Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The preparation and structure of Ge3F8 – a new mixed-valence fluoride of germanium, a convenient source of GeF2†
Andrew L.
Hector
,
Andrew
Jolleys
,
William
Levason
*,
David
Pugh
and
Gillian
Reid
Chemistry, University of Southampton, Southampton SO17 1BJ, UK. E-mail: wxl@soton.ac.uk; Tel: +44 (0)2380 593792
First published on 14th August 2014
Abstract
The new binary mixed-valence fluoride of germanium, Ge3F8, has been obtained by heating GeF4 with powdered Ge in an autoclave (390 K/4 bar/48 h). The structure contains pyramidal GeIIF3 and octahedral GeIVF6 units, linked by fluoride bridges. The new compound is the missing member of the series (GeF2)n·GeF4 (n = 2, 4, or 6). Sublimation of (GeF2)n·GeF4in vacuo provides a convenient source of GeF2 in ca. 30% overall yield.
Although germanium is technologically very important both as the element and in oxide or chalcogenide compounds, with key applications in electronics, ceramics and optics,1 its chemistry was neglected for many years compared with those of silicon and tin. It is now a very active area of main group chemistry and, in addition to the extensive chemistry of Ge(IV),2 recent work has identified a large and complex coordination chemistry of Ge(II);2,3 the latter contrasting with the limited coordination chemistry of Si(II).2 In Group 14 as well as the common tetrahalides MX4 (M = Si, Ge, Sn; X = F–I),4 there are dihalides MX2 (M = Ge, Sn), and the subhalides, GeBr and SnBr.5 Of these, the chemistry of GeF2 has been very little explored since it is not readily available commercially and its preparation by repeatedly passing GeF4 over heated germanium, is both inconvenient and time consuming,6 while the alternative method, involving the reaction of Ge with anhydrous HF in an autoclave, is hazardous. Both routes also require special equipment.7 A number of intermediate halides have also been identified.2 The latter are of two types; the most common are those with element–element (E–E) bonds, including Si2F6, Si2Cl6, Si3Cl8, Si6Cl14, Ge2Cl6 and Ge5Cl12,8 with structures analogous to the corresponding alkanes. Much rarer, and limited to Ge and Sn, are a second group of mixed-valence materials, including Sn3F8, Ge5F12, and Ge7F16, which are without direct E–E bonds, but are fluoride-bridged and contain distinct environments attributable to MII and MIV centres.9–11
We are currently developing new routes for electrodeposition of p-block materials from non-aqueous media, using reagents including halometallate anions as the p-block element source,12 and have recently reported the electrochemistry of [GeX3]− (X = Cl, Br or I) and [GeCl6]2− in CH2Cl2 solution.13 During the course of this work we have extended our studies to the fluoride systems. We report here the preparation and characterisation of a new binary, mixed-valence fluoride of germanium and its use to provide a convenient route to GeF2.
Depending upon the experimental conditions, repeatedly passing GeF4 at low pressure over heated germanium yields either GeF2,6 or mixed valence GeII–GeIV fluorides.10,11,14 Two of the latter identified by single crystal X-ray diffraction (XRD) studies are Ge5F12‡ and Ge7F16,10,11 which are members of the series (GeF2)n·GeF4.14 These flow reactions are inconvenient and low yielding, hence we have investigated the reduction of GeF4 with Ge powder in an autoclave under modest pressure (390 K/4 bar/48 h, see ESI†). Initial attempts at temperatures <370 K resulted in little reaction, but on increasing the temperature to 390 K/48 h, much of the GeF4 was consumed (as indicated by the drop in pressure), and upon opening the autoclave in a glove-box, a mass of white microcrystalline material was found on the cooler lid. The crystals are extremely moisture sensitive, converting into a pool of liquid immediately on exposure to air. Single crystal X-ray diffraction data were collected from one of the small crystals and the structure solution identified this product as Ge3F8, the missing third member of the series (GeF2)n·GeF4, with n = 2. Unit cell measurements on several other crystals confirmed these as the same compound. Powder X-ray diffraction (PXRD) data were also collected on the bulk material and that showed smaller amounts of Ge5F12 and Ge7F16, as well as traces of GeF2 were also present. The simulated and experimental powder XRD data from this mixture are shown in the ESI.†
Sublimation of the mixture (390 K/0.5 mm) gave ∼30% yield of GeF2 (based on elemental Ge used in the first step), which was identified by PXRD (see ESI†). Some involatile orange material (cf.ref. 6) was also formed.
Germanium difluoride has a polymeric chain structure based upon trigonal pyramidal GeF3 units (Ge–F = 1.79(2), 1.91(2), 2.09(2) Å), with a distant fourth fluoride at 2.57(2) Å that cross-links the chains.15 The new preparation is a convenient way to obtain GeF2 in useful quantity for further studies of its coordination and organometallic chemistry.
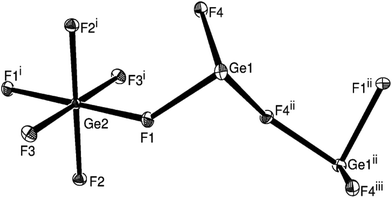
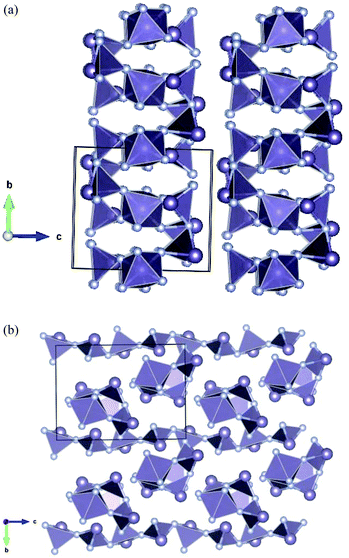
The single crystals of the mixed-valence Ge3F8 are isomorphous with Sn3F8,9 adopting the monoclinic space group P21/n. The structure is composed (Fig. 1) of slightly distorted GeF6 octahedra with four terminal Ge–F bonds (1.767(1), 1.782(1) Å), and two slightly longer Ge–F bonds (1.855(1) Å) that are involved in bridging to the GeII units. The germanium(II) core environment is trigonal pyramidal, composed of one terminal (Ge–F = 1.938(1) Å) and two bridging (Ge–F = 1.980(1), 2.010(1) Å) fluorides, one linked to GeIV and one to a second GeII centre. There are also longer GeII⋯F contacts (2.56 Å), and if these are included, the germanium(II) geometry is a distorted saw-horse shape, reminiscent of GeF2. Overall, the packing is best considered as sheets in the (101) planes (Fig. 2a), with each sheet being made up of puckered chains of GeF3 units along [010] connected together by the GeF6 octahedra (Fig. 2b).
 | ||
| Fig. 2 The Ge3F8 structure viewed along: (a) the b axis to observe the sheets, and (b) the a axis, showing the connectivity within the sheets. | ||
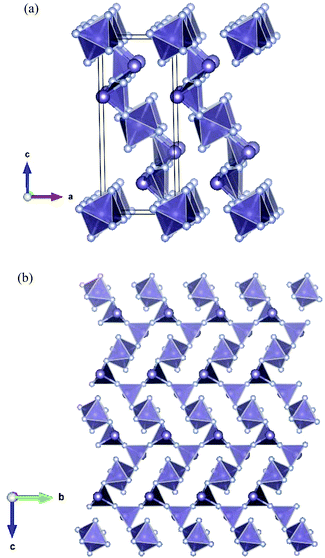
Considering the structures of Ge5F12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and Ge7F16,11 the same basic building blocks are present (trigonal pyramidal GeF3 and octahedral GeF6), but as the F/Ge ratio declines, the structures become more distorted to maintain the germanium coordination numbers. In Ge5F12, if we ignore the distant fourth fluoride at 2.44 Å, the GeF3 trigonal pyramids (Ge–F = 1.80(2), 1.99(2), 2.20(2) Å) form corrugated sheets in (001), based on GeF6 octahedra linked to dimers of two corner-linked GeF3 pyramids. As a consequence of the 4
10 and Ge7F16,11 the same basic building blocks are present (trigonal pyramidal GeF3 and octahedral GeF6), but as the F/Ge ratio declines, the structures become more distorted to maintain the germanium coordination numbers. In Ge5F12, if we ignore the distant fourth fluoride at 2.44 Å, the GeF3 trigonal pyramids (Ge–F = 1.80(2), 1.99(2), 2.20(2) Å) form corrugated sheets in (001), based on GeF6 octahedra linked to dimers of two corner-linked GeF3 pyramids. As a consequence of the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 GeII
1 GeII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GeIV constitution, the GeF6 units are linked to four dimers (rather than two as in Ge3F8) (Fig. 3a). The structure of Ge7F16 is complicated in that there are seven distinct germanium sites,11 but again, the building blocks are trigonal pyramidal GeF3 and octahedral GeF6 units. The structure is best described as chains of GeF3 pyramids along [001] with side chains of four GeF3 units terminated by a GeF6 octahedron attached to every second GeF3 of the main chain (Fig. 3b).
GeIV constitution, the GeF6 units are linked to four dimers (rather than two as in Ge3F8) (Fig. 3a). The structure of Ge7F16 is complicated in that there are seven distinct germanium sites,11 but again, the building blocks are trigonal pyramidal GeF3 and octahedral GeF6 units. The structure is best described as chains of GeF3 pyramids along [001] with side chains of four GeF3 units terminated by a GeF6 octahedron attached to every second GeF3 of the main chain (Fig. 3b).
In conclusion, the missing member of the unique series of mixed-valence germanium fluorides (GeF2)n·GeF4 (n = 2, 4, or 6) has been obtained by reaction of GeF4 and Ge powder under modest pressure and temperature, its structure determined and the structural relationships within the series established. Sublimation of the (GeF2)n·GeF4in vacuo provides a convenient route to the previously rather inaccessible GeF2. Further work to explore the chemistry of GeF2 formed by this route is underway and will be reported in due course.
Acknowledgements
We thank EPSRC for support (EP/1033394/1 and EP/1010890/1). The SCFED Project (http://www.scfed.net) is a multidisciplinary collaboration of British universities investigating the fundamental and applied aspects of supercritical fluids.Notes and references
- (a) D. D. Vaughn II and R. E. Schaak, Chem. Soc. Rev., 2013, 42, 2861 RSC; (b) S. Raoux, W. Welnic and D. Ielmini, Chem. Rev., 2010, 110, 240 CrossRef CAS PubMed; (c) D. V. Talapin, J. S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2010, 110, 389 CrossRef CAS PubMed.
- (a) J. Parr, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Oxford, 2004, vol. 3, p. 545 Search PubMed; (b) W. Levason, G. Reid and W. Zhang, Coord. Chem. Rev., 2011, 255, 1319 CrossRef CAS PubMed; (c) N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Butterworth, Oxford, 2nd edn, 1997 Search PubMed; (d) R. S. Ghadwal, H. W. Roesky, S. Merkel, J. Henn and D. Stalke, Angew. Chem., Int. Ed., 2009, 48, 5683 CrossRef CAS PubMed; (e) A. C. Filippou, O. Chernov and G. Schnakenburg, Angew. Chem., Int. Ed., 2009, 48, 5687 CrossRef CAS PubMed.
- For lead references on Ge(II) complexes see: (a) A. J. Arduengo III, H. V. Rasika Dias, J. C. Calabrese and F. Davidson, Inorg. Chem., 1993, 32, 1541 CrossRef; (b) P. A. Rupar, V. N. Staroverov, P. J. Ragogna and K. M. Baines, J. Am. Chem. Soc., 2007, 129, 15138 CrossRef CAS PubMed; (c) F. Cheng, A. L. Hector, W. Levason, G. Reid, M. Webster and W. Zhang, Angew. Chem., Int. Ed., 2009, 48, 5152 CrossRef CAS PubMed; (d) F. Cheng, J. M. Dyke, F. Ferrante, A. L. Hector, W. Levason, G. Reid, M. Webster and W. Zhang, Dalton Trans., 2010, 39, 847 Search PubMed; (e) F. Cheng, A. L. Hector, W. Levason, G. Reid, M. Webster and W. Zhang, Inorg. Chem., 2010, 49, 752 Search PubMed; (f) J. England and K. Wieghardt, Inorg. Chem., 2013, 52, 10067 CrossRef CAS PubMed; (g) P. A. Rupar, M. C. Jennings and K. M. Baines, Organometallics, 2008, 27, 5043 CrossRef CAS; (h) S. M. I. Al-Rafia, M. R. Momeni, R. McDonald, M. J. Ferguson, A. Brown and E. Rivard, Angew. Chem., Int. Ed., 2013, 52, 6390 CrossRef CAS PubMed.
- A. K. Wolf, J. Glinnemann and M. U. Schmidt, CrystEngComm, 2008, 10, 1364 RSC.
- (a) A. Schnepf and R. Köppe, Z. Anorg. Allg. Chem., 2002, 628, 2914 Search PubMed; (b) C. Schrenk, R. Köppe, I. Schellenberg, R. Pöttgen and A. Schnepf, Z. Anorg. Allg. Chem., 2009, 635, 1541 Search PubMed.
- N. Bartlett and K. C. Yu, Can. J. Chem., 1961, 39, 80 CrossRef CAS.
- E. L. Muetterties, Inorg. Chem., 1962, 1, 342 CrossRef CAS.
- (a) E. Hengge, in Halogen Chemistry, ed. V. Gutmann, Academic Press, NY, 1967, vol. 2, p. 169 Search PubMed; (b) D. Shriver and W. L. Jolly, J. Am. Chem. Soc., 1958, 80, 6692 CrossRef CAS; (c) I. R. Beattie, P. J. Jones, G. Reid and M. Webster, Inorg. Chem., 1998, 37, 6032 CrossRef CAS PubMed.
- M. F. A. Dove, R. King and T. J. King, J. Chem. Soc., Chem. Commun., 1973, 944 RSC.
- J. C. Taylor and P. W. Wilson, J. Am. Chem. Soc., 1973, 95, 1834 CrossRef CAS.
- J. Köhler and J.-H. Chang, Z. Anorg. Allg. Chem., 1997, 623, 596 CrossRef PubMed.
- (a) P. N. Bartlett, D. Cook, C. H. de Groot, A. L. Hector, R. Huang, A. Jolleys, G. P. Kissling, W. Levason, S. J. Pearce and G. Reid, RSC Adv., 2013, 3, 15645 RSC; (b) P. N. Bartlett, D. A. Cook, M. W. George, A. L. Hector, J. Ke, W. Levason, G. Reid, D. C. Smith and W. Zhang, Phys. Chem. Chem. Phys., 2014, 16, 9202 RSC.
- P. N. Bartlett, C. Y. Cummings, W. Levason, D. Pugh and G. Reid, Chem. – Eur. J., 2014, 20, 5019 CrossRef CAS PubMed.
- G. P. Adams, J. L. Margrave and P. W. Wilson, J. Inorg. Nucl. Chem., 1973, 33, 1301 CrossRef.
- (a) J. Trotter, M. Akhtar and N. Bartlett, J. Chem. Soc. A, 1966, 30 RSC; (b) G. Denes, J. Solid State Chem., 1989, 78, 52 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details for the syntheses of Ge3F8 and GeF2, and the PXRD data for all the products. Further details of the crystal structure investigation may be obtained from Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49)7247-808-666; e-mail: crysdata@fiz-karlsruhe.de, http://www.fiz-karlsruhe.de/request_for_deposited_data.html) on quoting CSD number 427896. See DOI: 10.1039/c4dt02265c |
| ‡ Ge5F12 was originally formulated as Ge2F5,14 the correct formula subsequently being established from the crystal structure determination.10 |
| This journal is © The Royal Society of Chemistry 2014 |