Nanochannel-based electrochemical sensor for the detection of pharmaceutical contaminants in water
Vinay J.
Nagaraj
a,
Michael
Jacobs
b,
Krishna Mohan
Vattipalli
b,
Venkata Praveen
Annam
c and
Shalini
Prasad
*b
aDepartment of Biochemistry, Midwestern University, 19555 North 59th Avenue, Glendale, Arizona 85308, USA
bDepartment of Bioengineering University of Texas, Dallas, 800 W Campbell Road, EC 39, Richardson, TX 75080, USA. E-mail: Shalini.Prasad@utdallas.edu
cBioengineering Program, College of Engineering, Wichita State University, 1745 N. Fairmount St., Wichita, KS 67260, USA
First published on 15th November 2013
Abstract
Effective real-time monitoring is the key to understanding and tackling the issue of pharmaceutical contamination of water. This research demonstrates the utility of an alumina nanochannel-based electrochemical sensor platform for the detection of ibuprofen in water derived from various sources. Our results indicate that the sensor is highly sensitive with a limit of detection at 0.25 pg mL−1. The novel sensor described here has potential for application as a simple, rapid, inexpensive and highly reliable method for real-time environmental water quality assessment.
Environmental impactPharmaceutical contaminants in fresh water systems pose a significant threat to humans as well as the ecosystems to which they are exposed. The sensor introduced in this manuscript reflects an innovative technique for identifying the presence of trace amounts of ibuprofen, a common pollutant found in aquatic environments. This technology demonstrates a quick and effective method for analyzing water samples not only at treatment plants but also out in the field. Real-time monitoring is the key to successfully maintaining the quality of water for safe public distribution and for the preservation of our local habitats. |
Introduction
An investigation of several major streams by the United States Geological Survey in the vicinity of agricultural and urban communities revealed a widespread occurrence of pharmaceutical contaminants in environmental fresh water samples.1 This is a direct consequence of improper disposal of unused pharmaceuticals and excretion of these compounds by humans and livestock. Pharmaceuticals in the fresh water environment, although present in trace amounts, are recognized as emerging contaminants since their persistent exposure can adversely affect human health and the environment. For example, pregnant women and children are at particular risk due to the possible effects that pharmaceutical contaminants can pose to a young child's physical development.2 Plant, animal, and microbial life are also adversely affected as a result of exposure to water contaminated with these drugs.3A frequently reported pharmaceutical contaminant found in fresh water environments across the United States is a non-steroidal anti-inflammatory drug called ibuprofen.4 Current analytical tools used to study the occurrence of ibuprofen and other contaminants in environmental water samples involve liquid or gas chromatographic separation followed by quantification using a mass spectrometer. Depending on the starting material, additional steps such as sample extraction, purification, concentration, and derivitization may be necessary prior to analysis.5 This process lacks efficiency due to the inability of being carried out in the field. The sample needs to be transported to a well-equipped laboratory and analyzed by highly skilled personnel. The entire process can be time consuming and expensive. Constraints associated with the rapid evaluation of large numbers of water samples limits the ability to routinely check for pharmaceutical contaminants in the environment. A field deployable monitoring device for the real-time analysis of water quality can have a significant impact on the proficiency with which public water is examined. Sensors based on electrochemical detection methods are particularly promising due to their relative simplicity compared to chromatography/mass spectrometry methods and can prove to be a direct, quick, reliable as well as cost-effective analytical tool.6
Electrochemical sensors have been extensively used for the detection of complex pharmaceutical and biological macromolecules such as proteins, nucleic acids, and glycans.7 When these macromolecules interact with a bio-recognition element on the electrode transducer surface, a measurable change in the electrical current or potential may occur. However, a majority of commonly found pharmaceutical contaminants in fresh water, including ibuprofen, are relatively small molecules found in trace amounts. The interaction of a small molecule with its bio-recognition element (e.g. an antibody) may not provide a large enough signal for detection at environmentally relevant concentrations. To overcome this challenge and achieve lower limits of detection, the use of sensor surfaces modified with nanostructured materials such as nanowires, nanotubes, or nanoparticles is becoming increasingly significant.6
In this study, we explored the applicability of a sensor surface integrated with nanochannels to enhance detection of ibuprofen in various water samples. The nanochannels provide a unique confined environment for the ibuprofen-specific capture antibody to interact with ibuprofen molecules. When the electrochemical immunoassay is performed in a high density array of nanoscale spaces, an amplified signal is created from the summation of numerous individual perturbations of the electrical double layer, allowing for detection of these molecules at lower concentrations.8 The sensor described here has the potential to be widely implemented in environmental monitoring systems for real-time, cost-effective water sample analysis. The impact of this technology will not only help protect our freshwater environments but also more closely monitor water quality prior to public disbursement for human consumption.
Materials and methods
Sensor design
The electrochemical sensor is comprised of three components which can be seen in Fig. 1(a)–(c). These include a printed circuit board (PCB) with a gold electrode sensing site, a nanoporous alumina membrane, and a transparent polydimethylsiloxane (PDMS) manifold for fluid confinement. The PCB contains two concentric circular gold electrodes, which constitute the working and reference electrodes. An input/output signal is transmitted to and from the electrodes through leads that are connected to a potentiostat. A commercially available nanoporous alumina membrane (Anodisc – Whatman, NJ), with a diameter of 13 mm and a thickness of 60 μm, is overlaid onto the sensing site of the printed circuit board platform. This membrane size was chosen because it completely covers the gold electrode pattern on the sensing site. The pore diameter was selected to be 200 nm because this size is large enough to function as a scaffold, allowing diffusion of one or more antibody–ibuprofen complexes to form in each nanoscale space. The interfacing of the alumina membrane with the metallic PCB electrodes resulted in the generation of a high density array of nanoscale confined spaces, referred to as nanochannels. Fig. 1(d) shows the three-dimensional rendering of a scanning electron microscope image of a 200 nm nanoporous alumina membrane. The third part of the sensor is the microfluidic manifold fabricated out of plasma treated PDMS. The elastomer encapsulant was designed to regulate the flow of reagents and confine them directly on top of the sensing site. The manifold was adhered to the PCB platform using a UV curable adhesive (Loctite – Westlake, OH).Electrochemical immunoassay preparation
Ibuprofen detection is achieved by measuring the change in electrical impedance that occurs as a result of binding of ibuprofen molecules to their anti-ibuprofen conjugate antibodies on the electrode surface. The first step in preparation of the immunoassay is the functionalization of anti-ibuprofen antibodies to the gold sensor surface via a thiol-based linker dithiobis succinimidyl propionate (DSP; Thermo Scientific – Waltham, MA). A 10 mM solution of DSP was prepared in dimethyl sulfoxide (DMSO; Fisher Scientific – Waltham, MA) and added onto the sensing site via the inlet of the PDMS manifold. After a 30 minute incubation at room temperature, unbound DSP was removed by passing DMSO through the interior of the manifold, followed by DI water. Next, the DSP activated surface was incubated with 5 μg mL−1 of anti-ibuprofen monoclonal antibodies (Abcam – Cambridge, MA) at room temperature for 15 minutes in a phosphate buffer saline solution. This amount of time was experimentally determined to allow sufficient time for covalent conjugation of the antibody to the linker via its ester groups.9,10 Any, excess unbound antibody was washed off using DI water. Finally, unbound linker molecules and unoccupied pores on the sensing site were blocked using Super Block T-20 (Thermo Fisher Scientific – Waltham, MA), which was also incubated for a period of 15 minutes at room temperature. This step helps to avoid any non-specific signal that could potentially be generated by ibuprofen interacting with DSP or the electrode surface. The total volume of each fluid used in the immunoassay preparation, as well as for ibuprofen detection experiments, was 100 μL.Experimental protocol for ibuprofen detection
Ibuprofen sodium salt (Sigma Aldrich – St. Louis, MO) was dissolved in deionized water, drinking water, and river water to determine the sensitivity and specificity of the sensor using each water source. DI water was distilled and deionized from the city of Wichita municipal water supply using a MegaPure MP6 purification system (Corning – Corning, NY). Commercial drinking water (Kroger Co, Cincinnati, OH) was purified using reverse osmosis filtration from a municipal source prior to bottling. River water samples were collected from the Arkansas River in Wichita, KS. This water was filtered through a 0.22 μm filter prior to use in experiments. A stock solution of 1 mg mL−1 ibuprofen was made in each of the three water samples and then further diluted to 0.001, 0.05, 0.25, 0.5, 1, 250, 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg mL−1.
000 pg mL−1.
Following the last immunoassay preparatory step, a water sample containing no ibuprofen was injected onto the antibody functionalized sensing site to determine a “baseline” impedance value using the potentiostat. After removal of the water, the lowest concentration of ibuprofen was applied to the sensor and allowed to incubate for 15 minutes at room temperature. Impedance measurements were again made via the potentiostat. The sensor was then flushed with DI water to remove all remnants of the sample. The next higher concentration of ibuprofen was introduced onto the same sensor and the process was repeated until the highest dilution was reached. Fig. 1(e) shows a detailed cross section of the mechanism of detection using the electrode functionalized immunoassay. Analysis of all concentrations of ibuprofen was repeated on three independent sensors for each water sample. In order to determine the specificity of the sensor for ibuprofen, diclofenac sodium salt (DSS; Sigma Aldrich – St. Louis, MO) was diluted in all three water samples and evaluated using an identical procedure to the ibuprofen sample testing.
Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy (EIS) was the strategy used to identify the drug ibuprofen in various water samples. This technique has been widely used in electrical biosensors for detecting various analytes.11,12 EIS works by applying a small AC voltage to the electrodes and measuring the resultant current. The impedance is then calculated by the ratio of these sinusoidal forms of current and voltage.13 Using a Reference 600® potentiostat (Gamry Instruments – Warminster, PA), a 10 mV AC voltage was applied to the electrodes with frequencies ranging from 50–1200 Hz. The input potential functioned as the electrical stimulus to direct the charged biomolecules onto the surface of the electrodes. The applied voltage is kept low to maintain a pseudo-linear system while at the same time preventing the denaturing of sensitive biomolecules.14 The frequencies utilized in the experiment were chosen because below 50 Hz, the impedance measurement is largely influenced by the diffusion of ions. Above 1200 Hz, the measured impedance is dominated more so by the resistance of the solution (Rs). A reliable impedance measurement must be completely based on the changes associated with the capacitive nature of the electrical double layer (Edl). When a molecule binds to the electrode surface it disturbs the charged layer, creating changes to the electrical double layer. This alteration in charge distribution results in a measurable change in impedance.15 The perturbations at the Edl occur within the first 50 nm of the electrode/solution interface. Fig. 1(f) shows the representative equivalent circuit model for the system. This modified Randles circuit illustrates a non-faradaic form of impedance spectroscopy, as there is no redox probe. The lack of a redox probe, as well as molecular labels, generates a much simpler platform for small analyte detection.16Results and discussion
The research presented here involves the development of an electrochemical sensor platform that integrates nanochannels onto a sensing surface to enhance detection of trace quantities of ibuprofen in various water sources. Sensors that incorporate nanomaterials into their device have shown potential in improving the detection capabilities of biosensors.6 We have previously found that sensor surfaces modified with nanochannels have superior performance in terms of sensitivity and selectivity for the detection of biomarkers.10 Here, we explored the utility of electrochemical immunoassays within nanochannels for improved detection of a common pharmaceutical contaminant in fresh water environments.Role of nanochannels
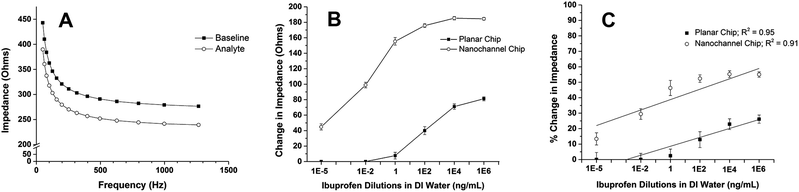
In order to demonstrate improved detection capability using nanochannels, performance of the sensor platform was tested with and without the porous alumina membrane. The comparative study was conducted using deionized (DI) water samples containing concentrations of ibuprofen ranging from 100 fg mL−1 to 1 mg mL−1. The impedance values measured at each frequency in the sweep were evaluated by computing the changes seen between each increasing concentration. It was determined that 100.4 Hz was the optimum frequency for analysis because it provided the largest change in impedance between the 100 fg mL−1 sample and the pure DI water sample. The data illustrating the resultant impedance across a range of frequencies can be seen in Fig. 2(a).The binding of ibuprofen molecules to their antibody conjugates on the electrode surface can be characterized by a decrease in measured impedance as the concentration of ibuprofen in the sample increased. Impedance measurements taken with untainted medium lacking the drug were assigned as the “baseline” measurement with which to compare impedance values corresponding to concentrated doses. The calculated impedance difference from the baseline measurement to the concentrated sample measurement was identified as the change in impedance.17 With each sample containing a greater amount of the target analyte, the representative impedance change correlating to that sample was greater compared to the lower concentrations. The graph in Fig. 2(b) demonstrates this trend for both sensors in the comparative study.
Fig. 2(c) shows the change in impedance represented as a percent change in impedance between the baseline and each pre-concentrated water sample. The data illustrates that the sensor without the alumina membrane interface did not yield a significant change in impedance from the baseline until the concentration of ibuprofen rose to at least 10 pg mL−1. The nanochannel-based sensor, on the other hand, was able to identify an altered impedance signal between the lowest ibuprofen concentration and the pure medium. The graph also shows that the nanochannel-based sensor generates an amplified electrical signal throughout the experiment compared to the sensor lacking the alumina membrane. The enhanced sensitivity of detection observed in the nanochannel-based sensor platform can possibly be attributed to the theory of nanoconfinement. Diffusion of these small molecules into the small space offered by the nanochannel simulates the confined volume of the cell, where biochemicals are known to be stable and interactive.18,19 Each of these nanochannels helps to improve the mobility of ibuprofen molecules to the surface of the antibody-functionalized electrodes. A greater number of ibuprofen molecules interacting with their capture antibody will result in a large number of individual capacitive changes due to charge perturbations at the Edl within each space. The collective summation of these signals allows for visible changes in electrical signal based on molecular interaction at the electrode interface. The correlation between nanochannels and the theory of nanoconfinement for biological sensing applications has been previously demonstrated in our prior work.8,10
Ibuprofen detection
To demonstrate the applicability of the sensing device, analysis of different water sources containing the target drug was achieved. Detection of ibuprofen was first tested in a pH neutral environment using DI water as the medium. Pre-concentrated samples of ibuprofen were prepared, with dilutions ranging from 1 fg mL−1 to 10 ng mL−1. Five impedance measurements were taken for each of these concentrations, and three independent experiments were completed with separate, unused sensors to identify any deviation between tests. A graphical representation of the data can be seen in Fig. 3(a). The difference in impedance was represented as a percentage change from the baseline. A notable change in impedance could be seen starting from the 0.25 pg mL−1 sample. As Fig. 2(c) previously demonstrated, the change in impedance continues to be more substantial as the concentration of the drug increases. This can be directly attributed to the amount of binding occurring at the Edl.20In addition to the DI water trials, ibuprofen diluted in drinking water was also examined. These dilutions were prepared over the same range of concentrations, from 1 fg mL−1 to 10 ng mL−1. Again, baseline impedance measurements were collected for an untainted drinking water sample. Fig. 3(b) displays the percent change in impedance with increased concentrations of ibuprofen in drinking water. When comparing the lower limits of detection in Fig. 3(a) and (b), it is evident that a significant change in impedance was again seen at the 0.25 pg mL−1 concentration. However, over the course of the experiment, the drinking water samples display lower overall impedance changes compared to the tests using DI water. This could possibly be attributed to the presence of salts that are commonly added to commercial drinking water. Certain salts can actually interfere with a proteins ability to interact appropriately with other molecules.21
Since the goal of this work is to demonstrate a platform for monitoring environmental water samples, river water containing the same range of ibuprofen concentrations was analyzed with the sensor. Apart from basic filtering, river water was not processed in any other way before the analysis of ibuprofen on the sensor. Fig. 3(c) details the results from these trials. The lower limit of detection can again be seen around the 0.25 pg mL−1 concentration. Interestingly though, the change in impedance seen for ibuprofen concentrations in river water are greater when comparing equivalent doses in the other mediums. One major factor that can alter the molecular interaction on the sensing site is the slightly decreased pH of river. Also, the presence of organic and inorganic matter may influence the binding of ibuprofen to its antibody as well as contribute to the electrical signal.15,21 Pre-processing of river water to remove molecules that interfere with detection may help to obtain more accurate results.
The overall outcome of the water screening experiments indicates the excellent potential of the sensor for detection of pharmaceutical contaminants in environmental samples.
Sensor specificity
The sensor's ability to distinguish ibuprofen from other molecules was tested using a chemically similar molecule. By analyzing concentrations of DSS with the same immunoassay preparatory setup, any possible cross-reactivity that may exist will become apparent. Also, by evaluating the amount of signal created in these experiments, it is possible to identify the noise of the system that is attributed to non-specific molecular interaction on the electrode surface.22 Figure panel 3 shows the plots representing the sensor performance in detecting ibuprofen and DSS from DI water, drinking water, and river water, respectively. The DI water and the river water show very little impedance change generated from cross-reactivity of DSS with the ibuprofen capture antibody. This not only demonstrates feasibility of the sensor to specifically detect ibuprofen in these two mediums, but it also shows that the signal produced in the river water is not attributed to interference by particulate matter. Some signal is generated at higher concentrations of DSS but this interaction is inevitable due to the large number of molecules settling on top of the antibody-functionalized electrodes at the bottom of the nanochannels. The drinking water, however, displays a considerable amount of impedance change compared to the other two mediums. This could possibly be attributed to the greater number of ions found in drinking water. The higher concentration of ions could be increasing the amount of charge transfer within the system.23 Although the drinking water samples displayed issues with non-specific binding, the sensor showed viability as an environmental water monitoring device by specifically detecting ibuprofen in river water.Conclusions
The sensor presented in this paper has shown feasibility as a platform capable of identifying trace quantities of pharmaceutical contaminants in different water sources. The sensor was successful at demonstrating improved detection by combining the strategies of electrochemical impedance spectroscopy and nanochannel integration. Increased sensitivity of the sensor can be credited directly to the nanochannels, as demonstrated by the comparison study without the alumina membrane. Nanochannels provide the ideal space for interaction between the antibodies and their target ibuprofen molecules. The collective signal produced from the sum of the reactions occurring in the nanochannels provides the amplified electrical signal generated throughout the range of sample concentrations.In order for a technology to be employed as a water quality assessment tool in the field, it would be necessary for the device to give results quickly as well as demonstrate sensitivity, selectivity, reliability, low cost, and ease of use. The nanochannel-based sensor was able to detect the presence of ibuprofen in a 100 μL sample within 15 minutes, without the use of a redox probe or molecular tags. These advantages result in lower cost, lower volumes, and the ability to rapidly screen multiple samples. Also, these preliminary tests show that the limits of detection achieved with this sensor are lower than the existing method of liquid/gas chromatography coupled with mass spectrometry.24 Significant specificity was apparent as well. The cross-reactivity studies showed that the sensor's output signal is very low when molecules other than ibuprofen are found in the medium. This was especially evident when the sensor was used in the analysis of water directly from the environment.
Contrary to the existing technologies of water quality assessment, which are confined to a laboratory environment, this sensing platform can be made portable. The ability to take sensor devices out of the laboratory and into the field would allow for greater efficiency when testing for possible water contamination. The other aspect of this technology that is being further investigated is the ability of multi-plexed detection. Screening for multiple types of contaminants simultaneously from the same sample would greatly improve effectiveness and speed of overall water quality evaluation. Although additional research is needed to make the sensor more proficient at fully analyzing water samples, preliminary studies show its feasibility as a sensitive, reliable, and cost-effective tool for identifying trace quantities of specific pharmaceutical contaminants in water samples.
Acknowledgements
Bernardo Chavira, Morgan Zingsheim, and Anjan Panneer Selvam for technical help with experiments and manuscript preparation.References
- D. W. Kolpin,
et al., Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A National Reconnaissance, Environ. Sci. Technol., 2002, 36(6), 1202–1211 CrossRef CAS PubMed.
A National Reconnaissance, Environ. Sci. Technol., 2002, 36(6), 1202–1211 CrossRef CAS PubMed. - A. C. Collier, Pharmaceutical contaminants in potable water: potential concerns for pregnant women and children, EcoHealth, 2007, 4(2), 164–171 CrossRef.
- K. Kümmerer, Pharmaceuticals in the Environment, Annu. Rev. Environ. Resour., 2010, 35(1), 57–75 CrossRef.
- K. E. Murray, S. M. Thomas and A. A. Bodour, Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment, Environ. Pollut., 2010, 158(12), 3462–3471 CrossRef CAS PubMed.
- C. Girardi, et al., Microbial degradation of the pharmaceutical ibuprofen and the herbicide 2,4-D in water and soil – Use and limits of data obtained from aqueous systems for predicting their fate in soil, Sci. Total Environ., 2013, 444, 32–42 CrossRef CAS PubMed.
- N. Sanvicens, et al., Biosensors for pharmaceuticals based on novel technology, TrAC, Trends Anal. Chem., 2011, 30(3), 541–553 CrossRef CAS.
- J. Li, S. Li and C. F. Yang, Electrochemical Biosensors for Cancer Biomarker Detection, Electroanalysis, 2012, 24(12), 2213–2229 CrossRef CAS.
- S. Brandigampala, et al., Designing better nanobiosensors: Role of pore confinement of biomolecules, in Nanotechnology (IEEE-NANO), 2011 11th IEEE Conference on, 2011 Search PubMed.
- K.-C. Lin, et al., Biogenic nanoporous silica-based sensor for enhanced electrochemical detection of cardiovascular biomarkers proteins, Biosens. Bioelectron., 2010, 25(10), 2336–2342 CrossRef CAS PubMed.
- K. Vattipalli, et al., Study of Nanoporous membranes with applications in the enhanced detection of cardiovascular biomarker proteins, Nano LIFE, 2010, 1(3–4), 175–183 CrossRef CAS.
- M. G. V. V. Bothara, R. K. K. Reddy, T. W. Barrett, J. Carruthers and S. Prasad, Nanomonitors: Electrical immunoassays for protein biomarker profiling, Nanomedicine, 2008, 3(4), 423–436 CrossRef CAS PubMed.
- R. K. Reddy, M. G. Bothara, T. W. Barrett, J. Carruthers and S. Prasad, Nanomonitors: Protein biosensors for rapid analyte analysis, IEEE Sens. J., 2008, 8(6), 720–723 CrossRef CAS.
- J. R. Macdonald, Impedance spectroscopy, Ann. Biomed. Eng., 1992, 20(3), 289–305 CrossRef CAS PubMed.
- E. P. Randviir and C. E. Banks, Electrochemical impedance spectroscopy: an overview of bioanalytical applications, Anal. Methods, 2013, 5(5), 1098–1115 RSC.
- C. Berggren, B. Bjarnason and G. Johansson, Capacitive Biosensors, Electroanalysis, 2001, 13(3), 173–180 CrossRef CAS.
- A. Panneer Selvam, K. M. Vattipalli and S. Prasad, Design of a high sensitive non-faradaic impedimetric sensor, in Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE, 2012 Search PubMed.
- S. Prasad, et al., A nanomonitor compared to ELISA for C-reactive protein detection in patient blood, in Nanotechnology (IEEE-NANO), 2011 11th IEEE Conference on, 2011 Search PubMed.
- S. B. Zimmerman and A. P. Minton, Macromolecular Crowding: Biochemical, Biophysical, and Physiological Consequences, Annu. Rev. Biophys. Biomol. Struct., 1993, 22(1), 27–65 CrossRef CAS PubMed.
- N. A. Chebotareva, B. I. Kurganov and N. B. Livanova, Biochemical effects of molecular crowding, Biochemistry (Moscow), 2004, 69(11), 1239–1251 CrossRef CAS.
- A. Panneer Selvam and S. Prasad, Nanosensor electrical immunoassay for quantitative detection of NT-pro brain natriuretic peptide, Future Cardiol., 2012, 9(1), 137–147 CrossRef PubMed.
- J. H. Jensen, Calculating pH and salt dependence of protein–protein binding, Curr. Pharm. Biotechnol., 2008, 9(2), 96–102 CAS.
- A. Hassibi, H. Vikalo and A. Hajimiri, On noise processes and limits of performance in biosensors, J. Appl. Phys., 2007, 102(1), 014909 CrossRef.
- M. A. Vorotyntsev, J.-P. Badiali and G. Inzelt, Electrochemical impedance spectroscopy of thin films with two mobile charge carriers: effects of the interfacial charging, J. Electroanal. Chem., 1999, 472(1), 7–19 CrossRef CAS.
- M. Starek and J. Krzek, A review of analytical techniques for determination of oxicams, nimesulide and nabumetone, Talanta, 2009, 77(3), 925–942 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |