Environmental specimen banks as a resource for mercury and mercury isotope research in marine ecosystems
Rusty D.
Day
*ab,
Paul R.
Becker
a,
Olivier F. X.
Donard
b,
Rebecca S.
Pugh
a and
Stephen A.
Wise
c
aNational Institute of Standards and Technology, Chemical Sciences Division, Hollings Marine Laboratory, 331 Fort Johnson Road, Charleston, South Carolina 29412, USA. E-mail: russell.day@nist.gov; Fax: +1 (843)762 8742; Tel: +1 (843)762 8904
bInstitut Pluridisciplinaire de Recherche sur l'Environnement et les Materiaux, Equipe de Chimie Analytique BioInorganique et Environnement, Universite de Pau et des Pays de l'Adour, CNRS UMR 5254, Helioparc, 64053 Pau, France
cNational Institute of Standards and Technology, Analytical Chemistry Division, 100 Bureau Drive, Gaithersburg, Maryland 20899, USA
First published on 28th October 2013
Abstract
Environmental specimen banks (ESBs) have been a fundamental tool for many nations to monitor contaminant temporal and spatial trends, study fate and transport, and assess the severity and risks of pollution. The specimens archived in ESBs are among the longest time-series, most geographically robust, and highest integrity samples available for performing environmental research. Mercury (Hg) remains one of the world's most ubiquitous environmental contaminants, and ESBs have played a prominent role in Hg research. Historically this has involved measuring concentrations of Hg species in various environmental matrices, but the emerging field of Hg stable isotope research provides a new analytical approach that can augment these traditional techniques. Signatures of Hg isotope fractionation have been effectively used for source apportionment and for elucidating Hg biogeochemical cycling. As the research surrounding Hg stable isotopes continues to mature, ESBs can play a useful role in analytical quality control, provide a robust and economical sample archive to expand and diversify the inventory of Hg isotope measurements, and be used to develop and test hypotheses to evaluate whether broadly prevailing paradigms are supported. Samples archived in ESBs are available for request by external collaborators in order to perform high impact research, and should be utilized more effectively to address emerging global environmental concerns.
Environmental impactThis article reviews how environmental specimen banks (ESBs) have historically been used to enhance environmental research and management, and provides insight into how investigators can utilize archived samples in ESBs for innovative research applications on a global scale. Mercury is used as a case study, exemplifying how the temporal, geographical, and taxonomic robustness of sample archives in ESBs are an invaluable resource to advance research in the emerging field of mercury stable isotope chemistry. |
History and rationale for environmental specimen banking
The environmental sciences use a wide variety of tools and approaches in order to characterize ecosystems, study how they function, and assess the impacts of anthropogenic activities on ecosystem condition. Environmental specimen banks (ESBs) are facilities that are dedicated to the systematic, long-term archival of biotic or abiotic environmental samples for monitoring and research. Various types of environmental samples have been collected and used in past centuries to study environmental processes and how they change through time.1 Zoological and museum specimens were among the first collections that were utilized to retrospectively document time trends in the environment using archived samples such as feathers, fish, bones, teeth, or hair.1 Recognition of the importance of ESBs as a formal and dedicated monitoring and research tool gained momentum in the late 1970's, punctuated with the International Workshop on Monitoring Environmental Materials and Specimen Banking in Berlin in 1978.2 In the decades following this workshop, ESB programs were initiated around the globe and developed more sophisticated and well-defined methodologies to ensure the integrity of specimens. Twenty-three formal ESBs now exist in 15 countries (Table 1), with new facilities in other countries currently under development. The underlying premise is to collect and process samples without altering their original composition, and preserve them in a stable environment over extremely long time-frames. This typically means processing samples under clean room conditions using carefully chosen materials and detailed protocols, storing specimens under cryogenic temperatures, and using well-designed sample inventory and tracking systems.| Country | Name | Location |
|---|---|---|
| Canada | Canadian Wildlife Service Specimen Bank | National Wildlife Research Centre |
| Canada | National Aquatic Biological Specimen Bank and Database | Canada Centre for Inland Waters, Environment Canada |
| China | Yangtze Environmental Specimen Bank | Tongji University, Jiaxing |
| Denmark | Tissue and Data Bank for Greenland | National Environmental Research Institute |
| Denmark | Faroe Islands Environmental Specimen Bank | Environment Agency, Traðagøta, Faroe Islands |
| Finland | Paljakka Environmental Specimen Bank | Finnish Forest Research Institute |
| France | Observatoire de Recherche sur l'Environnement (ORQUE) | University of Pau |
| France | ANDRA Observatoire Perenne de l'Environnement (OPE) | University of Pau |
| France | Mytilothèque | French Research Institute for Exploitation of the Sea (Ifremer), |
| Germany | German Environmental Specimen Bank | Federal Environment Agency, Dessau-Roßsslau |
| Italy | Mediterranean Marine Mammal Tissue Bank | University of Padua |
| Italy | Antarctic Environmental Specimen Bank (BCAA) | Genoa |
| Japan | Environmental Specimen Bank for Global Monitoring (es-Bank) | Ehime University |
| Japan | Time Capsule for Environment and Endangered Wildlife | National Institute of Environmental Studies |
| Norway | Norwegian Environmental Specimen Bank | Oslo Centre for Interdisciplinary Environmental and Social Research |
| Spain | Biscay Bay Environmental Biospecimen Bank | University of the Basque Country, Plentzia |
| Spain | Environmental Specimen Bank of Galicia | University of Santiago De Compostela |
| South Africa | Biological Resource Bank | National Zoological Gardens |
| South Korea | National Environmental Specimen Bank | National Institute of Environmental Sciences, Seoul |
| South Korea | South Sea Research Institute (SSRI) | Geoje |
| Sweden | Environmental Specimen Bank | Swedish Museum of Natural History |
| UK | National Fish Tissue Archive | Centre for Ecology and Hydrology |
| USA | Marine Environmental Specimen Bank | National Institute of Standards and Technology Charleston, SC |
| USA | CDC and ASTDR Specimen Packaging, Inventory, and Repository | Centers for Disease Control and Prevention |
| USA | Alaska Frozen Tissue Collection | Museum of the North, University of Alaska, Fairbanks |
In general, archived samples can be used for a variety of purposes, including clinical, medical, and preservation of cell-lines or genetic diversity. Historically, most environmental specimen banks have focused primarily on environmental contaminants. The collection of samples for ESBs may be performed by dedicated field teams, or using networks of trained volunteers with partner institutions or citizen scientists who are geographically dispersed. Specimens collected for banking are typically processed and divided into aliquots which can be individually retrieved for different analyses. Most aliquots will be stored for long periods before they are analyzed, but coordinating long-term banking with real-time analyses of samples can provide valuable data that can feed back into further refining the design of long-term specimen banking programs. These real-time data may be informative for defining the frequency of collection, the types and numbers of specimens needed, and the most appropriate spatial distribution of sampling in order to provide samples that are representative of the diverse conditions in the environment.3,4 Sample aliquots in ESBs are not only intended for internal use by ESBs and their immediate monitoring or research objectives. Formal tissue access policies typically exist that provide a mechanism to distribute aliquots of archived samples to external researchers who have an appropriate scientific objective. Considering the funding constraints that the research community faces in today's financial environment, leveraging ESBs to conduct research is an economical and under-utilized alternative to obtaining new funding and conducting costly field studies.
ESBs have been created to meet a variety of goals, but some of the most commonly stated objectives for ESBs regarding chemical contaminants are (1) to determine baseline concentrations, (2) to perform analytical quality control, (3) to evaluate compliance with environmental regulations, (4) to assess exposure and health risks to humans or wildlife, (5) to document temporal changes in contaminants due to policy actions, the emergence of new chemical contaminants, or environmental crises, and (6) to study the spatial distribution, transport, and cycling of contaminants. The types of environmental samples archived in ESBs are as diverse as the objectives for which they are used and the countries from which they come. The species, tissue, or matrix that is selected for banking should be carefully chosen so that it integrates the particular environmental compartment, time period, or geographic region of interest. The criteria for selecting appropriate monitoring matrices have been discussed extensively since formal environmental monitoring programs began.2 In some cases the choice of what matrix to collect, analyze, or bank may be easy. For example, if the goal is to monitor contaminant concentrations in fish in order to assess human health risks from seafood, then banking and analysis of routinely consumed fish species is clearly required. Similarly, if the mandate is to monitor water or sediment to assess compliance with regulatory guidelines, then these specific matrices must be banked and/or analyzed. If an animal health assessment is the primary objective, then the target species may be selected based on their conservation status and the severity of the presumed threats. The aforementioned examples refer to scenarios where the species or matrix (water or sediment) is in itself the primary target. However in other cases the goal may be to use a biomonitoring species as a passive or active sampling tool to assess the relative differences in environmental contamination in a given area or for a discrete period of time.
Biomonitoring, when an organism is used to integrate the bioavailable fraction of a contaminant in the environment, is perhaps the most common type of contaminant field study. Discrete research efforts have collected and analyzed countless species and declared them to be a potential indicator species. However candidate species for systematic real-time monitoring or long-term archival in ESBs should be thoroughly vetted to ensure they have suitable life history and physiological characteristics to integrate the specific segment of the environment and temporal period to meet the desired goals. Species whose biology and ecology have been thoroughly studied are the most appropriate candidates. Even well-studied species may require additional experimental work such as pharmacokinetic studies for dynamic tissues like blood,5–8 seasonal sampling to assess temporal biases,9,10 or repeat sample collections to assess the methodological biases of sample collection techniques. Since the goal is to use a selected matrix as an indicator of the contamination in a given environment, other species or matrices from the same ecosystem should be simultaneously sampled during the program design phase in order to corroborate that the trends observed in the indicator species are representative of the environment at large. For example, temporal Hg trends in Germany's major rivers were corroborated using both bream and mussels.11
Systematic monitoring of contaminants in the environment will continue to be a priority for managers, and ESBs can serve as valuable resource toward this goal. ESB sample inventories are under-utilized for research by external collaborators and efforts should be made to improve visibility and increase interactions. Considering the global nature of today's environmental challenges, broad-scale collective efforts utilizing ESBs may provide an effective research tool. The following sections provide a concise review of Hg as a global pollutant, highlight the strengths of ESBs for contaminant research, and discuss factors that should be considered when selecting target matrices and designing banking/monitoring programs. The discussion will focus on the use of ESBs for Hg research, with particular emphasis on the potential utility of banking and biomonitoring in the emerging field of Hg stable isotope research. We will focus primarily on marine ecosystems, and conclude with a discussion of the challenges and limitations for using ESBs for environmental research.
Mercury as a global pollutant
Mercury (Hg) has long been recognized as one of the most ubiquitous and toxic contaminants in the environment, and a serious threat to the health of humans and wildlife. A wide variety of modes of Hg toxicity have been demonstrated in mammals, birds, fish, and reptiles including neurotoxicity, impaired growth and development, reduced reproductive success, liver and kidney damage, immunomodulation, genotoxicity, and endocrine disruption.12–16 The primary route of MeHg exposure for humans and wildlife is from consumption of contaminated aquatic organisms. In the United States 76% of fish consumption advisories in 2003 were due at least in part to Hg, for a total of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289
289![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 hectares of lakes and 1
020 hectares of lakes and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 986
986![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081 river miles.17 Unlike many legacy compounds like DDT or PCBs that have been phased out of use in many countries and are declining in the environment, Hg levels have not systematically declined, and have remained stable or increased in many areas.18 Based on the reliance of much of the world population on fossil fuels for electricity, Hg will likely remain a contaminant of concern for decades to come. Unlike man-made contaminants, Hg is a naturally occurring heavy metal that is part of the Earth's geology and is released into the hydrosphere and atmosphere through normal environmental processes such as weathering, erosion or geothermal activity. While anthropogenic activity does not create new Hg, it does dramatically alter the distribution and cycling of Hg between the lithosphere and more bioactive environmental compartments. Since Hg is present in most geogenic materials, human activities that accelerate erosion, such as mining, will accelerate the liberation of Hg from geogenic reservoirs. Mining of Hg-rich ores such as cinnabar, or gold mining that uses Hg for amalgamation are significant sources of Hg pollution to local environments.19,20 Mercury has numerous industrial applications such as chlorine production, fluorescent lights, and in the medical and electronics industry. Mercury from these applications may be discharged as waste directly into local watersheds, or released into the atmosphere through medical or refuse incineration, but the largest anthropogenic source of Hg is fossil fuel combustion.18 Once released into the environment, Hg undergoes complex chemical speciation, biogeochemical cycling, and isotope fractionation which has been thoroughly reviewed elsewhere.21,22 While in aquatic systems, Hg may undergo biotic or abiotic methylation, to form methylmercury (MeHg). The MeHg species is more lipophilic and toxic than inorganic Hg (iHg), and is efficiently bioaccumulated by organisms and biomagnified up aquatic food webs where concentrations and toxicity are highest at upper tropic levels.
081 river miles.17 Unlike many legacy compounds like DDT or PCBs that have been phased out of use in many countries and are declining in the environment, Hg levels have not systematically declined, and have remained stable or increased in many areas.18 Based on the reliance of much of the world population on fossil fuels for electricity, Hg will likely remain a contaminant of concern for decades to come. Unlike man-made contaminants, Hg is a naturally occurring heavy metal that is part of the Earth's geology and is released into the hydrosphere and atmosphere through normal environmental processes such as weathering, erosion or geothermal activity. While anthropogenic activity does not create new Hg, it does dramatically alter the distribution and cycling of Hg between the lithosphere and more bioactive environmental compartments. Since Hg is present in most geogenic materials, human activities that accelerate erosion, such as mining, will accelerate the liberation of Hg from geogenic reservoirs. Mining of Hg-rich ores such as cinnabar, or gold mining that uses Hg for amalgamation are significant sources of Hg pollution to local environments.19,20 Mercury has numerous industrial applications such as chlorine production, fluorescent lights, and in the medical and electronics industry. Mercury from these applications may be discharged as waste directly into local watersheds, or released into the atmosphere through medical or refuse incineration, but the largest anthropogenic source of Hg is fossil fuel combustion.18 Once released into the environment, Hg undergoes complex chemical speciation, biogeochemical cycling, and isotope fractionation which has been thoroughly reviewed elsewhere.21,22 While in aquatic systems, Hg may undergo biotic or abiotic methylation, to form methylmercury (MeHg). The MeHg species is more lipophilic and toxic than inorganic Hg (iHg), and is efficiently bioaccumulated by organisms and biomagnified up aquatic food webs where concentrations and toxicity are highest at upper tropic levels.
Global sources of Hg emissions have decreased in North America and Europe but are rapidly increasing in developing nations in Asia.18 Given the propensity for extended residence times and long-range transport,23,24 atmospheric Hg pollution is a global phenomenon. Increases in emissions from distant Hg sources means that Hg concentrations in local environments may not be immediately responsive to local or regional changes in emissions. This presents a challenge for those charged with assessing the degree to which new management policies improve environmental conditions. Multi-decadal monitoring and specimen banking programs combined with state-of-the-art analytical tools offer an effective means to meet these challenges.
Mercury pollution has traditionally been monitored by measuring the concentrations of Hg species in biotic and abiotic matrices. However the advent of multi-collector inductively coupled plasma mass spectrometry (MC-ICPMS) has led to a growing number of studies documenting natural variability of Hg stable isotope fractionation in environmental samples. Isotope systems of other elements have been used for decades for forensics, source apportionment, or to study environmental processes. However the high atomic mass of Hg results in a much lower relative mass difference between isotopes compared to lighter elements, and a resulting smaller degree of mass dependent isotope fractionation. Therefore the small differences in Hg isotope abundances in natural samples cannot be adequately resolved without the high precision isotope ratio measurement capability of MC-ICPMS. Mercury isotope ratios are reported using delta (δ) notation, which references all measured isotope ratios to a universally used delta standard (NIST SRM 3133), and provides a relative measure of the degree of Hg mass-dependent fractionation (MDF). Mercury is among a small group of elements which also exhibits mass-independent fractionation (MIF) that is based on the parity (odd versus even) of the isotope, which is reported using capital delta (Δ).25 One area of research using Hg isotopes has been source apportionment, which distinguishes Hg from a given source location or source type from ambient Hg by measuring the distinct Hg isotope signature. Various geologic and environmental matrices have been characterized to inventory the isotope signatures of different types of source materials, and document the ranges in Hg MDF and MIF in materials around the globe. This has included host rocks, Hg-bearing minerals, hydrothermal fields, sediments and soils,26–28 coal,29 and aquatic biota.30–36 The Hg isotope signatures in these materials were recently reviewed in Yin et al.26 and Sonke,22 and highlight distinct Hg MDF and MIF in a variety of source materials (Fig. 1). Gradients in Hg concentrations and isotope signatures have been associated with Hg point sources such as chlor-alkali plants,31 atmospheric Hg emissions,37–39 mines,19,20 or natural geogenic sources.40 To improve the utility of isotopic source apportionment we need to expand and improve data inventories of Hg MDF and MIF values for a variety of sample types and locations. This is one function where the robust sample archives in ESBs can play a particularly useful role.
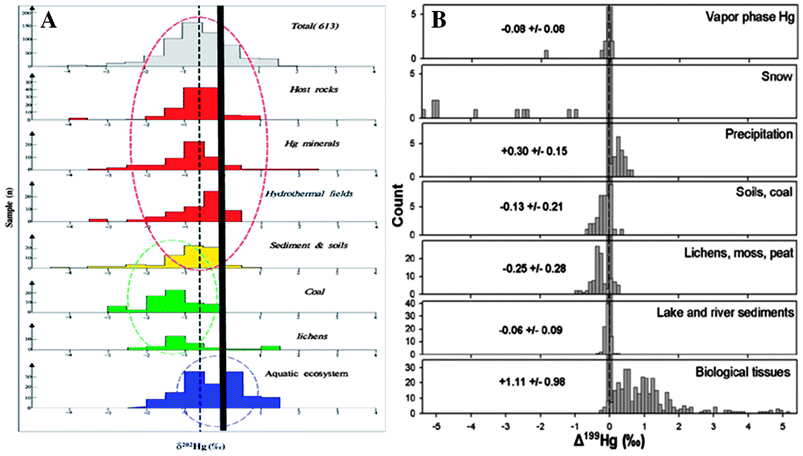 | ||
| Fig. 1 Review of (A) δ202Hg, representing MDF,26 and (B) Δ199Hg, representing MIF,22 reported in various types of natural samples and the number of observations for each. ESBs represent a diverse and cost effective means to expand the body of work describing the natural variability of Hg isotope fractionation in the environment. | ||
The cycling of Hg in marine systems has been thoroughly reviewed elsewhere,21 and shows the complexity of Hg dynamics in the environment and the lack of adequate data to fully describe these processes. Another application of measuring Hg stable isotopes in the environmental is to study Hg biogeochemical cycling. Isotope fractionation has been observed in numerous physical, chemical, and biological transformations of Hg, including redox reactions (photolysis, microbial, or chemical pathways), evaporation/condensation, volatilization, methylation, absorption, and diffusion.32,34,41–45 MDF is the most common form of fractionation, and is driven by small differences in physicochemical properties such as diffusivity and chemical bond strength that results from isotopes having slightly different atomic masses. Some elements such as oxygen, sulfur, and Hg also undergo MIF, which is caused by differences in isotope parity (even versus odd isotopes) instead of mass. MIF is induced by the magnetic isotope effect or nuclear field shift effect which causes preferential enrichment/depletion of odd and even isotopes during some chemical processes, particularly those involving radicals. Photodegradation of methylmercury (MeHg) and photoreduction of inorganic mercury (iHg) have been experimentally shown to induce MIF in the presence of organic ligands.32,46 The photoreduction of iHg into more volatile Hg0 is an important processes mediating the exchange of Hg species between aquatic and atmospheric reservoirs,47 and is believed to be the most important mechanism inducing the Hg MIF that has been observed in aquatic biota.22,32 Measuring Hg fractionation in key compartments of the environment and using experimentally derived rate constants for various fractionation reactions makes Hg isotopes a new tool for modeling global Hg cycles.22
Utility of ESBs
Analytical quality assurance/quality control
The analytical instrumentation and methodology used to quantify environmental contaminants is constantly evolving as new technology and innovations are developed. This evolution invariably improves the reliability of more recent data, however it may create potential biases when meta-analyses are performed that include different analytical methods. This may be particularly problematic for very long-term datasets that transcend periods where substantial changes in analytical practices occurred. Ideally all environmental samples that are analyzed are accompanied by matrix-matched certified reference materials (CRMs) with certified concentrations for analytes of interest. However, readily available matrix-matched CRMs may not exist for some sample types, which could introduce undetectable matrix-based analytical artifacts during the measurement of environmental samples. CRMs are typically measured by metrology laboratories using multiple independent methods that represent the best available technology at that time. However, these methods too may change over time, and the inventories of some CRM materials may be consumed and replaced by new materials, providing little opportunity to directly compare analytical methods and sample stability in the same material over very long time periods. Some monitoring and banking programs, such as NOAA's National Status and Trends Mussel Watch, are supported by the production of matrix-matched CRMs (e.g., mussels and oysters) that are also archived for long-term QA/QC and stability testing. This provides a high degree of long-term quality assurance/quality control (QA/QC) while avoiding the consumption of precious environmental samples through repeated reanalysis.Since the modern conception of environmental specimen banking, the value of ESBs for maintaining QA/QC has been recognized. Real-time monitoring programs without some specimen banking component may have a more difficult time confirming the long-term continuity of datasets. This is particularly important where significant analytical advances have been made in a field. Total Hg can be one of the more difficult elements to quantify because of high reactivity, volatility, and long wash-out times. However cold vapor and combustion sample introduction techniques have improved these measurements and increased sample throughput for total Hg quantification, and this measurement is now routine for a wide range of matrices. The past decade has seen significant measurement improvements for organo-Hg species (methylmercury, ethylmercury) using hyphenated techniques such as GC-ICPMS and speciated isotope dilution.48 This method allows the inter-conversion of Hg species during sample preparation to be tracked, and corrected, eliminating a significant source of measurement error for some types of sample matrices. Archived samples that were previously analyzed using less precise methods can now be reanalyzed to ensure method biases did not contribute to the observed trends.
The relatively recent availability of MC-ICPMS has created a growing field of research on Hg stable isotopes. The relatively heavy mass of Hg means that differences in atomic mass among the seven Hg stable isotopes are small relative to the overall mass. Therefore the degree of Hg isotopic fractionation in natural samples is small (maximum range of ≈8‰, and typically much smaller within studies)26,49–52 compared to lighter isotopes. The high instrumental precision of MC-ICPMS has proven to be adequate to discriminate the variability in Hg isotope ratios in environmental samples. Perhaps the largest analytical challenges stem from the same properties that made Hg problematic for total elemental analysis and speciation; its reactivity. The most commonly used sample preparation method for measuring Hg isotope ratios is an acid digestion (often microwave assisted) to fully oxidize all MeHg into Hg2+ followed by a cold vapor sample introduction by reducing Hg2+ to volatile Hg0 (typically using stannous chloride). Incomplete digestion or reduction can induce MDF,42 so it is critical to confirm the recoveries of these reactions to ensure no analytical artifacts are introduced. The chemical constituents in complex biological matrices can impair the efficiency of cold vapor generation if they are present in the digestant at high concentrations, so samples should be diluted so they are similar in matrix content to the delta bracketing standard. One approach to eliminate the issue of the biological matrix is to perform a separation where the Hg is captured on and re-eluted from a column or amalgamator, but these processes must also be 100% efficient to avoid isotope fractionation artifacts. These matrix-related analytical challenges are compounded by the fact that currently the Hg isotope research community relies on inorganic inter-laboratory comparison standards such as the informally distributed UM-Almaden material for QA/QC validation. QA/QC practices typically include reporting the measured δxxxHg and ΔxxxHg in UM-Almaden compared to the accepted values, but there is rarely any isotopically characterized biological control material (and even more rarely a matrix-matched material) measured in conjunction with unknown environmental samples to verify the validity of measurements. Inevitably, the analytical methodologies being used in this field will also continue to evolve and improve. Proper archival of samples in ESBs from defining studies being conducted now will allow future reanalysis and verification of findings using new and improved methods, thereby further advancing this field using the most accurate data possible.
Environmental compliance/management/remediation/response
The well-documented protocols used by ESBs for sample collection, processing, and analyses make them useful tools for environmental programs where management, policy, or litigation action may be involved. This may include permitting, risk assessment, remediation, environmental impact assessments, or responses to major environmental crises. Some ESBs have explicitly stated that one of their primary functions is to monitor and archive samples in order to assess and document compliance with environmental regulations. The German ESB has such a mandate, and collects and analyzes biota, suspended particulate matter, or water for comparison to legislated Hg Environmental Quality Standards (EQS).11 Thus far the preferred tissues to monitor surface waters for this purpose have been bream (Abramis brama L.), zebra mussels (Dreissena polymorpha), and suspended particulate matter. The Galician ESB used macroalgae (Fucus spp.) to monitor Hg and other metals in coastal waters in order to demonstrate compliance with the European Marine Strategy Framework Directive (Directive 2008/56/EC). The Marine Mammal Tissue Bank (NMMTB) housed within the NIST Marine ESB was mandated by the U.S. Congress through the Marine Mammal Health and Stranding Response Act (Public Law 102-587) to cryogenically archive marine mammal tissues. These very pragmatic and clearly defined functions give ESBs strong relevance for both policy development and management action.Responding to and documenting the impacts and recovery from environmental incidents is also a key role that ESBs can play. The NIST Marine ESB has banked samples collected in response to the Deepwater Horizon/BP oil spill in the Gulf of Mexico in 2010 (personal observation) and the Exxon Valdez Damage Assessment program in 1989.53 Thorough documentation of collection and processing protocols and chain of custody control is critical in these scenarios where samples may be used as evidence in court proceedings to assess financial reparations. In the aftermath of environmental incidents, there is often a realization that archived samples from before the crisis that can serve as a baseline do not exist. This makes assessing damages and documenting recovery difficult.54 These lessons provide further justification to maintain, or expand, a comprehensive archive of samples even when funding is not available for real-time analyses.
While Hg is not a priority contaminant in oil spills, other industrial activities such as chlor-alkali plants, gold and Hg mining, and coal-fired power plants are important point sources of Hg. Releases from these point sources are less acute, however systematic sampling prior to (e.g., during permit requests), during, and after these operations allows for an assessment of the Hg pollution associated with these activities. Establishing a definitive link between a presumed Hg source and the ultimate environmental sink can be challenging because of the ubiquitous nature of Hg pollution and the myriad of factors that control its fate and bioavailability. Documenting that elevated Hg concentrations are temporally or spatially associated with a point source provides one line of evidence to make this link. Mercury stable isotope data provide another means to chemically differentiate Hg from a point source from ambient background Hg present in the region. This has been successfully done with mine tailings,19 coal-fired power plant emissions,37 and chlor-alkali plant pollution31 due to the distinct isotopic patterns in the Hg source materials. Using archived samples for isotopic source apportionment of Hg provides a promising new tool for use in environmental impact assessments.
Health risks to humans and wildlife
The impetus for environmental monitoring and regulations is to protect humans and wildlife from the adverse effects of chemical contaminants. The degree of emphasis on human tissue collection and archival in ESBs varies globally. In some cases, agencies such as the U.S. Center for Disease Control whose explicit mandate is human health, take a leading role in the archival of human tissues. However human liver samples were one of the earliest samples housed in the NIST Marine ESB, and the German and Japanese ESBs have ongoing programs archiving blood, urine, and hair for Hg analysis. One underlying assumption is that higher Hg contamination in a local environment will result in higher exposure and health risks to humans in that area. Since food (in particular seafood) is the main route of MeHg exposure, the link between local environmental Hg contamination and human exposure will be strongest for subsistence fishermen in rural regions and indigenous peoples who consume locally caught species instead of imported food products. Monitoring Hg exposure in these demographics provides data on those most closely linked to the local environment and those with the highest Hg exposure and toxicological risks. Other types of human banking and monitoring programs may be more appropriate for assessing occupational, medical, or atmospheric Hg exposure. In the U.S., the FDA has the regulatory responsibility for monitoring food safety, and plays a leading role in screening commercial seafood products such as fish for Hg. However these efforts rarely include subsistence food items such as marine mammal tissues that have Hg concentrations far higher than typically reported in fish.55,56 ESBs monitoring programs have targeted liver, kidney, and muscle from commonly hunted marine mammals, and eggs from seabirds that make up a routine part of human diets in some northern regions.55,57,58Wildlife species with high Hg concentrations are also subject to toxicological risk themselves. In practice, many monitoring programs target species that have relevance for both human and wildlife health. ESBs that include wildlife health among their primary objectives will choose target species for banking that have the highest contaminant exposure, greatest toxicological sensitivity, and most tenuous conservation status. In some cases this means working with protected species that have restricted access to tissues and limited or no direct toxicological data to establish impact thresholds. Instead, data from model species are considered in conjunction with correlative field studies of exposure and health indicators, and in vitro and in vivo experimental work to establish a weight of evidence approach for assessing toxicological risk.13 Field-based health assessments, lab-based toxicology, and specimen banking and monitoring can be combined to construct a population-level risk assessment like that performed for persistent organic pollutant exposures in bottlenose dolphins in the southeast U.S.59 Samples from ESBs can provide baseline data on contaminant exposure in species of interest to assess risk broadly and determine where additional research is merited.1,55
Fate, transport, and biogeochemical cycling
Monitoring and banking programs inevitably incorporate a spatial component to their designs. Generating baseline Hg concentration data, assessing toxicological risk, and longitudinal sampling at multiple sites all lend themselves to comparing geographic patterns in Hg contamination among sites. Sampling locations for ESB programs may reflect localized urban or industrial activity or relatively pristine conditions. Monitoring and banking performed on this scale allows for meaningful comparisons within or among watersheds to investigate anthropogenic sources and natural transport and biochemical cycling within regions. This type of approach is not unique to ESBs, as discrete research projects often focus on localized scales to address specific research questions. However, the longevity and broader scope of ESB programs means that the spatial scales at which samples are collected and research questions are asked can be much larger. The Japanese es-Bank has used archived samples collected worldwide to determine regional and global distributions and trends for various contaminant classes in developed and developing nations.60 The NIST Marine ESB has seabird egg samples from the Seabird Tissue Archival and Monitoring Program (STAMP) that span from the Arctic Ocean to the Hawaiian islands. These banked samples have recently been used to reveal geographic gradients in Hg fractionation related to photochemical processes and to Hg sources (Fig. 2).36,40 Seabird eggs from the families Alcidae and Laridae are globally distributed and are routinely banked in the U.S., Canada, Germany, Denmark, Sweden, and Norway. The availability and high quality of archived seabird eggs make it possible to conduct a truly global study on Hg isotopes, butyltins, or other contaminants of concern by utilizing the international network of ESBs (Fig. 3). A similar approach could also be performed using other broadly distributed and commonly banked taxa that represent lower and high trophic levels, such as bivalves and delphinids. There are approximately 300 U.S. Mussel Watch sites that span the entire continental U.S. coastline, Great Lakes, Alaska, Puerto Rico, and Hawaii.61 European ESBs have excellent broad-scale coverage of this continent using bivalves, and specimen banks in Japan have numerous bivalve collection sites in Asia. Delphinids have a global distribution and a broad latitudinal range and are also routinely banked during strandings, harvest, and health assessments. Tissues from delphinids in the Atlantic have been collected for the NOAA Marine Mammal Health and Stranding Response Program from New Brunswick, Canada to the Gulf of Mexico.62 The Japanese es-Bank has an extensive archive of delphinid tissues throughout the Pacific and Indian Oceans (Table 2). Like seabirds, delphinids have ranges that integrate a broad area, and may be representative of regional conditions (opposed to highly localized) in large-scale geographic studies.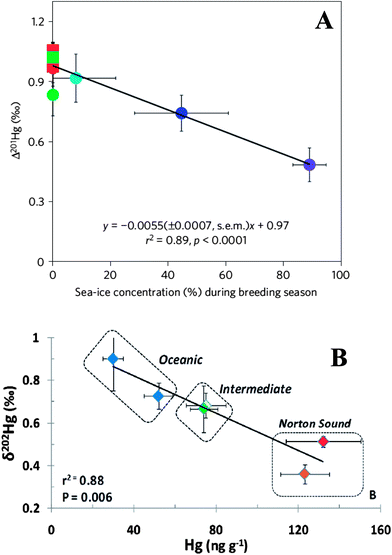 | ||
| Fig. 2 Two studies using Hg isotope fractionation in ESB samples to study biogeochemical cycling and sources of Hg. (A) Influence of sea ice on Hg MIF (indicated by Δ201Hg) in seabird eggs across the Alaskan Arctic and subarctic.36 Sea ice in the Arctic acts as a barrier to Hg photochemical reduction and exchange between oceanic and atmospheric reservoirs, resulting in lower Hg MIF. (B) Gradient in Hg MDF (indicated by δ202Hg) and Hg concentrations in seabird eggs from the coastal embayment of Norton Sound to oceanic colonies in the Bering Sea40 (reprinted with permission from ref. 40. Copyright 2012 American Chemical Society). Elevated Hg concentrations in Norton Sound are the result of high fluxes of terrestrially derived Hg from the Yukon River and other watersheds during the spring melt. This is supported by the more terrestrial Hg isotopic source signature, higher Hg concentration, and terrestrial carbon signature in Norton Sound compared to offshore. | ||
| Species | Tissue | Year | Ecosystem | Region | Publications |
|---|---|---|---|---|---|
| NIST Marine ESB | |||||
| Mussels, Mytilus spp., Dreissena spp. and oysters, Crassostrea virginica | Soft tissue | 1986– | Coastal/estuarine and Great Lakes | Continental USA, Hawaii, Alaska | http://ccma.nos.noaa.gov/about/coast/nsandt/musselwatch.aspx |
| Common murre, Uria aalge, and thick-billed murre, U. lomvia | Egg | 1999– | Coastal/marine | Alaska, Pacific Northwest, Hawaiian Islands | 22, 36, 40, 57 and 75–80 |
| Sea otter, Enhydra lutris | Liver, kidney, serum | 1997–2007 | Coastal/marine | SE Alaska, Cook Inlet | — |
| California sea lion, Zalophus californianus | Blubber, liver, kidney | 1993–2007 | Coastal/marine | California | 81 |
| Northern fur seal Callorhinus ursinus | Liver, kidney, muscle | 1987–2007 | Coastal/marine | St. Paul Island, Alaska | 55, 58 and 82–88 |
| Bearded seal, Erignathus barbatus | Liver, kidney, blubber | 1989–2007 | Coastal/marine | Norton Sound, Chukchi Sea, Alaska | 84 and 87–90 |
| Spotted seal, Phoca largha | Liver, kidney, blubber | 1991–2007 | Coastal/marine | Kotzebue Sound, Alaksa | 84 and 90 |
| Harbor seal, Phoca vitulina | Liver, kidney, blubber | 1990–2005 | Coastal/marine | Cook Inlet, Alaska | 55, 87 and 88 |
| Ringed seal, Phoca hispida | Liver, kidney, blubber | 1988–2007 | Coastal/marine | Chukchi Sea, Norton Sound | 55, 82–84, 87 and 89–92 |
| Pacific walrus, Odobenus rosmarus divergens | Liver, kidney, blubber | 1993–2006 | Coastal/marine | Bering Sea, Chukchi Sea | 55, 88 and 92 |
| Harbor porpoise, Phocoena phocoena | Liver, kidney, blubber | 1990–2006 | Coastal/marine | Northwest Atlantic, (New England, Mid-Atlantic, New Brunwick Canada), Pacific NW | 56, 84 and 93 |
| Common dolphin, Delphinus delphis | Liver, kidney, blubber | 1996–2007 | Coastal/marine | New England (MA) and Mid Atlantic | 86 |
| Rough-toothed dolphin, Steno bredanensis | Liver, kidney, blubber | 1997–2005 | Marine | Florida (GOM, Keys, St. Lucie) | 94–97 |
| White-sided dolphin, Lagenorhynchus acutus | Liver, kidney, blubber | 1993–2008 | Coastal/marine | Massachusetts | 56, 84, 93 and 97–99 |
| Bottlenose dolphin, Tursiops truncatus | Liver, kidney, blubber | 1997–2010 | Coastal/marine | Mid-Atlantic to Gulf of Mexico (most from Carolinas) | 100–104 |
| Bowhead whale, Balaena mysticetus | Liver, kidney, blubber | 1992–2000 | Marine | Chukchi Sea, Alaska | 88, 90, 105 and 106 |
| Pygmy sperm whale, Kogia simus | Liver, kidney, blubber | 1998–2010 | Marine | North Carolina, South Carolina, north/mid Florida | 107 |
| Beluga whale Delphinapterus leucas | Blubber, liver, kidney | 1989–2006 | Marine | Alaska (Cook Inlet, Chukchi Sea) | 55, 82–84, 88–90 and 108–112 |
| Pilot whale Globicephala melas | Liver, kidney, blubber | 1990–2005 | Marine | Northwest Atlantic | 56, 84, 93 and 108 |
| Polar bear, Ursus maritimus | Liver, kidney, adipose | 1996–2007 | Marine | Bering Sea, Chukchi Sea | 55, 88 and 91 |
| German ESB | |||||
| Sediments | 1991 | Limnic | Germany | — | |
| Brown algae, Fucus spp. | Whole | 1982– | Coastal/estuarine | North Sea, Baltic Sea | 63 and 113 |
| Zebra mussels, Dreissena polymorpha | Soft tissue | 1985– | Limnic | Germany | 11 |
| Common mussel, Mytilus edulis | Soft tissue | 1985– | Coastal/estuarine | North Sea, Baltic Sea | 63 and 113 |
| Bream, Abramis brama | Muscle, liver | 1985– | Limnic | Germany | 11 and 114–117 |
| Eel-pout, Zoarces viviparus | Muscle, liver | 1992– | Coastal/marine | North Sea, Baltic Sea | 63 and 118 |
| Herring gull, Larus argentatus | Egg | 1988– | Coastal/marine | North Sea, Baltic Sea | 63 and 119–121 |
| Swedish ESB | |||||
| Blue mussels, Mytilus edulis | Soft tissue | 1987– (BS), 1980– (NS) | Coastal/marine | North Sea, Baltic Sea | 64, 122 and 123 |
| Herring, Clupea harengus | Muscle, liver | 1972– (BS), 1980– (NS) | Coastal/marine | North Sea, Baltic Sea | 64, 124 and 125 |
| Cod, Gadus morhua | Muscle, liver | 1980– (BS), 1979– (NS) | Coastal/marine | North Sea, Baltic Sea | 64 |
| Dab, Limanda limanda | Muscle, liver | 1981– | Coastal/marine | North Sea | 64 |
| Flounder, Platichthys flesus | Muscle, liver | 1980– | Coastal/marine | North Sea | 64 |
| Perch, Perca fluviatilis | Muscle, liver | 1980– | Coastal/marine | Baltic Sea | 64 |
| Eelpout, Zoarces viviparus | Muscle, liver | 1988– (NS), 1995– (BS) | Coastal/estuarine | North Sea, Baltic Sea | 64 and 118 |
| Common murre, Uria aalge | Egg | 1968– (BS), 1991– (NS) | Coastal/marine | North Sea (NS), Baltic Sea (BS) | 64 and 125–129 |
| Peregrine falcon, Falco peregrinus | Egg | 1974–2008 | Terrestrial | Sweden | 130 |
| Grey seal, Halichoerus grypus | Liver | 1974–2008 | Coastal/marine | Baltic Sea | 131 |
| Canada National Wildlife Specimen Bank | |||||
| Herring gull, Larus argentatus | Egg | 1972– (Atlantic), 1974– (Great Lakes) | Coastal/marine | Canadian Atlantic, Great Lakes | 132–136 |
| Thick-billed murre, Uria lomvia | Egg | 1975– (PLI), 1993– (HB) | Coastal/marine | Canadian Arctic/Subarctic, Prince Leopold Island (PLI), Hudson Bay (HB) | 72, 74 and 137–139 |
| Northern fulmar, Fulmaris glacialis | Egg | 1975– | Coastal/marine | Canadian Arctic (PLI) | 72, 74, 138 and 139 |
| Black-legged kittiwake, Rissa tridactyla | Egg | 1975– | Coastal/marine | Canadian Arctic (PLI) | 72, 74 and 137 |
| Ivory gulls, Pagophila eburnea | Egg | 1976–2004 | Coastal/marine | Canadian Arctic (Seymour Island) | 140–142 |
| Black guillemot, Cepphus grylle | Egg | 1993– | Coastal/marine | Canadian Arctic (PLI) | Braune, unpublished data |
| Glaucous gull, Larus hyperboreus | Egg | 1993– | Coastal/marine | Canadian Arctic (PLI) | Braune, unpublished data |
| Double-crested cormorant, Phalacrocorax auritus | Egg | 1972–(Atlantic), 1970 (BC) | Coastal/marine | Canadian Atlantic, British Columbia | 143 |
| Storm petrel, Oceanodroma furcata | Egg | 1971– | Marine | British Columbia | 144 |
| Great blue heron, Ardea herodias | Egg | 1977– | Coastal | British Columbia | 143, 145 and 146 |
| Ancient murrelet, Synthliboramphus antiquus | Egg | 1968– | British Columbia | — | |
| Rhinoceros auklet, Cerorhinca monocerata | Egg | 1970– | Coastal/marine | British Columbia | 142 |
| Osprey, Pandion haliaetus | Egg | 1991– | British Columbia | 143, 147 and 148 | |
| Leach's storm petrel, Oceanodroma leucorhoa | Egg | 1968– | Marine | Canadian Atlantic, British Columbia | 142 |
| Atlantic puffin, | Egg | 1972– | Coastal/marine | Canadian Atlantic | 142 |
| Canada's National Aquatic Biological Specimen Bank | |||||
| Plankton (153 μm) | Bulk sample | 1982– | Limnic | Great Lakes | 149 and 150 |
| Mysis diluviana | Bulk sample | 1981– | Limnic | Great Lakes | 149 and 150 |
| Diporeia hoyi | Bulk sample | 1983– | Limnic | Great Lakes | 149 and 150 |
| Walleye, Sander vitreus | Whole body homogenate | 1977– (GL), 2005– (CAN) | Limnic | Great Lakes (GL), Canadian (CAN) | 151–154 |
| Lake trout, Salvelinus namaycush | Whole body homogenate | 1977– (GL), 2005– (CAN) | Limnic | Great Lakes (GL), Canadian (CAN) | 152 and 155–157 |
| Rainbow smelt, Osmerus mordax | Whole body homogenate | 1977– | Limnic | Great Lakes | 149 and 154 |
| Alewife, Alosa pseudoharengus | Whole body homogenate | 1986– | Limnic | Great Lakes | 149 and 150 |
| Slimy sculpin, Cottus cognatus | Whole body homogenate | 1977– | Limnic | Great Lakes | 149 and 150 |
| Japan es-Bank | |||||
| Black-footed albatross, Diomedea nigripes | Muscle | 1998 | Marine | Pacific Ocean | — |
| Laysan albatross, Diomedea immutabilis | Muscle | 1998 | Marine | Pacific Ocean | — |
| Northern fulmar, Fulmarus glacialis | Muscle | 1999 | Marine | Pacific Ocean | — |
| Black-tailed gull, Larus crassirostris | Muscle | 1999 | Coastal/marine | Japan | — |
| Steller's sea-eagle, Haliaeetus pelagicus | Muscle | 1994 | Coastal/marine | Japan | — |
| Common cormorant Phalacrocorax carbo | Muscle | 2001 | Limnic | Japan | — |
| Striped dolphin, Stenella coeruleoalba | Blubber, muscle, liver, kidney | 1978–2007 | Coastal/marine | North Pacific, Japan | — |
| Finless porpoise, Neophocaena phocaenoides | Blubber, muscle, liver kidney | 1979–2011 | Marine | China, Hong Kong, Japan | — |
| Pacific white-sided dolphin, Lagenorhynchus obliquidens | Blubber, muscle, liver kidney | 1980–2011 | Marine | North Pacific, Japan | — |
| Indo-Pacific humpback dolphin, Sousa chinensis | Blubber, muscle, liver kidney | 1989–2001 | Marine | India, Bay of Bengal, Hong Kong | — |
| Japan Time Capsule Program | |||||
| Bivalves | Soft tissues | 1994– | Coastal/marine | Japan | — |
| Black-tailed gull, Larus crassirostris | Egg | 1983– | Coastal/marine | Japan | — |
The ability to look at larger scale patterns is one of the true strengths of ESBs compared to other types of contaminant research efforts. Comprehensive sampling programs combined with detailed documentation and database management allows sample archives to be mined for the most appropriate specimens to infer the distribution, biogeochemical cycling, or isotope fractionation of Hg in the environment. The field of Hg stable isotope research is relatively new, so diverse observations provide valuable data to improve our current understanding of the Hg fractionation in nature. Samples from ESBs are ideal to generate baseline data, provide a broad sense of context, and look for patterns that can frame and test hypotheses regarding the variability and processes that define Hg fractionation.
Temporal trends
One of the primary strengths of ESBs is the ability to conduct retrospective time-trend analyses on samples that have been systematically collected and archived. Changing technology and industry can lead to the release of new classes of contaminants, or create the ability to measure previously undetectable or unknown compounds in the environment. No historical data exists for new and emerging contaminants to compare to current data, so archived samples in ESBs provide the most powerful tool available to retrospectively assess temporal trends. Mercury has been included in routine contaminant monitoring for decades, but the new ability to measure Hg stable isotopes has created a completely new analytical target for which no historical data exist. For traditional Hg concentration studies, shifts in urban or industrial activity, regulatory action, or climate change can create a need to generate temporal data from areas previously considered a low priority. Thoughtfully designed banking programs often include samples that do not merit real-time analysis at the time of collection, but become a valuable resource as patterns in human impacts and research priorities change.As discussed above, selecting an appropriate sample type is critical to maximizing the power of your monitoring program to detect temporal trends. One of the most important criteria to consider is the time-period through which your sample integrates the ambient environmental Hg. On one end of the spectrum is a water sample which provides a snap-shot of Hg in the aquatic environment. Some complications are that water may exhibit low concentrations and high short-term variability, which means that assessing annual differences may require a tremendous number of samples. On the other end of the spectrum is a sample such as marine mammal liver. Mercury accumulates in this organ over decades of the animal's life, which introduces potential confounding effects related to the individual's life history status if one wishes to evaluate temporal trends. This limits sampling to individuals of similar age and sex, makes assumptions and corrections for bioaccumulation with age, or requires extremely long monitoring durations before a reasonable change in environmental Hg levels can be detected.
The ideal specimen for monitoring annual changes in environmental Hg reflects the Hg assimilated during the same period in the same location each year. This requires knowledge of the behavior, physiology, pharmacokinetics, and life history of the species and tissue in question. Seabird eggs are being used by numerous specimen banks to track Hg temporal trends, and have proven to be very effective at tracking long-term changes in environmental Hg concentrations (Fig. 4A). ESBs currently banking seabird eggs include the Canadian Wildlife Service Specimen Bank,54 German Environmental Specimen Bank,63 Environmental Specimen Bank at the Swedish Museum of Natural History,64 and the NIST Marine Environmental Specimen Bank.40 Many seabird species are income breeders,65 so the Hg deposited into eggs each year reflects the Hg consumed in prey by nesting females once they arrive at the breeding grounds. Mercury in bird eggs has been found to reflect Hg in the blood or diet of nesting females,66,67 and eggs are generally a well characterized tissue for banking. Macroalgae have also been used by ESBs in Poland, Germany, Denmark, and Spain.68 Macroalgae have a limited ability to regulate metal ion uptake,69 and provide a time-integrated measure of the Hg concentrations in their environment. Macroalgae are also sedentary, widespread, and easy to collect. Other matrices that reflect discrete periods of Hg exposure and may be suitable to bank for temporal studies are plankton, yearling fish, blood, or keratinized structures such as feathers or hair that have known growth patterns. Fish filets (Fig. 4B) and mussels from ESBs have also successfully been used to track temporal trends.11,61 Since the Hg concentrations in fish filets and mussels may be confounded by bioaccumulation with age, the size/age class that is sampled for each species must be restricted to minimize this effect.
 | ||
| Fig. 4 Temporal Hg trends from ESB samples for (A) thick-billed murres (Uria lomvia) eggs from the Canadian Arctic74 and (B) bream (Abramis brama) muscle from a German river.11 | ||
The temporal studies discussed above have measured trends in Hg concentrations, but these same matrices may also be suitable to track temporal changes in Hg isotope patterns in the environment. Temporal changes in atmospheric Hg isotope ratios have been reconstructed over century time scales using dated cores from peat28 and lake sediments,70 and suggest that anthropogenic emissions have caused a shift to isotopically lighter Hg deposition into aquatic and terrestrial environments. This approach can yield valuable data, however, there is also evidence that varying degrees of diagenesis across these cores may alter the source Hg isotope signature, thereby introducing artifacts in the temporal trend. Therefore corroborating these results with independent observations in other sample matrices would further strengthen these findings. ESB samples that are collected and stored in a manner suitable to preserve the Hg isotope ratios offer a complementary alternative to coring to detect potential temporal evolution of Hg isotope signatures in the environment. Dietz et al.71 compiled temporal data on Hg in hard structures of biota (teeth, hair, and feathers) from the Arctic that dated back to 1200 to estimate the proportion of anthropogenic Hg in present day biota. These sample types that exist in long-term archives present a valuable opportunity to explore Hg isotope temporal trends, and should be priorities for analysis and continued banking. Samples dating back 3 to 4 decades are more common in ESBs, and have been collected, processed, and stored under more controlled conditions. The Swedish ESB, Canadian ESB, and German ESB contain seabird eggs from the 1960s, 1970s and 1980s,63,64,72 and the NIST Marine ESB maintains the NOAA Mussel Watch archive that represents one of the longest time-series (1986 to present) in the U.S.53 Mercury isotopes in fish samples from the German ESB have recently been measured, and provide a twenty-year temporal trend documenting changing Hg isotope fractionation in rivers and lakes across Germany (personal communication). Ringed seal livers archived in the NIST Marine ESB were also used to document shifts in Hg isotope patterns in sea ice food webs in the Arctic where multi-year ice has decreased.73 A comprehensive compilation of the most prominent sample archives in ESBs worldwide is shown in Table 2. These collections represent the longest ongoing tissue collections that are currently available for request by external collaborators. Selecting the desired region, ecosystem, species, and tissue from this vast library of samples make it possible to investigate temporal trends of Hg isotopes or other emerging contaminants across numerous environmental compartments.
In addition to monitoring changes in anthropogenic Hg sources, Hg isotope fractionation may be useful for tracking temporal changes in the release and cycling of Hg in natural reservoirs in response to climate change. Seabird eggs from the NIST Marine ESB have been effective in using Hg isotope signatures to differentiate whether Hg in marine food webs is derived from terrestrial geogenic sources versus oceanic reservoirs.40 Eggs banked from the coastal embayment of the Norton Sound had a distinct terrestrial/geogenic isotope signature for carbon and Hg that reflects the influence of the Yukon River. As permafrost melts in northern watersheds such as the Yukon, increased erosion and flushing of soils and peat is expected to increase the inputs of fluvial Hg into coastal ecosystems. This process could be quantified by measuring long-term changes in Hg concentrations and Hg fractionation patterns in banked seabird eggs. Rising global temperatures have many other anticipated effects on environmental processes, including diminishing the extent of sea ice coverage. Sea ice acts as a physical barrier to the photodegradation of MeHg and photoreduction of iHg that induce MIF. Livers from Arctic ringed seals banked in the NIST Marine ESB have been analyzed for Hg isotopes, providing a temporal trend dating back to 1988 which spans significant reductions in sea ice (personal communication). Alaskan seabird eggs from the NIST Marine ESB were able to detect significant differences in MIF at northern sites that experience seasonal sea ice compared to southern sites that are ice-free all year.36 As sea ice continues to recede, MIF values in banked seabird eggs and other tissues can be used to monitor increases in Hg photoreduction, and model the resulting increase in Hg evasion from oceanic surface waters to the atmosphere.
Challenges and limitations regarding ESBs
The previous discussion highlights how specimen banks can be a valuable resource to investigate Hg and Hg isotopes in the marine environment. However there are practical limitations to the types and scope of research that can be conducted using archived samples. Retrospective temporal trend analysis is one of the strengths of ESBs, but the time frame of most sample sets is currently limited to several decades. Further hind-casting of Hg in the environment requires the use of alternative methods such as coring or identifying informally but suitably stored samples that pre-date ESBs. For some Hg species and matrices, sample stability over century time-scales could be an issue. There is no data available to inform these concerns, but this would probably be most problematic for Hg species in water, sediment, soil, or surrogate air samples, and least problematic for tissues such as fish or eggs where Hg and MeHg are more strongly bound to proteins. Hg concentration data from archived samples should include moisture content analyses to ensure dehydration of fresh-frozen samples does not induce a systematic temporal bias. Mercury stable isotope fractionation in samples archived for extended periods should not be an issue as long as loss of Hg through transpiration or diffusion through the sample container does not occur. Potential isotope fractionation induced by sample preparation procedures such as cryo-homogenization and freeze-drying should be evaluated in a range of sample types to ensure no measurable artifacts are induced.There are also several challenges regarding data interpretation that must be considered with studies utilizing ESBs. In the case of biological samples from indicator species, the validity of the data interpretation is only as good as the complementary information and/or assumptions regarding the life history, ecology, migratory behavior, and physiology of the organism. These biological factors influence the spatial and temporal scales which the sample integrates and determine whether the Hg measured in the sample reflects the intended environmental compartment and time period. This means that the selection of the indicator species and the availability of comprehensive complementary biological data are critical to maintaining continuity in long-term sample sets. This may require including routine measurements of key parameters such as carbon and nitrogen stable isotopes, fatty acid profiles, or total protein content that inform us of shifts in foraging behavior or nutritional status that could affect Hg uptake, metabolism, and deposition/binding in target tissues.
There are additional limitations to the types of conclusions that can be drawn from analysis of ESB samples. A well-characterized indicator species and comprehensive complementary data can provide conclusive evidence that differences in Hg concentrations or Hg isotope patterns has occurred in the environment. However our ability to deduce the cause of the observed difference is often limited to inspecting the correlative relationships that we observe with other parameters measured in the same sample, or information that is available about the study site or other components of the ecosystem from which our sample came. Robust, multi-compartmental sampling in an ecosystem is typically too costly and time-consuming to repeat year after year as part of routine banking. Therefore in most cases long-term monitoring and banking programs choose one, or possibly two, key matrices to represent a given environment. The benefits of this stream-lined strategy include the potential for an increased spatial and temporal scope. However one of the costs is that a comprehensive understanding of the processes and interactions that occur are more difficult to discern compared to a discrete, intensive ecosystem-based study. Definitively characterizing mechanisms of Hg biogeochemical cycling, or Hg isotope fractionation using limited correlative data alone is problematic because of the host of biotic and abiotic covariates that exist. Intensive ecosystem-based field campaigns, manipulative field experiments, and controlled laboratory studies are needed to refine our understanding of Hg cycling and fractionation and demonstrate causative relationship between processes and analytical endpoints. To address these limitations, ESBs should consider periodically (every 5 or 10 years) collecting and banking a more comprehensive suite of samples from representative ecosystems/sites in order to “ground-truth” the observations made in key indicators species or matrices, and allow for retrospective investigation of complex ecosystem interactions.
Despite this limitation, designing exploratory studies that mine the vast sample archives in ESBs can provide invaluable data to investigate relationships between Hg and other environmental parameters and to develop and test hypotheses regarding the processes driving these trends. These data can provide a broad context from a variety of systems and frame the objectives of more experimental field and laboratory studies. Research on Hg stable isotopes is fairly new, and therefore this function is particularly important for this field in generating and testing cohesive theories and directing future research. For example, ESBs provide a readily available resource of samples to expand the inventory of Hg stable isotope measurements across a wide range of sample types and locations to evaluate how broadly prevailing hypotheses are supported. A more robust inventory of Hg isotope data is also very informative for characterizing materials for source apportionment and biota that accumulate Hg. It is in the best interests of ESBs managers to promote their sample archives as a resource for external collaborators, have a clearly defined and efficient sample access policy to improve availability for high priority research objectives, and explore how complementary sample collections in ESBs worldwide could be used to investigate global-scale research questions. ESBs represent a particularly valuable resource for researchers in the new area of Hg stable isotopes, and should be more fully utilized for this purpose.
References
- Environmental Biomonitoring, ed. K. S. Subramanian and G. V. Iyengar, American Chemical Society, Washington, DC, 1997 Search PubMed.
- Monitoring Environmental Materials and Specimen Banking, ed. N. P. Luepke, Martinus Hijhoff Publishers, The Hague, 1979 Search PubMed.
- C. E. Hebert and D. V. C. Weseloh, Ecotoxicology, 2003, 12, 141–151 CrossRef CAS.
- F. Riget, R. Dietz and M. Cleeman, Sci. Total Environ., 2000, 245, 249–259 CrossRef CAS.
- S. Bearhop, G. D. Ruxton and R. W. Furness, Environ. Toxicol. Chem., 2000, 19, 1638–1643 CrossRef CAS.
- F. Fournier, W. H. Karasov, K. P. Kenow, M. W. Meyer and R. K. Hines, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2002, 133, 703–714 CrossRef.
- L. R. Monteiro and R. W. Furness, Environ. Sci. Technol., 2001, 35, 739–746 CrossRef CAS.
- J. A. Schwenter, Master of Science, College of Charleston, 2007.
- L. Zhang, L. M. Campbell and T. B. Johnson, Environ. Pollut., 2012, 161, 178–184 CrossRef CAS PubMed.
- G. Blanvillain, J. A. Schewenter, R. D. Day, D. Point, S. J. Christopher, W. A. Roumillat and D. W. Owens, Environ. Toxicol. Chem., 2007, 26, 1441–1450 CrossRef CAS.
- P. Lepom, U. Irmer and J. Wellmitz, Chemosphere, 2012, 86, 202–211 CrossRef CAS PubMed.
- J. Wiener, D. Krabbenhoft, G. Heinz and A. Scheuhammer, in Handbook of Ecotoxicology, ed. D. Hoffman, B. A. Rattner, G. A. Burton Jr and J. Cairns Jr, CRC Press, Boca Raton, 2nd edn, 2003, pp. 409–463 Search PubMed.
- R. D. Day, A. Segar, M. D. Arendt, A. M. Lee and M. M. Peden-Adams, Environ. Health Perspect., 2007, 115, 1421–1428 CAS.
- C. S. A. Pereira, S. I. A. G. Guilherme, C. M. M. Barroso, L. Verschaeve, M. G. G. Pacheco and S. A. L. V. Mendo, Arch. Environ. Contam. Toxicol., 2010, 58, 112–122 CrossRef PubMed.
- P. E. Drevnick and M. B. Sandheinrich, Environ. Sci. Technol., 2003, 37, 4390–4396 CrossRef CAS.
- J. T. Zelikoff, R. Smialowicz, P. E. Bigazzi, R. A. Goyer, D. A. Lawrence, H. I. Maibach and D. Gardner, Fundam. Appl. Toxicol., 1994, 22, 1–7 CrossRef CAS.
- USEPA, Office of Water, Fact Sheet: National Listing of Fish Advisories, EPA-823-F-04-016, Washington, DC, 2004 Search PubMed.
- AMAP, AMAP Assessment 2011: Mercury in the Arctic, Arctic Monitoring and Assessment Programme (AMAP), Olso, Norway, 2011 Search PubMed.
- G. E. Gehrke, J. D. Blum and M. Marvin-DiPasquale, Geochim. Cosmochim. Acta, 2011, 75, 691–705 CrossRef CAS PubMed.
- D. Foucher, N. Ogrinc and H. Hintelmann, Environ. Sci. Technol., 2009, 43, 33–39 CrossRef CAS.
- W. F. Fitzgerald, C. H. Lamborg and C. R. Hammerschmidt, Chem. Rev., 2007, 107, 641–662 CrossRef CAS PubMed.
- J. E. Sonke, Geochim. Cosmochim. Acta, 2011, 75, 4577–4590 CrossRef CAS PubMed.
- W. H. Schroeder and J. Munthe, Atmos. Environ., 1998, 32, 809–822 CrossRef CAS.
- N. E. Selin, D. J. Jacob, R. J. Park, R. M. Yantosca, S. Strode, L. Jaegle and D. Jaffe, J. Geophys. Res., 2007, 112, D02308 CrossRef.
- J. D. Blum and B. A. Bergquist, Anal. Bioanal. Chem., 2007, 388, 353–359 CrossRef CAS PubMed.
- R. Yin, X. Feng and W. Shi, Appl. Geochem., 2010, 25, 1467–1477 CrossRef CAS PubMed.
- X. Feng, D. Foucher, H. Hintelmann, H. Yan, T. He and G. Qiu, Environ. Sci. Technol., 2010, 44, 3363–3368 CrossRef CAS PubMed.
- S. WenFang, X. Feng, G. Zhang, L. Ming, R. Yin, Z. Zhao and J. Wang, Chin. Sci. Bull., 2011, 56, 877–882 CrossRef PubMed.
- A. Biswas, J. D. Blum, B. A. Bergquist, G. J. Keeler and Z. Xie, Environ. Sci. Technol., 2008, 42, 8303–8309 CrossRef CAS.
- N. Gantner, H. Hintelmann, W. Zheng and D. C. G. Muir, Environ. Sci. Technol., 2009, 43, 9148–9154 CrossRef CAS PubMed.
- V. Perrot, V. N. Epov, M. V. Pastukhov, V. I. Grebenshchikova, C. Zouiten, J. E. Sonke, S. Husted, O. F. X. Donard and D. Amouroux, Environ. Sci. Technol., 2010, 44, 8030–8037 CrossRef CAS PubMed.
- B. A. Bergquist and J. D. Blum, Science, 2007, 318, 417–420 CrossRef CAS PubMed.
- D. B. Senn, E. J. Chesney, J. D. Blum, M. S. Bank, A. Maage and J. P. Shine, Environ. Sci. Technol., 2010, 44, 1630–1637 CrossRef CAS PubMed.
- L. Laffont, J. E. Sonke, L. Maurice, H. Hintelmann, M. Pouilly, Y. S. Bacarreza, T. Perez and P. Behra, Environ. Sci. Technol., 2009, 43, 8985–8990 CrossRef CAS PubMed.
- V. Perrot, M. V. Pastukhov, V. N. Epov, S. Husted, O. F. X. Donard and D. Amouroux, Environ. Sci. Technol., 2012, 46, 5902–5911 CrossRef CAS PubMed.
- D. Point, J. E. Sonke, R. D. Day, D. G. Roseneau, K. A. Hobson, S. S. Vander Pol, A. J. Moors, R. S. Pugh, O. F. X. Donard and P. R. Becker, Nat. Geosci., 2011, 4, 188–194 CrossRef CAS.
- T. A. Jackson and D. C. G. Muir, Sci. Total Environ., 2012, 417–418, 189–201 CrossRef CAS PubMed.
- N. Estrade, J. Carignan and O. F. X. Donard, Environ. Sci. Technol., 2010, 44, 6062–6067 CrossRef CAS PubMed.
- N. Estrade, J. Carignan and O. F. X. Donard, Environ. Sci. Technol., 2011, 45, 1235–1242 CrossRef CAS PubMed.
- R. D. Day, D. G. Roseneau, S. Berail, K. A. Hobson, O. F. X. Donard, S. S. Vander Pol, R. S. Pugh, A. J. Moors, S. E. Long and P. R. Becker, Environ. Sci. Technol., 2012, 46, 5327–5335 CrossRef CAS PubMed.
- K. Kritee, J. D. Blum, M. W. Johnson, B. A. Bergquist and T. Barkay, Environ. Sci. Technol., 2007, 41, 1889–1895 CrossRef CAS.
- L. Yang and R. E. Sturgeon, Anal. Bioanal. Chem., 2009, 393, 377–385 CrossRef CAS PubMed.
- W. Zheng, D. Foucher and H. Hintelmann, J. Anal. At. Spectrom., 2007, 22, 1097–1104 RSC.
- N. Estrade, J. Carignan, J. E. Sonke and O. F. X. Donard, Geochim. Cosmochim. Acta, 2009, 73, 2693–2711 CrossRef CAS PubMed.
- P. Rodriguez-Gonzalez, V. N. Epov, R. Bridou, E. Tessier, R. Guyoneaurd, M. Monperrus and D. Amouroux, Environ. Sci. Technol., 2009, 43, 9183–9188 CrossRef CAS PubMed.
- W. Zheng and H. Hintelmann, Geochim. Cosmochim. Acta, 2009, 73, 6704–6715 CrossRef CAS PubMed.
- E. M. Sunderland and R. P. Mason, Global Biogeochem. Cycles, 2007, 21, GB4022 CrossRef.
- D. Point, W. C. Davis, J. I. G. Alonso, M. Monperrus, S. J. Christopher, O. F. X. Donard, P. R. Becker and S. A. Wise, Anal. Bioanal. Chem., 2007, 389, 787–798 CrossRef CAS PubMed.
- D. Foucher and H. Hintelmann, Anal. Bioanal. Chem., 2006, 384, 1470–1478 CrossRef CAS PubMed.
- C. N. Smith, S. E. Kesler, J. D. Blum and J. J. Rytuba, Earth Planet. Sci. Lett., 2008, 269, 399–407 CrossRef PubMed.
- C. N. Smith, B. Klaue, S. E. Kesler and J. D. Blum, Geology, 2005, 33, 825–828 CrossRef CAS PubMed.
- J. E. Sonke, J. Schafer, J. Chmeleff, S. Audry, G. Blanc and B. Dupre, Chem. Geol., 2010, 279, 90–100 CrossRef CAS PubMed.
- P. R. Becker, S. A. Wise, L. K. Thorsteinson, B. J. Koster and T. Rowles, Chemosphere, 1997, 34, 1889–1906 CrossRef CAS.
- B. J. Wakeford and M. T. Kasserra, Chemosphere, 1997, 34, 1933–1938 CrossRef CAS.
- P. R. Becker, Mar. Pollut. Bull., 2000, 40, 819–829 CrossRef CAS.
- E. A. Mackey, R. Demiralp, P. R. Becker, R. R. Greenberg, B. J. Koster and S. A. Wise, Sci. Total Environ., 1995, 175, 25–41 CrossRef CAS.
- R. D. Day, D. G. Roseneau, S. S. Vander Pol, K. A. Hobson, O. F. X. Donard, R. S. Pugh, A. J. Moors and P. R. Becker, Environ. Pollut., 2012, 166, 226–232 CrossRef CAS PubMed.
- P. R. Becker, B. J. Koster, S. A. Wise and R. Zeisler, Biol. Trace Elem. Res., 1990, 26–27, 329–334 CrossRef CAS.
- L. H. Schwacke, E. O. Voit, L. J. Hansen, R. S. Wells, G. B. Mitchum, A. A. Hohn and P. A. Fair, Environ. Toxicol. Chem., 2002, 12, 2752–2764 Search PubMed.
- S. Tanabe, J. Environ. Monit., 2006, 8, 782–790 RSC.
- K. L. Kimbrough, W. E. Johnson, G. G. Lauenstein, J. D. Christensen and D. A. Apeti, An Assessment of Two Decades of Contaminant Monitoring in the Nation's Coastal Zone, NOAA Technical Memorandum NOS NCCOS 74, Silver Spring, MD, 2008 Search PubMed.
- R. S. Pugh, M. B. Ellisor, A. J. Moors, P. R. Becker, B. J. Porter, E. A. Mackey, M. M. Schantz, R. Demiralp and S. A. Wise, The National Marine Mammal Tissue Bank Specimen Inventory, NISTIR 7372, National Institute of Standards and Technology, Gaitersburg, MD, 2006 Search PubMed.
- H. Emons, J. D. Schladot and M. J. Schwuger, Chemosphere, 1997, 34, 1875–1888 CrossRef CAS.
- T. Odsjo, A. Bignert, M. Olsson, L. Asplund, U. Eriksson, L. Haggberg, K. Litzen, C. de Wit, C. Rappe and K. Alslund, Chemosphere, 1997, 34, 2059–2066 CrossRef CAS.
- K. A. Hobson, J. Sirois and M. L. Gloutney, Auk, 2000, 117, 760–774 Search PubMed.
- A. Kambamamonli-Dimou, A. Kamarianos and S. Kilikidis, Bull. Environ. Contam. Toxicol., 1991, 46, 128–133 CrossRef.
- D. C. Evers, K. M. Taylor, A. Major, R. J. Taylor, R. H. Poppenga and A. M. Scheuhammer, Ecotoxicology, 2003, 12, 69–81 CrossRef CAS.
- I. G. Viana, J. R. Aboal, J. A. Fernandez, C. Real, R. Villares and A. Carballeira, Water Res., 2010, 44, 1713–1724 CrossRef CAS PubMed.
- R. Fuge and K. H. James, Mar. Pollut. Bull., 1974, 5, 9–12 CrossRef CAS.
- T. A. Jackson and D. C. G. Muir, Environ. Sci. Technol., 2004, 38, 2813–2821 CrossRef CAS.
- R. Dietz, P. M. Outridge and K. A. Hobson, Sci. Total Environ., 2009, 407, 6120–6131 CrossRef CAS PubMed.
- B. Braune, G. M. Donaldson and K. A. Hobson, Environ. Pollut., 2001, 114, 39–54 CrossRef CAS.
- J. Masbou, D. Point, J. E. Sonke and P. R. Becker, Mineral. Mag., 2012, 76, 2077 Search PubMed.
- B. Braune, Environ. Pollut., 2007, 148, 599–613 CrossRef CAS PubMed.
- S. S. Vander Pol, K. A. Hobson, P. R. Becker, R. D. Day, M. B. Ellisor, R. S. Pugh and D. G. Roseneau, J. Environ. Monit., 2011, 13, 699–705 RSC.
- R. D. Day, S. S. Vander Pol, S. J. Christopher, W. C. Davis, R. S. Pugh, K. S. Simac, D. G. Roseneau and P. R. Becker, Environ. Sci. Technol., 2006, 40, 659–665 CrossRef CAS.
- S. J. Christopher, S. S. Vander Pol, R. S. Pugh, R. D. Day and P. R. Becker, J. Anal. At. Spectrom., 2002, 17, 780–785 RSC.
- S. S. Vander Pol, P. R. Becker, B. Sylvain, R. D. Day, O. F. X. Donard, K. A. Hobson, A. J. Moors, R. S. Pugh, L. B. Rust and D. G. Roseneau, Seabird Tissue Archival and Monitoring Project: Egg Collections and Analytical Results for 2006–2009, NIST Interagency/Internal Report NISTIR 7872, 2012 Search PubMed.
- S. S. Vander Pol, P. R. Becker, J. R. Kucklick, R. S. Pugh, D. G. Roseneau and K. S. Simac, Environ. Sci. Technol., 2004, 38, 1305–1312 CrossRef CAS.
- S. S. Vander Pol, P. R. Becker, R. D. Day, M. B. Ellisor, A. Guichard, A. J. Moors, D. Point, R. S. Pugh and D. G. Roseneau, Seabird Tissue Archival and Monitoring Project: Egg collections and analytical results for 2002–2005, NISTIR 7562, National Institute of Standards and Technology, Gaithersburg, MD, 2009, 145 p Search PubMed.
- H. Stapleton, N. G. Dodder, J. R. Kucklick, C. M. Reddy, M. M. Schantz, P. R. Becker, F. Gulland, B. J. Porter and S. A. Wise, Mar. Pollut. Bull., 2006, 52, 522–531 CrossRef CAS PubMed.
- R. Zeisler, R. Demiralp, B. J. Koster, P. R. Becker, M. Burow, P. Ostapczuk and S. A. Wise, Sci. Total Environ., 1993, 139/140, 365–386 CrossRef.
- M. M. Schantz, B. J. Koster, S. A. Wise and P. R. Becker, Sci. Total Environ., 1993, 139–140, 323–345 CrossRef CAS.
- P. R. Becker, E. A. Mackey, R. Demiralp, M. M. Schantz, B. J. Koster and S. A. Wise, Chemosphere, 1997, 34, 2067–2098 CrossRef CAS.
- S. Mossner, T. R. Spraker, P. R. Becker and K. Ballschmiter, Chemosphere, 1992, 24, 1171–1180 CrossRef.
- S. Mossner, I. Barudio, T. S. Spraker, G. Antonelis, G. Early, J. R. Geraci, P. R. Becker and K. Ballschmiter, Fresenius' J. Anal. Chem., 1994, 349, 708–716 CrossRef.
- M. M. Krahn, P. R. Becker, K. L. Tilbury and J. E. Stein, Chemosphere, 1997, 34, 1889–1906 CrossRef.
- R. A. Ponce, G. M. Egeland, J. P. Middaugh and P. R. Becker, Twenty years of trace element analyses of marine mammals: Evaluation and summation of data from Alaska and other Arctic regions, State of Alaska Epidemiology Bulletin, Anchorage, Alaska, 1997, vol. 1, no. 3, 15 p Search PubMed.
- E. A. Mackey, P. R. Becker, R. Demiralp, R. R. Greenberg, B. J. Koster and S. A. Wise, Arch. Environ. Contam. Toxicol., 1996, 30, 503–512 CrossRef CAS.
- P. R. Becker, E. A. Mackey, M. M. Schantz, R. Demiralp, R. R. Greenberg, B. J. Koster, S. A. Wise and D. C. G. Muir, Concentrations of chlorinated hydrocarbons, heavy metals and other elements in tissues banked by the Alaska Marine Mammal Tissue Archival Project, NISTIR 5620, National Institute of Standards and Technology, Gaithersburg, MD, 1995, 115 p Search PubMed.
- J. R. Kucklick, W. D. J. Struntz, P. R. Becker, G. W. York and J. E. Bohonowych, Sci. Total Environ., 2002, 287, 45–49 CrossRef CAS.
- J. R. Kucklick, M. M. Krahn, P. R. Becker, B. J. Porter, M. M. Schantz, G. W. York, T. M. O'Hara and S. A. Wise, J. Environ. Monit., 2006, 8, 848–854 RSC.
- P. R. Becker, B. J. Porter, E. A. Mackey, M. M. Schantz, R. Demiralp and S. A. Wise, National Marine Mammal Tissue Bank and Quality Assurance Program: Protocols, Inventory, and Analytical Results, NISTIR 6279, National Institute of Standards and Technology, Gaithersburg, MD, 1999, 183 p Search PubMed.
- J. R. Kucklick, W. D. J. Struntz, P. R. Becker, M. M. Schantz, E. A. Mackey, R. Demiralp, M. Epstein, B. J. Porter, S. A. Wise, W. E. McFee and M. K. Stolen, Persistent organochlorine pollutants and elements determined in tissues of rough-toothed dolphin (Steno bradendensis) banked from a mass stranding event, NISTIR 6857, National Institute of Standards and Technology, Gaithersburg, MD, 2002, 57 p Search PubMed.
- E. A. Mackey, R. D. Oflaz, M. Epstein, B. Buehler, B. J. Porter, T. Rowles, S. A. Wise and P. R. Becker, Arch. Environ. Contam. Toxicol., 2003, 44, 523–532 CrossRef CAS PubMed.
- W. D. J. Struntz, J. R. Kucklick, M. M. Schantz, P. R. Becker, W. E. McFee and M. K. Stolen, Mar. Pollut. Bull., 2004, 48, 1305–1312 CrossRef PubMed.
- K. J. Tuerk, J. R. Kucklick, P. R. Becker, H. Stapleton and J. E. Baker, Environ. Sci. Technol., 2005, 39, 692–698 CrossRef CAS.
- K. J. Tuerk, J. R. Kucklick, W. E. McFee, R. S. Pugh and P. R. Becker, Environ. Toxicol. Chem., 2005, 24, 1079–1087 CrossRef CAS.
- A. M. Peck, R. S. Pugh, A. J. Moors, M. B. Ellisor, B. J. Porter, P. R. Becker and J. R. Kucklick, Environ. Sci. Technol., 2008, 42, 2650–2655 CrossRef CAS.
- J. E. Yordy, R. S. Wells, B. C. Balmer, L. H. Schwacke, T. Rowles and J. R. Kucklick, Sci. Total Environ., 2010, 408, 2163–2172 CrossRef CAS PubMed.
- J. E. Yordy, R. S. Wells, B. C. Balmer, L. H. Schwacke, T. Rowles and J. R. Kucklick, Environ. Sci. Technol., 2010, 44, 4789–4795 CrossRef CAS PubMed.
- J. E. Yordy, D. A. Pabst, W. A. McLellan, R. S. Wells, T. Rowles and J. R. Kucklick, Environ. Toxicol. Chem., 2010, 29, 1263–1273 CrossRef CAS PubMed.
- B. C. Balmer, L. H. Schwacke, R. S. Wells, R. C. George, J. Hoguet, J. R. Kucklick, S. M. Lane, A. Martinez, W. A. McClellan, P. E. Rosel, K. Sparks, T. Speakman, E. S. Zolman and D. A. Pabst, Sci. Total Environ., 2011, 409, 2094–2101 CrossRef CAS PubMed.
- J. R. Kucklick, L. H. Schwacke, R. S. Wells, A. A. Hohn, A. Guichard, J. E. Yordy, L. J. Hansen, E. S. Zolman, R. Wilson, J. Litz, D. Nowacek, T. Rowles, R. S. Pugh, B. C. Balmer, C. Sinclair and P. E. Rosel, Environ. Sci. Technol., 2011, 45, 4270–4277 CrossRef CAS PubMed.
- C. A. Krone, P. A. Robisch, K. L. Tilbury, J. E. Stein, E. A. Mackey, P. R. Becker, T. M. O'Hara and L. M. Philo, Mar. Mamm. Sci., 1999, 15, 123–142 CrossRef.
- T. M. O'Hara, M. M. Krahn, D. Boyd and P. R. Becker, J. Wildl. Dis., 1999, 35, 741–752 CrossRef CAS PubMed.
- C. E. Bryan, W. C. Davis, W. E. McFee, C. Neumann, G. D. Bossart and S. J. Christopher, Chemosphere, 2012, 89, 556–562 CrossRef CAS PubMed.
- P. R. Becker, E. A. Mackey, R. Demiralp, R. Suydam, R. Early, B. J. Koster and S. A. Wise, Mar. Pollut. Bull., 1995, 30, 262–271 CrossRef CAS.
- M. M. Krahn, D. G. Burrows, J. E. Stein and P. R. Becker, Journal of Cetacean Resource Management, 1999, 1, 239–249 Search PubMed.
- P. R. Becker, M. M. Krahn, E. A. Mackey, R. Demiralp, M. M. Schantz, M. Epstein, M. K. Donais, B. J. Porter, D. C. G. Muir and S. A. Wise, J. Wildl. Dis., 2000, 62, 81–98 Search PubMed.
- J. L. Reiner, S. G. O'Connel, J. R. Kucklick, P. R. Becker and J. M. Keller, Environ. Sci. Technol., 2011, 45, 8129–8136 CrossRef CAS PubMed.
- J. Hoguet, J. L. Reiner, J. M. Keller, J. R. Kucklick, C. E. Bryan, A. J. Moors, R. S. Pugh and P. R. Becker, Sci. Total Environ., 2013, 449, 285–294 CrossRef CAS PubMed.
- P. Ostapczuk, M. Burow, K. May, C. Mohl, M. Froning, B. Subenbach, E. Waidmann and H. Emons, Chemosphere, 1997, 34, 2049–2058 CrossRef CAS.
- A. Wenzel, W. Bohmer, J. Muller, H. Rudel and C. Schroter-Kermani, Environ. Sci. Technol., 2004, 38, 1654–1661 CrossRef CAS.
- H. Rudel, W. Bohmer and C. Schroter-Kermani, J. Environ. Monit., 2006, 8, 812–823 RSC.
- R. Klein, M. Bartel, X. He, J. Muller and M. Quack, Environ. Res., 2005, 98, 55–63 CrossRef CAS PubMed.
- D. Teubner, K. Tarricone, M. Veith, G. Wagner, M. Bartel-Steinbach, R. Klein, A. Korner, M. Quack and M. Paulus, Ecol. Indic., 2011, 11, 1487–1489 CrossRef CAS PubMed.
- J. E. Hedman, H. Rudel, J. Gercken, S. Bergek, J. Strand, M. Quack, M. Appelberg, L. Forlin, A. Tuvikene and A. Bignert, Mar. Pollut. Bull., 2011, 62, 2015–2029 CrossRef CAS PubMed.
- S. Esslinger, R. Becker, C. Jung, C. Schroter-Kermani, W. Bremser and I. Nehl, Chemosphere, 2011, 83, 161–167 CrossRef CAS PubMed.
- C. Schroter-Kermani, E. Schmolz, T. Herrmann and O. Papke, Organohalogen Compd., 2005, 67, 1295–1299 Search PubMed.
- R. Klein, M. Bartel-Steinbach, J. Koschorreck, M. Paulus, K. Tarricone, D. Teubner, G. Wagner, T. Weimann and M. Veith, Environ. Sci. Technol., 2012, 46, 5273–5284 CrossRef CAS PubMed.
- K. Lofstrand, A. Malmvarn, P. Haglund, A. Bignert, A. Bergman and L. Asplund, Environ. Sci. Pollut. Res., 2010, 17, 1460 CrossRef PubMed.
- A. Malmvarn, Y. Zebuhr, S. Jensen, L. Kautsky, E. Greyerz, T. Nakano and L. Asplund, Environ. Sci. Technol., 2005, 39, 8235–8242 CrossRef.
- K. Lundstedt-Enkel, R. Bjerselius, L. Asplund, K. Nylund, Y. Liu and M. Sodervall, Environ. Sci. Technol., 2010, 44, 9018–9023 CrossRef CAS PubMed.
- K. Lundstedt-Enkel, M. Tysklind, J. Trygg, P. Schuller, L. Asplund, U. Eriksson, L. Haggberg, T. Odsjo, M. Hjelmberg, M. Olsson and J. Orberg, Environ. Sci. Technol., 2006, 39, 8395–8402 CrossRef.
- H. Jorundsdottir, A. Bignert, J. Svararsson, T. Nygard, P. Weihe and A. Bergman, Sci. Total Environ., 2009, 407, 4174–4183 CrossRef PubMed.
- K. Lundstedt-Enkel, L. Asplund, K. Nylund, A. Bignert, M. Tysklind, M. Olsson and J. Orberg, Chemosphere, 2006, 65, 1591–1599 CrossRef CAS PubMed.
- K. Lundstedt-Enkel, A. Johansson, M. Tysklind, L. Asplund, K. Nylund, M. Olsson and J. Orberg, Environ. Sci. Technol., 2005, 39, 8630–8637 CrossRef CAS.
- A. Bignert, K. Litzen, T. Odsjo, M. Olsson, W. Persson and L. Reutergardh, Environ. Pollut., 1995, 89, 27 CrossRef CAS.
- K. Holmstrom, A. Johansson, A. Bignert, P. Lindberg and U. Berger, Environ. Sci. Technol., 2010, 44, 4083–4088 CrossRef PubMed.
- J. Kratzer, L. Ahrens, A. Roos, B.-M. Backlin and R. Ebinghaus, Chemosphere, 2011, 85, 253–261 CrossRef CAS PubMed.
- C. E. Hebert, Ecol. Appl., 1998, 8, 669–679 CrossRef.
- R. J. Norstrom, M. Simon, J. Moisey, B. J. Wakeford and D. V. C. Weseloh, Environ. Sci. Technol., 2002, 36, 4783–4789 CrossRef CAS.
- W. A. Gebbink, C. E. Hebert and R. J. Letcher, Environ. Sci. Technol., 2009, 43, 7443–7449 CrossRef CAS.
- L. T. Gauthier, C. E. Hebert, D. V. C. Weseloh and R. J. Letcher, Environ. Sci. Technol., 2008, 42, 1524–1530 CrossRef CAS.
- N. M. Burgess, A. L. Bond, C. E. Hebert, E. Neugebauer and L. Champoux, Environ. Pollut., 2013, 172, 216–222 CrossRef CAS PubMed.
- B. Braune, G. M. Donaldson and K. A. Hobson, Environ. Pollut., 2002, 117, 133–145 CrossRef CAS.
- C. M. Butt, S. A. Mabury, D. C. G. Muir and B. Braune, Environ. Sci. Technol., 2007, 41, 3521–3528 CrossRef CAS.
- B. M. Braune and R. J. Letcher, Environ. Sci. Technol., 2013, 47, 616–624 CrossRef CAS PubMed.
- B. Braune, M. L. Mallory and H. G. Gilchrist, Mar. Pollut. Bull., 2006, 52, 969–987 CrossRef PubMed.
- B. Braune, M. L. Mallory, H. G. Gilchrist, R. J. Letcher and K. G. Drouillard, Sci. Total Environ., 2007, 378, 403–417 CrossRef CAS PubMed.
- Patterns and Trends of Organic Contaminants in Canadian Seabird Eggs 1968–90, ed. J. E. Elliot, D. G. Noble, R. J. Norstrom, P. E. Whitehead, M. Simon, P. A. Pearce and D. B. Peakall, Pergamon Press, Oxford, 1992, ch. 8, pp. 181–194 Search PubMed.
- J. E. Elliot, L. Wilson and B. J. Wakeford, Environ. Sci. Technol., 2005, 39, 5584–5591 CrossRef.
- J. E. Elliot and A. M. Scheuhammer, Mar. Pollut. Bull., 1997, 34, 794–801 CrossRef.
- J. E. Elliot, M. L. Harris, L. Wilson, P. E. Whitehead and R. J. Norstrom, Ambio, 2001, 30, 416–428 Search PubMed.
- M. L. Harris, J. E. Elliot, R. W. W. Butler and L. K. Wilson, Environ. Pollut., 2003, 121, 207–227 CrossRef CAS.
- M. F. Guigueno, K. H. Elliot, J. Levan, M. Wayland and J. E. Elliot, Environ. Int., 2012, 40, 24–32 CrossRef CAS PubMed.
- J. E. Elliot, J. Levac, M. F. Guigueno, D. P. Shaw, M. Wayland, C. A. Morrissey, D. C. G. Muir and K. H. Elliot, Environ. Sci. Technol., 2012, 46, 9681–9689 CrossRef PubMed.
- G. T. Tomy, W. Budakowski, T. Halldorson, D. M. Whittle, M. J. Keir, C. H. Marvin, G. MacInnis and M. Alaee, Environ. Sci. Technol., 2004, 38, 2298–2303 CrossRef CAS.
- J. W. Martin, D. M. Whittle, D. C. G. Muir and S. A. Mabury, Environ. Sci. Technol., 2004, 38, 5379–5385 CrossRef CAS.
- S. B. Gewurtz, D. J. McGoldrick, M. G. Clark, M. J. Keir, M. M. Malecki, M. Gledhill, M. Sekela, J. Syrgiannis, M. S. Evans, A. Armellin, J. Pomeroy, J. Waltho and S. M. Backus, Environ. Toxicol. Chem., 2011, 30, 1564–1575 CrossRef CAS PubMed.
- S. P. Bhavsar, S. B. Gewurtz, D. J. McGoldrick, M. J. Keir and S. M. Backus, Environ. Sci. Technol., 2010, 44, 3273–3279 CrossRef CAS PubMed.
- B. A. Monson, D. F. Staples, S. P. Bhavsar, T. M. Holsen, C. S. Schrank, S. K. Moses, D. J. McGoldrick, S. M. Backus and K. A. Williams, Ecotoxicology, 2011, 20, 1555–1567 CrossRef CAS PubMed.
- D. V. C. Weseloh, D. J. Moore, C. E. Hebert, S. R. de Solla, B. M. Braune and D. J. McGoldrick, Ecotoxicology, 2011, 20, 1644–1658 CrossRef CAS PubMed.
- S. B. Gewurtz, A. O. De Silva, S. M. Backus, D. J. McGoldrick, M. J. Keir, J. Small, L. Melymuk and D. C. G. Muir, Environ. Sci. Technol., 2012, 46, 5842–5850 CrossRef CAS PubMed.
- J. W. Martin, D. M. Whittle, D. C. G. Muir and S. A. Mabury, Environ. Sci. Technol., 2004, 38, 5379 CrossRef CAS.
- S. B. Gewurtz, R. Lega, P. W. Crozier, D. M. Whittle, L. Fayez, E. J. Reiner, P. A. Helm, C. H. Marvin and G. T. Tomy, Environ. Toxicol. Chem., 2009, 28, 921 CAS.
| This journal is © The Royal Society of Chemistry 2014 |