radioactive waste is a serious concern. Further, with the exception of 14C, the other isotopes could only be applied in the study of functionalized fullerenes. As an alternative, the long-lived radioactive isotope 14C (t1/2 5730 years), which is appropriate for long-term tracing studies, has been often used for labeling fullerene. However, the whole procedure of synthesis, detection and waste treatment of 14C–fullerene is quite difficult.
Given the disadvantages of radioactive labeling under the strict conditions of radioactivity operation and the generation of radioactive waste, stable isotope labeling is a good choice in order to avoid these issues.18,22–26 Generally, stable isotope labeling is combined with isotope ratio mass spectrometry (IRMS), which is a specialization of mass spectrometry and can precisely measure the relative abundance of stable isotopes. Carbon isotope ratios are measured relative to the internationally recognized C standard Vienna Pee Dee Belemnite (VPDB) and are reported in delta notation, δ.26 As a simple but powerful technique, stable isotopic labeling has been employed in some areas of biomedical research.22–26 We have demonstrated previously the stable isotopic labeling of carbon nanotubes (CNTs) and carbon quantum dots for in vivo biodistribution studies.27–29 Fullerene shares a structure similar to CNTs, therefore 13C might be an ideal choice for the quantification of fullerene. For the first time, we attempt to explore this technique in the study of fullerene.
Herein, we labeled fullerene C60 with 13C isotopes on the skeleton and quantified the 13C-enriched C60 in mice (Fig. 1). 13C-enriched C60 was prepared by the arc discharge method and dispersed in tween 80 aqueous solution for the animal experiments. After i.v. injection, blood and tissue samples were collected to analyze the 13C-enriched C60 content. The isotope ratio mass spectrometry (IRMS) results indicated that 13C-enriched C60 cleared from blood quickly and was trapped in the organs of the reticuloendothelial system (RES). The 13C-labeling technique provides a means to simply and directly assess the bio-behavior of unaltered carbon nanomaterials (including fullerene), which would largely benefit evaluation of the biosafety and environmental fate studies of carbon nanomaterials.
Experimental methods
Preparation of 13C-enriched C60
13C-enriched C60 was prepared by the arc discharge method. Amorphous carbon powder 99.9% 13C was purchased from Cambridge Isotopes. Briefly, the anode electrodes were fabricated by coring the natural-abundance carbon rods and packing the core with isotopically enriched amorphous carbon powder (the mass ratio of 13C-powder/12C-rod was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85).30–32 The cathode was a pure graphite rod. The arc discharge was performed under Ar atmosphere (135 Torr) with the following parameters: interelectrode distance: 5 mm; current: 110 A; voltage 27 V. The ash was refluxed in CS2 for 10 h to extract the 13C-enriched C60. The 13C-enriched C60 was further purified by HPLC. Unlabeled C60 was prepared following the same protocol by using normal carbon as the starting material. The obtained sample was characterized by MS (Autoflex, Bruker, Germany), infrared spectroscopy (IR, Tensor27, Bruker, Germany) and Raman spectroscopy (Renishaw inVia plus, Renishaw, UK). The 13C-contents were determined by IRMS (MAT-253, Thermo Electron-Finnigan, USA).
85).30–32 The cathode was a pure graphite rod. The arc discharge was performed under Ar atmosphere (135 Torr) with the following parameters: interelectrode distance: 5 mm; current: 110 A; voltage 27 V. The ash was refluxed in CS2 for 10 h to extract the 13C-enriched C60. The 13C-enriched C60 was further purified by HPLC. Unlabeled C60 was prepared following the same protocol by using normal carbon as the starting material. The obtained sample was characterized by MS (Autoflex, Bruker, Germany), infrared spectroscopy (IR, Tensor27, Bruker, Germany) and Raman spectroscopy (Renishaw inVia plus, Renishaw, UK). The 13C-contents were determined by IRMS (MAT-253, Thermo Electron-Finnigan, USA).
Dispersion of 13C-enriched C60
13C-enriched C60 (30 mg) was dissolved in CS2 and then 300 mg tween 80 was added. After vigorous stirring for 10 min, CS2 was removed by vacuum distillation. The sample was further dried under vacuum at 80 °C to remove the residual CS2. The dispersion was obtained by adding 15 mL deionized water and sonicating for 30 min. Large aggregates were removed by centrifugation at 300 rpm for 2 min. The dispersion was characterized using scanning electron microscopy (SEM, S-4800, Hitachi, Japan), transmission electron microscopy (TEM, Tecnai G2 20 S-TWIN, FEI, USA) and Nanosizer (Zetasizer 3000 HS, Malvern, UK).Quantification of 13C-enriched C60 in tissues
About 2 mL deionized water was added to 0.5 g tissue sample. The sample was then homogenized thoroughly on a homogenizer. The homogenate was lyophilized and the dry powder was collected. To quantify the 13C-enriched C60, IRMS analysis was performed and the result was given in the form of a δ value. The δ value was converted into 13C/12C ratio (r) following eqn (1), where the 13C/12C ratio of the VPDB standard sample [(13C/12C)standard] was 0.0112372. The r value was then converted into the percentage of 13C in mass (ω13C) following eqn (2). The amount of 13C-enriched C60 in samples (m13C-fullerene) could be calculated from eqn (3), where ωcarbon from IRMS was the content of carbon in the dry sample; msample was the weight of sample; mdry and mwet were the weights of tissue before and after drying, which was determined following our previous report;28 the percentage of 13C in 13C-enriched C60 was found to be 9.108%. | (1) |
 | (2) |
 | (3) |
For blood samples, the concentration of 13C-enriched C60 was expressed in μg C60 per mL blood (eqn (4)). For other tissues, the content of 13C-enriched C60 was expressed as %ID g−1 (percentage of injected dose per gram tissue) (eqn (5)) or %ID (percentage of injected dose) (eqn (6)), where dose was the amount of injected 13C-enriched C60.
 | (4) |
 | (5) |
 | (6) |
Blood clearance of 13C-enriched C60
All animal experiments were performed in compliance with the institutional ethics committee regulations and guidelines on animal welfare and approved by the Animal Center of Southwest University for Nationalities. Male KM mice (~25 g) were obtained from Sichuan University Animal Center and raised in a plastic cage (5 mice per cage). Each mouse was injected with 400 μL of 13C-enriched C60 dispersion (2.2 mg mL−1) via a single tail vein injection. The mice were sacrificed at specified time intervals. Blood samples were collected and stored at −18 °C before sampling. The control mice were injected with 400 μL of normal C60 dispersion and the control samples were collected following the same protocol. The one-compartmental (eqn (7)) and two compartmental (eqn (8)) models were adopted to analyze the data.27 In eqn (7), c0 is the initial blood concentration; c is the blood concentration; t is the time and k is the elimination rate constant. In eqn (8), α is the distributional rate constant; β is the eliminative rate constant; c is the blood concentration; t is the time; A and B are empirical constants.| c = c0 ⋅ e−kt | (7) |
| c = A ⋅ e−αt + B ⋅ e−βt | (8) |
Biodistribution of 13C-enriched C60
Each mouse was injected with 400 μL of 13C-enriched C60 dispersion (2.2 mg mL−1) via a single tail vein injection. The mice were sacrificed and dissected at specified time intervals. Tissues and organs, including heart, liver, spleen, stomach, kidneys, lungs, brain, small intestine, large intestine, muscle (hind leg), bone (shank) and skin, were collected and stored at −18 °C before sampling. The control samples were collected following the same protocol by administration with 400 μL of normal C60 dispersion.Statistical analysis
All data were expressed as the mean of four individual observations with standard deviation. Significance was calculated using Student's t-test, where p < 0.05 was taken as the statistical significance.Results and discussion
Skeleton labeling of C60
13C isotopes were labeled on the skeleton of C60. According to the IRMS analyses, the C60 samples were enriched to around 9.108 wt% 13C. The isotope effect difference was clearly indicated in the MS spectra, where the partially enriched C60 with a statistical distribution of isotopes was observed. As shown in Fig. 2a, in comparison with the normal C60, the highest peak of 13C-enriched C60 shifted from 720 (m/z) to 725.71 (m/z). The isotopic distributions in the MS spectra (range from 720 to 735 m/z) displayed the Poisson distribution extremely well . This experimental evidence is consistent with that in the literature.30–32 Normal C60 has a pronounced peak at 720 (m/z), due to the high abundance of 12C in nature. The enrichment of 13C was also observed in the molecular vibrational spectrum. In the IR spectra, several peaks shifted toward lower wavenumbers. For example, the peak at 527 cm−1 shifted to 523 cm−1 after 13C was incorporated. Peak shifts were also observed in the Raman spectra. The G-band of the graphene structure shifted from 1468 cm−1 to 1461 cm−1 upon incorporation of 13C. Mass and vibration spectral analyses of the 13C-enriched C60 suggested that 13C-enriched C60 still retained the characteristic structure of fullerene itself. Incorporation of 13C resulted only in a shift of the characteristic peaks; it did not change its intrinsic structure.33,34 Overall, the above results collectively indicated that our C60 sample was 13C enriched. | ||
| Fig. 2 Characterization of 13C-enriched C60. (a) MS of 13C-enriched C60; (b) MS of unlabelled C60; (c) IR spectra; (d) Raman spectra. | ||
The purified 13C-enriched C60 was not water dispersible, thus, it was unsuitable for animal experiments. We dispersed C60 in tween 80 aqueous solution assisted by CS2. Previous reports showed that the aqueous dispersion of C60 could be achieved by adding a solution of C60 in an organic solvent dropwise to water under sonication. However, following such protocol led to the appearance of a trace amount of remnant CS2, which could not be removed even after vacuum evaporation for 24 h. CS2 is a toxic chemical, which might affect the biological evaluation. We therefore improved the protocol by first dispersing C60 in CS2 and then adding tween 80. CS2 could be thoroughly removed and C60 was well coated by tween 80. Finally, the coated C60 was dispersed by water. Following our protocol, nanorods of 13C-enriched C60 were obtained. It should be stated that this method has not been adopted previously for the production of fullerene products. The advantage of this method was that it avoid the toxicity of an organic solvent, which might lead to false positive results.35,36 As shown in Fig. 3a, rod-like C60 aggregates were present. The surface of the rods was fuzzy, which should be attributed to the soft chains of tween 80. The lengths of C60 rods were found to be about 90 nm and the diameters were around 30 nm by SEM. After thorough washing, most of the tween 80 was removed and the surface of the rods was distinguishable. The small dots of C60 aggregates in the nanorods could be recognized (inset in Fig. 3a). The small aggregates were also confirmed by TEM images (data not shown). The microscopy results were consistent with the size measurement on a Nanosizer. The average size of the C60 rods was 64 nm. A very tiny peak was observed at 980 nm (0.2% number). Tween 80 has been approved by US Food and Drug Administration (FDA) for i.v. injection, therefore the dispersed C60 is suitable for i.v. studies. The dispersion was injected to mice immediately after the sonication.
 | ||
| Fig. 3 Characterization of C60 dispersion. (a) SEM; (b) DLS. Inset of (a) is the SEM image of the thoroughly washed C60. | ||
Blood clearance of 13C-enriched C60
Stable isotopes 13C were incorporated into the skeleton of C60, hence possessing high stability in vivo. Skeleton labeling retains the intrinsic structure, ensuring that the labels reflect the real properties of C60. Thus, 13C-enriched C60 was ideal for the in vivo quantification of chemically unmodified C60. We demonstrated the feasibility by injecting 13C-enriched C60 into mice. The blood concentration of 13C-enriched C60 was determined by IRMS post i.v. injection. As shown in Fig. 4, the blood concentration decreased sharply in the first 30 min. Nearly all of the injected C60 was cleared at 24 h post-exposure, where the C60 concentration was only 2.56 μg mL−1. The data were better described by the two-compartmental model. The parameters are listed in Table 1. The short first-phase half-life (t1/2α) indicated that C60 was distributed in mice quickly. The eliminative half-life (t1/2β) was 191 min, suggesting that the thorough clearance of C60 in blood circulation was of moderate speed. The steady-state volume of distribution (Vss) was 32.6 mL, larger than the total body water content (17.5 mL).37 This suggested that C60 was distributed extensively in blood and extracellular fluid. The total clearance (CL) was 0.12 mL min−1, lower than the hepatic blood perfusion speed (1.2 mL min−1),37 suggesting that the clearance of C60 in blood circulation was not that quick. In addition, we fitted the data to the one-compartmental model, where the blood circulation half-life (t1/2) was obtained for the comparison with other reports. The t1/2 of C60 was 14 min.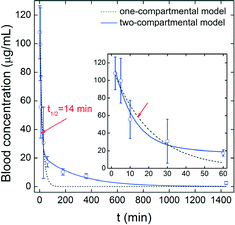 | ||
| Fig. 4 Time-dependent blood concentrations of 13C-enriched C60 in mice after i.v. administration (n = 4). | ||
There are several reports on the blood clearance of fullerene materials in vivo. For unmodified fullerene, the blood clearance depends more on the dispersant and dispersion method. Kubota et al. found that a C60-liposome solution was undetectable in blood at 1 day post-exposure.15 Moussa et al. reported the blood concentration of C60 as 179 mg L−1 at 1 day, which further decreased to 1.10 mg L−1 after 6 days.10 Yamago et al. labeled water-miscible C60 with 14C; its uptake was only 1.6%ID at 1 h and the radioactivity remained undetectable at 160 h.38 Nikolic et al. prepared 125I–C60 nanocrystals by a tetrahydrofuran (THF) method and determined the blood concentration.16 At 90 min, the blood concentration was significantly higher than that of free Na125I. Similarly, functionalized fullerene compounds were found to be cleared rather quickly from the blood circulation within a short period.39–43
The 13C-labeling technique is a sensitive, reliable and non-destructive method for tracing carbon nanomaterials in vivo. All of these reports further demonstrate that our results of the pharmacokinetics of 13C-enriched C60 are reasonable. The chemically pristine (chemically unmodified) C60 rapidly (within a few minutes) cleared from the blood circulation and is then captured by the tissues of the whole body regardless of the exposure pathways. However, the surface chemistry and/or chemical environment of fullerene does influence its blood circulation. The slight differences in the data reported above potentially resulted from the different exposure pathways, times or doses. In future studies, the influence of the fast blood clearance on the applications and toxicity of fullerene should be addressed.
Biodistribution of 13C-enriched C60
After being cleared from the blood circulation, 13C-enriched C60 was mainly trapped in the liver, spleen and lungs (Fig. 5). In other tissues, the C60 concentration decreased along with the decrease of blood concentration. This is reasonable, since many studies have demonstrated that nanoparticles tend to be trapped in RES organs. The C60 concentration was higher in the spleen and lungs than in the liver. The accumulation in the kidneys was statistically significant at 6 h post-exposure, but no significant value was measured at 24 h. The accumulation in the small intestine was statistically significant during 24 h. Trapping in the brain was observed too, however, the short accumulation was statistically significant only at 5 min. This might be attributed to the high blood concentration.The total contents of C60 in the liver, spleen and lungs were calculated. As shown in Fig. 6, the major accumulation was in the liver, where the content peaked at 6 h post-exposure (37.2%ID). At 24 h, the content decreased to 23.7%ID, suggesting that C60 could be eliminated from the liver. The maximum contents were found at 1 h in both the spleen and lungs. The contents in the spleen were nearly constant which was consistent with the results of tween 80 dispersed CNTs.27 In the lungs, the decrease of content with time was observed. A possible excretion pathway might be via mucus through mucociliary transport. The considerably high RES uptake of tween 80 dispersed C60 suggested that future toxicological studies of even well dispersed fullerene should be focused on the damage to RES organs.
The biodistribution of C60 has been measured by several groups. For non-covalently dispersed fullerene, the RES uptake dominates. Nikolic et al. reported that the uptake of 125I–C60 nanocrystals by the THF method was higher than that of Na125I in liver, although the accumulation level was still very low.16 Sumner et al. reported polyvinylpyrrolidone (PVP) coated 14C–C60 was trapped in the liver and spleen, after i.v. injection to pregnant and lactating rats.17 The difference occurred in lungs, where a very low concentration was observed in pregnant rats and a high concentration was found in lactating rats. Kubota et al. found that C60-liposome solution was trapped in RES organs.15 The concentration in lungs was extremely high (254 μg g−1 at 1 day post-exposure), much higher than those in the spleen (53.0 μg g−1 at 1 day) and liver (25.5 μg g−1 at 1 day). After that, C60 gradually cleared from the lungs and liver, but the accumulation in the spleen increased. Moussa et al. reported that only 0.7% C60 uptake was detected in the liver and 0.5% in the spleen at 1 day.10 The uptake increased at 2 day to 1.0% for the liver and 2.4% for the spleen. In our study, we also found that tween 80 dispersed C60 was trapped in RES organs. In summary, the different distributions resulted from the different methods of dispertion, reagents and exposure conditions (such as exposure pathway, times or doses). The influence of surface chemistry on the biodistribution of fullerene is also evident in studies of functionalized fullerene.19,20,39–42
Moreover, the biodistribution of tween 80 dispersed C60 was very similar to that of tween 80 dispersed CNTs,27 where RES organs were the accumulating organs. It seemed to be that the dispersant or surface modified chemistry mastered the biodistribution of nanomaterials, much more than the properties of nanomaterials themselves. Similar results were reported in the studies of hydroxylated C60 and hydroxylated CNTs.18 In this regard, we suggest more attention should be paid to the important role of surface chemistry on the biodistribution of nanomaterials.
Conclusions
In summary, we successfully prepared C60 labeled with 13C on the carbon skeleton with high stability, which could be used as a powerful tool for absolutely quantifying in vivo carbon nanomaterials such as fullerene. The 13C-labeling technique does not damage the unique intrinsic structure and properties of carbon nanomaterials. Unlike isotope labeling with iodine, copper and other conventionally used elements, 13C labeling does not introduce foreign atoms onto the carbon-networks. Thus, the present results unambiguously demonstrate for the first time that the 13C labeling technique can be applied for the tracing of fullerene in in vivo absorption, distribution, metabolism, and excretion (ADME) studies. According to the IRMS measurement of the present study, the 13C-enriched C60 cleared quickly from the blood stream. The blood circulation of C60 was calculated using a two-compartmental model. The highest C60 accumulation levels occurred in the RES organs, and slight decreases in the liver and lungs were observed at 24 h post-exposure. Presumably, the toxicological effects of the long-term exposure, the consequence of the surface functionalization and the potential in vivo metabolism of fullerene will be the most necessary and interesting topics for further investigations. Meanwhile, we believe that our results would largely benefit the ongoing biological/environmental safety studies of carbon nanomaterials by revealing information on the uptake, accumulation level, translocation and elimination of carbon nanomaterials.Acknowledgements
We are grateful to Prof. Yuanfang Liu at Peking University and Prof. Xueyun Gao at Institute of High Energy Physics, Chinese Academy of Sciences, for their enthusiastic support and instructive suggestions. We also thank Prof. Xian Zhang at Institute of Urban Environment, Chinese Academy of Sciences, for the IRMS analyses. We thank the National Natural Science Foundation of China (no. 11005116 and 21307101) and the National Basic Research Program of China (973 program: 2012CB932601 and 2013CB932703) for financial support.References
- J. Wang, C. Chen, B. Li, H. Yu, Y. Zhao, J. Sun, Y. Li, G. Xing, H. Yuan, J. Tang, Z. Chen, H. Meng, Y. Gao, C. Ye, Z. Cai, C. Zhu, B. Ma, X. Fang and L. Wan, Biochem. Pharmacol., 2006, 71, 872 CrossRef CAS PubMed.
- W. Zhang, B. Sun, L. Zhang, B. Zhao, G. Nie and Y. Zhao, Nanoscale, 2011, 3, 2636 RSC.
- X. Liang, H. Meng, Y. Wang, H. He, J. Meng, J. Lu, P. Wang, Y. Zhao, X. Gao, B. Sun, C. Chen, G. Xing, D. Shen, M. M. Gottesman, Y. Wu, J. Yin and L. Jia, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 7449 CrossRef CAS PubMed.
- S. Kang, G. Zhou, P. Yang, Y. Liu, B. Sun, T. Huynh, H. Meng, L. Zhao, G. Xing, C. Chen, Y. Zhao and R. Zhou, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15431 CrossRef CAS PubMed.
- C. Chen, G. Xing, J. Wang, Y. Zhao, B. Li, J. Tang, G. Jia, T. Wang, J. Sun, L. Xing, H. Yuan, Y. Gao, H. Meng, Z. Chen, F. Zhao, Z. Chai and X. Fang, Nano Lett., 2005, 5, 2050 CrossRef CAS PubMed.
- N. Gharbi, M. Pressac, M. Hadchouel, H. Szwarc, S. R. Wilson and F. Moussa, Nano Lett., 2005, 5, 2578 CrossRef CAS PubMed.
- T. Baati, F. Bourasset, N. Gharbi, L. Njim, M. Abderrabba, A. Kerkeni, H. Szwarc and F. Moussa, Biomaterials, 2012, 33, 4936 CrossRef CAS PubMed.
- J. Zhang, K. Liu, G. Xing, T. Ren and S. Wang, J. Radioanal. Nucl. Chem., 2007, 272, 605 CrossRef CAS PubMed.
- J. J. Ryan, H. R. Bateman, A. Stover, G. Gomez, S. K. Norton, W. Zhao, L. B. Schwartz, R. Lenk and C. L. Kepley, J. Immunol., 2007, 179, 665 CAS.
- F. Moussa, M. Pressac, E. Genin, S. Roux, F. Trivin, A. Rassat, R. Ceolin and H. Szwarc, J. Chromatogr. B: Biomed. Sci. Appl., 1997, 696, 153 CrossRef CAS.
- F. Moussa, S. Roux, M. Pressac, E. Genin, M. Hadchouel, F. Trivin, A. Rassat, R. Ceolin and H. Szwarc, New J. Chem., 1998, 22, 989 RSC.
- F. Moussa, F. Trivin, R. Ceolin, M. Hadchouel, P. Y. Sizaret, V. Greugny, C. Fabre, A. Rassat and H. Szwarc, Fullerene Sci. Technol., 1996, 4, 21 CrossRef CAS.
- N. Shinohara, T. Nakazato, M. Tamura, S. Endoh, H. Fukui, Y. Morimoto, T. Myojo, M. Shimada, K. Yamamoto, H. Tao, Y. Yoshida and J. Nakanishi, Toxicol. Sci., 2010, 118, 564 CrossRef CAS PubMed.
- R. Kubota, M. Tahara, K. Shimizu, N. Sugimoto, A. Hirose and T. Nishimura, Bull. Natl. Inst. Health Sci., 2009, 127, 65 CAS.
- R. Kubota, M. Tahara, K. Shimizu, N. Sugimoto, A. Hirose and T. Nishimura, Toxicol. Lett., 2011, 206, 172 CrossRef CAS PubMed.
- N. Nikolic, S. Vranjes-Duric, D. Jankovic, D. Dokic, M. Mirkovic, N. Bibic and V. Trajkovic, Nanotechnology, 2009, 20, 385102 CrossRef PubMed.
- S. C. J. Sumner, T. R. Fennell, R. W. Snyder, G. F. Taylorc and A. H. Lewin, J. Appl. Toxicol., 2010, 30, 354 CAS.
- H. Wang, S.-T. Yang, A. Cao and Y. Liu, Acc. Chem. Res., 2013, 46, 750 CrossRef CAS PubMed.
- Y. Li, X. Huang, R. Liu, Q. Li, X. Zhang and W. Li, J. Radioanal. Nucl. Chem., 2005, 265, 127 CrossRef CAS.
- J. Xu, Q. Li, J. Li, T. Ran, S. Wu, W. Song, S. Chen and W. Li, Carbon, 2007, 45, 1865 CrossRef CAS PubMed.
- D. W. Cagle, S. J. Kennel, S. Mirzadeh, J. M. Alford and L. J. Wilsonin, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 5182 CrossRef CAS.
- J. Eagles, S. J. Fairweather-Tait and R. Self, Anal. Chem., 1985, 57, 469 CrossRef CAS.
- A. E. Mutlib, Chem. Res. Toxicol., 2008, 21, 1672 CrossRef PubMed.
- M. Li, W. E. Huang, C. M. Gibson, P. W. Fowler and A. Jousset, Anal. Chem., 2012, 85, 1642 CrossRef PubMed.
- M.-N. Croteau, D. J. Cain and C. C. Fuller, Environ. Sci. Technol., 2013, 47, 3424 CAS.
- Z. Muccio and G. P. Jackson, Analyst, 2009, 134, 213 RSC.
- S.-T. Yang, W. Guo, Y. Lin, X. Deng, H. Wang, H. Sun, Y. Liu, X. Wang, W. Wang, M. Chen, Y. Huang and Y.-P. Sun, J. Phys. Chem. C, 2007, 111, 17761 CAS.
- S.-T. Yang, K. A. S. Fernando, J.-H. Liu, J. Wang, H. Sun, Y. Liu, M. Chen, Y. Huang, X. Wang, H. Wang and Y.-P. Sun, Small, 2008, 4, 940 CrossRef CAS PubMed.
- S.-T. Yang, X. Wang, H. Wang, F. Lu, P. G. Luo, L. Cao, M. J. Meziani, J.-H. Liu, Y. Liu, M. Chen, Y. Huang and Y.-P. Sun, J. Phys. Chem. C, 2009, 113, 18110 CAS.
- T. W. Ebbesen, J. Tabuchi and K. Tanigaki, Chem. Phys. Lett., 1992, 191, 336 CrossRef CAS.
- P. W. Dunk, N. K. Kaiser, C. L. Hendrickson, J. P. Quinn, C. P. Ewels, Y. Nakanishi, Y. Sasaki, H. Shinohara, A. G. Marshall and H. W. Kroto, Nat. Commun., 2012, 3, 855 CrossRef PubMed.
- J. M. Hawkins, A. Meyer, S. Loren and R. Nunlist, J. Am. Chem. Soc., 1991, 113, 9394 CrossRef CAS.
- W. Kratschmer, K. Fostiropoulos and D. R. Huffman, Chem. Phys. Lett., 1990, 170, 167 CrossRef.
- M. C. Martin, J. Fabian, J. Godard, P. Bernier, J. M. Lambert and L. Mihaly, Phys. Rev. B: Condens. Matter, 1995, 51, 2844 CrossRef CAS.
- G. Andrievsky, V. Klochkov and L. Derevyanchenko, Fullerenes, Nanotubes, Carbon Nanostruct., 2005, 13, 363 CrossRef CAS.
- N. Gharbi, M. Pressac, M. Hadchouel, H. Szwarc, S. R. Wilson and F. Moussa, Nano Lett., 2005, 5, 2578 CrossRef CAS PubMed.
- L. E. Gerlowski and R. K. Jain, J. Pharm. Sci., 1983, 72, 1103 CrossRef CAS.
- S. Yamago, H. Tokuyama, E. Nakamura, K. Kikuchi, S. Kananishi, K. Sueki, H. Nakahara, S. Enomoto and F. Ambe, Chem. Biol., 1995, 2, 385 CrossRef CAS.
- R. Bullard-Dillard, K. E. Creek, W. A. Scrivens and J. A. Tour, Bioorg. Chem., 1996, 24, 376 CrossRef CAS.
- Q. Li, T. Xiu, X. Zhang, R. Liu, Q. Du, X. Sun, S. Chen and W. Li, Chin. Sci. Bull., 2001, 49, 1615 CrossRef.
- T. Maksin, D. Djokic, D. Jankovic, A. Djordjevica and O. Neskovic, J. Optoelectron. Adv. Mater., 2007, 9, 2571 CAS.
- Q. Li, Y. Xiu, X. Zhang, R. Liu, Q. Du, X. Shun, S. Chen and W. Li, Nucl. Med. Biol., 2002, 29, 707 CrossRef CAS.
- P. Rajagopalan, F. Wud, R. F. Schinazi and F. D. Boudinot, Antimicrob. Agents Chemother., 1996, 40, 2262 CAS.
| This journal is © The Royal Society of Chemistry 2014 |