Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Soft diphosphine and diarsine complexes of niobium(V) and tantalum(V) fluorides: synthesis, properties, structures and comparisons with the corresponding chlorides†
William
Levason
*,
Mark. E.
Light
,
Gillian
Reid
and
Wenjian
Zhang
School of Chemistry, University of Southampton, Southampton SO17 1BJ, UK. E-mail: wxl@soton.ac.uk
First published on 7th May 2014
Abstract
The reactions of the soft diphosphines o-C6H4(PMe2)2, Me2P(CH2)2PMe2, Et2P(CH2)2PEt2 or o-C6H4(PPh2)2 with NbF5 or TaF5 in anhydrous MeCN solution produce [MF4(diphosphine)2][MF6] (M = Nb or Ta), which have been characterised by microanalysis, IR, 1H, 19F{1H}, 31P{1H} and 93Nb NMR spectroscopy. X-ray crystal structures are reported for the isomorphous [MF4{o-C6H4(PMe2)2}2][MF6], which confirm the presence of eight-coordinate (distorted dodecahedral) cations. The corresponding reactions using o-C6H4(AsMe2)2 produced [MF4{o-C6H4(AsMe2)2}2][MF6] which were similarly characterised, including by the X-ray structure of [NbF4{o-C6H4(AsMe2)2}2][NbF6]. These are very rare examples of arsine complexes of high valent metal fluorides. The chloro complexes [NbCl4{o-C6H4(PMe2)2}2]Cl, [TaCl4{o-C6H4(PMe2)2}2][TaCl6], [NbCl4{Me2P(CH2)2PMe2}2][NbCl6] and [MCl4{o-C6H4(AsMe2)2}2][MCl6] were prepared and their structural and spectroscopic properties compared with the fluoride analogues. Attempts to prepare diphosphine complexes of NbOF3 were unsuccessful, but the NbOCl3 complexes, [{{Me2P(CH2)2PMe2}NbOCl3}2{μ-Me2P(CH2)2PMe2}] and [{o-C6H4(PMe2)2}NbOCl3(μ-O)NbCl3(CH3CN){o-C6H4(PMe2)2}] were obtained. X-Ray structures are also reported for [NbCl4{o-C6H4(PMe2)2}2]Cl, [NbCl4{o-C6H4(AsMe2)2}2][NbCl5(OEt)], [NbCl4{o-C6H4(PMe2)2}2][NbOCl4(CH3CN)], [{{Me2P(CH2)2PMe2}NbOCl3}2{μ-Me2P(CH2)2PMe2}] and [{o-C6H4(PMe2)2}NbOCl3(μ-O)NbCl3(CH3CN){o-C6H4(PMe2)2}].
Introduction
Niobium(V) and tantalum(V) fluorides are white crystalline solids which have a tetranuclear structure in the solid state, composed of six-coordinate metal centres at the corners of a square, with single fluoride bridges along each edge.1 They are very strong Lewis acids and have been explored as constituents of superacid media, although they are weaker than SbF5 or AsF5 in this respect.2 Despite the strong fluoride bridges, they dissolve easily in many O- or N-donor solvents and in halocarbon solvents in the presence of hard donor ligands.3 A wide range of adducts with O-donor Lewis bases have been thoroughly characterised, and in addition to simple adduct formation, C–O, C–H or C–C bond cleavage, rearrangements and polymerisation reactions have been observed.3–5 A smaller range of N-donor adducts are also known.3–5 As would be expected for very hard Lewis acids, complexes with soft Lewis bases are rare. We have reported extremely moisture sensitive thioether complexes of types [MF5(SR2)] (M = Nb or Ta), [MF4(SR2)4][MF6] and [MF4{RS(CH2)SR}2][MF6]; the last two types contain eight-coordinate cations.6,7 Unstable complexes with selenoethers also form, but these decompose in a few hours with fluorination of the ligand.6,7 There appear to be no characterised examples of these pentafluorides complexed to neutral phosphorus or arsenic ligands.3 The heavier halides MX5 (X = Cl or Br) are also strong Lewis acids which form many complexes with both hard and soft donor ligands, including P- or As-donor ligands.8 It is notable that hydrolysis of the adducts with the heavier halides, or sometimes O-abstraction from neutral ligands, results in oxide-halide complexes,5,8,9 whereas similar O/F exchange does not occur with the pentafluorides.3,4 Complexes of NbOF3 were reported very recently, formed by reaction of MF5, hard donor ligands including OPR3, OSMe2, 2,2′-bipyridyl or 1,10-phenanthroline, and the siloxane (Me3Si)2O (HMDSO),5 although the tantalum analogues remain unknown. Here we report the synthesis of complexes of NbF5 and TaF5 with diphosphine and diarsine ligands, attempts to make NbOF3 adducts, and comparable data on complexes with NbCl5, TaCl5 and NbOCl3.Results and discussion
[MF4(diphosphine)2][MF6] complexes
Initial attempts to form complexes of alkyldiphosphines (L–L) (L–L = o-C6H4(PMe2)2, Me2P(CH2)2PMe2 or Et2P(CH2)2PEt2) used reaction of the appropriate MF5 with the diphosphine in anhydrous CH2Cl2 solution, similar conditions to those used to make thioether complexes,6,7 and diphosphine complexes of TiF4 from [TiF4(MeCN)2].10 However, this approach failed; the major products were phosphonium salts of the well known [MF6]− anions, and some other unidentified species. It is likely that the CH2Cl2 is activated towards reaction with the phosphines by the strong Lewis acidity of the pentafluorides. In contrast, reaction of MF5 with the diphosphines in anhydrous MeCN gave colourless solutions, which on concentration or precipitation with anhydrous diethyl ether, deposited [MF4(L–L)2][MF6] as white microcrystalline powders, except for [TaF4{Et2P(CH2)2PEt2}2][TaF6], which was a waxy solid. The complexes [MF4{o-C6H4(PPh2)2}2][MF6] formed similarly, but PMe3 did not react cleanly with the MF5 in anhydrous MeCN, the isolated products containing substantial amounts of coordinated nitrile (IR and 1H NMR spectroscopic evidence) as well as some PMe3. The [MF5]4 dissolve in MeCN with formation of [MF4(MeCN)4][MF6],4g which may be intermediates in the reactions; however since [MF4(RS(CH2)2SR)2][MF6] form from reaction of MF5 with RS(CH2)2SR in CH2Cl2,6,7 a donor solvent is not essential to break up the tetrameric pentafluorides. It seems that the soft PMe3 cannot compete successfully with the hard MeCN for the metal centres, at least when the nitrile is present in large excess as solvent (whilst use of chlorocarbon solvents is ruled out by the reactivity described above). Note that PMe3 also failed to cleanly displace MeCN from [TiF4(MeCN)2].10 Several chloride analogues, [NbCl4{o-C6H4(PMe2)2}2]Cl, [TaCl4{o-C6H4(PMe2)2}2][TaCl6] and [NbCl4{Me2P(CH2)2PMe2}2][NbCl6] were made for comparison purposes from reaction of the corresponding MCl5 with the diphosphine in anhydrous MeCN. The structure of [TaCl4{Me2P(CH2)2PMe2}2]+ has also been reported.11 The niobium chloro complexes are orange, while those with tantalum are white or pale yellow.The diphosphine complexes of the metal fluorides are moisture sensitive solids, modestly soluble in anhydrous MeCN, in which they retain their integrity (NMR evidence see below), although they decompose in CH2Cl2, and are very readily hydrolysed in solution. Hydrolysis produces a mixture of free diphosphine and the phosphonium hexafluorometallate(V), e.g. [Me2P(CH2)2PMe2H][NbF6] from [NbF4{Me2P(CH2)2PMe2}2][NbF6], identified by their characteristic NMR spectra. The chloro-complexes are less moisture sensitive and generally poorly soluble in non- or weakly coordinating solvents.
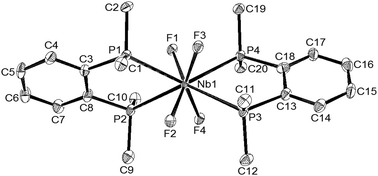
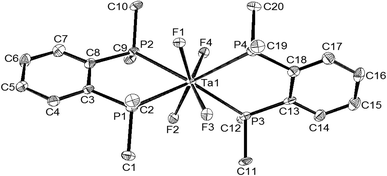
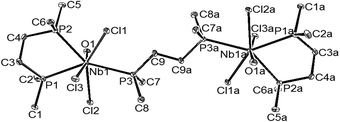
It is convenient to discuss the X-ray structures first and then interpret the spectroscopy and reactions in terms of the complex units present. The two diphosphine complexes [MF4{o-C6H4(PMe2)2}2][MF6] (M = Nb or Ta) are isomorphous (Table 1) and contain eight coordinate distorted dodecahedral cations and the familiar octahedral anions. The cation geometries (Fig. 1 and 2) show essentially identical M–F and M–P distances for the two metal centres, with the former slightly longer (by ∼0.07 Å) than those found in the [MF6]− anions. In part this can be attributed to the increase in coordination number, but it is notable that the d(M–F) in the cations are longer (by ∼0.02 Å) than those found in the eight-coordinate cations in [MF4{RS(CH2)2SR}2]+ or [MF4{MeO(CH2)2OMe}2]+,4b,6,7 possibly indicating some steric crowding by the phosphorus centres which carry three substituents, as against two in the Group 16 donor ligands. Comparison of the cation geometry in [NbCl4{o-C6H4(PMe2)2}2]Cl (Fig. 3) with those in the fluorides shows d(Nb–P) has increased by ∼0.04 Å, which could be due to steric crowding, but may also reflect weaker Lewis acidity of the tetrachloroniobium(V) centre compared to the fluoride analogue.
| Compound | [NbF4{o-C6H4(PMe2)2}2] [NbF6] | [TaF4{o-C6H4(PMe2)2}2] [TaF6] | [NbF4{o-C6H4(AsMe2)2}2] [NbF6] |
|---|---|---|---|
| Formula | C20H32F10Nb2P4 | C20H32F10P4Ta2 | C20H32As4F10Nb2 |
| M | 772.16 | 948.24 | 947.96 |
| Crystal system | Triclinic | Triclinic | Orthorhombic |
| Space group (no.) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2) (no. 2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2) (no. 2) |
Fddd (no. 70) |
| a/Å | 12.246(4) | 12.253(2) | 12.989(4) |
| b/Å | 12.325(4) | 12.348(2) | 21.329(6) |
| c/Å | 12.458(4) | 12.481(2) | 22.051(6) |
| α/° | 114.100(3) | 114.629(2) | 90 |
| β/° | 111.026(2) | 110.486(3) | 90 |
| γ/° | 101.266(2) | 101.38(3) | 90 |
| U/Å3 | 1467.5(6) | 1471.6(4) | 6109(3) |
| Z | 2 | 2 | 8 |
| μ(Mo-Kα)/mm–1 | 1.072 | 7.720 | 5.121 |
| F(000) | 768 | 896 | 3648 |
| Total number reflns | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 736 736 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828 828 |
5513 |
| R int | 0.0520 | 0.0457 | 0.0273 |
| Unique reflns | 5703 | 5150 | 1510 |
| No. of params, restraints | 333, 0 | 333, 0 | 99, 25 |
| R 1, wR2 [I > 2σ(I)]b | 0.0639, 0.1839 | 0.0577, 0.1840 | 0.0516, 0.1595 |
| R 1, wR2 (all data) | 0.0694, 0.1862 | 0.0625, 0.1857 | 0.0564, 0.1708 |
| Compound | [{{Me2P(CH2)2PMe2}NbOCl3}2-μ-{Me2P(CH2)2PMe2}] | [NbCl4{o-C6H4(PMe2)2}2] Cl·CH3CN | [{o-C6H4(PMe2)2}NbOCl3-μ-O-NbCl3(CH3CN){o-C6H4(PMe2)2}] |
|---|---|---|---|
| a Common items: T = 100 K; wavelength (Mo-Kα) = 0.71073 Å; θ(max) = 27.5°. b R 1 = ∑||Fo| − |Fc||/∑|Fo|; wR2 = [∑w(Fo2 − Fc2)2/∑wFo4]1/2. | |||
| Formula | C18H48Cl6Nb2O2P6 | C22H35Cl5NNbP4 | C22H35Cl6NNb2O2P4 |
| M | 880.90 | 707.55 | 867.91 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group (no.) | P21/c (no. 14) | C2/m (no. 12) | P21/c (no. 14) |
| a/Å | 9.096(5) | 21.764(6) | 11.845(8) |
| b/Å | 10.987(6) | 10.178(2) | 33.518(19) |
| c/Å | 18.064(11) | 14.770(4) | 8.596(6) |
| α/° | 90 | 90 | 90 |
| β/° | 90.158(15) | 109.431(9) | 92.35(3) |
| γ/° | 90 | 90 | 90 |
| U/Å3 | 1805.3(18) | 3085.6(13) | 3410(4) |
| Z | 2 | 4 | 4 |
| μ(Mo-Kα)/mm–1 | 1.362 | 1.044 | 1.352 |
| F(000) | 892 | 1440 | 1736 |
| Total number reflns | 8255 | 9182 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
| R int | 0.0550 | 0.0280 | 0.1619 |
| Unique reflns | 3444 | 3191 | 6683 |
| No. of params restraints | 160, 0 | 180, 3 | 342, 332 |
| R 1, wR2 [I > 2σ(I)]b | 0.0577, 0.1332 | 0.0327, 0.0650 | 0.1030, 0.2254 |
| R 1, wR2 (all data) | 0.0699, 0.1442 | 0.0362, 0.0663 | 0.2117, 0.2966 |
The IR spectra of the [MF4(L–L)2][MF6] show the presence of the L–L, the absence of phosphine oxide groups, and strong overlapping features in the range 620–550 cm−1 assigned as terminal M–F stretching vibrations.5–7 The 1H NMR spectra (Experimental section) show the expected resonances for the neutral ligand, shifted to high frequency on coordination. In the diphosphine complexes 2JPH couplings were usually not clearly resolved. Multinuclear NMR spectra (19F, 31P) are much more informative. The 19F{1H} NMR spectra in MeCN solution at ambient temperatures show the characteristic 10 line multiplet at δ = +103 ppm for [NbF6]− and a singlet at δ = +38 ppm for [TaF6]−.5,6 The 19F{1H} resonances of the diphosphine containing cations appear as broad lines to low frequency of the resonances for the corresponding anions (complexes with N-, S- or O-donor ligands usually have resonances to high frequency of the corresponding [MF6]− anion4–7). Under higher resolution, binomial quintet couplings are apparent on the cation resonances, which are assigned as 2JPF ∼ 40–60 Hz (Table 2) due to coupling with the four equivalent phosphorus centres (Fig. 4). In most cases the couplings are clearly resolved, although in [NbF4{o-C6H4(PMe2)2}2][NbF6] and [NbF4{o-C6H4(PPh2)2}2][NbF6] they appear as shoulders on a single broad resonance. Apart from small temperature drifts, the 19F{1H} change little on cooling the solutions to 223 K, showing exchange processes are slow even at room temperature. This contrasts with the thioether complexes,6,7 which showed only a single very broad resonance at room temperature due to rapid dissociative ligand exchange, and which exhibited separate resonances for cation and anion only at low temperatures. The 31P{1H} NMR spectra of the diphosphine complexes each show a single resonance, with very large high frequency coordination shifts (Table 2). In some cases under high resolution these resonances show binomial quintet patterns due to 2JPF, although these were poorly resolved in the spectra of several of the niobium cations.
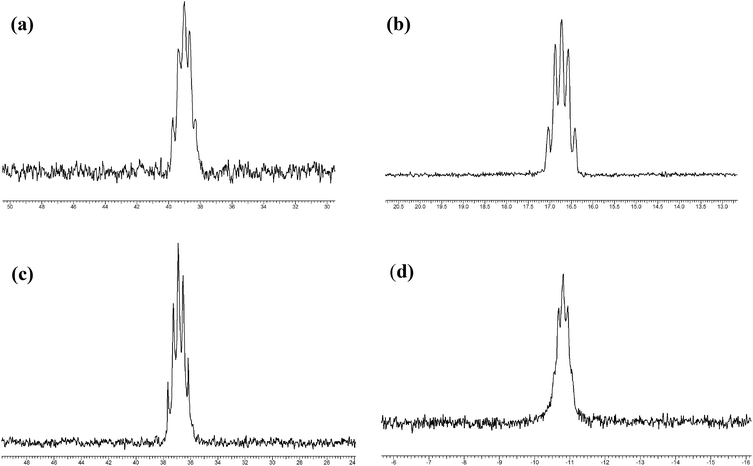 | ||
| Fig. 4 NMR spectra of the cations: (a) 31P{1H} of [TaF4{o-C6H4(PMe2)2}2]+; (b) 19F{1H} of [TaF4{o-C6H4(PMe2)2}2]+; (c) 31P{1H} of [NbF4{Me2P(CH2)2PMe2}2]+; (d) 19F{1H} of [NbF4{Me2P(CH2)2PMe2}2]+. | ||
| Complex | δ(19F{1H})a/ppm | δ(31P{1H})a/ppm | ΔP (δ(complex) − δ(ligand)) | Diffuse reflectance UV/Vis datac/cm−1 |
|---|---|---|---|---|
| a Recorded in MeCN–CD3CN solution 295 K unless indicated otherwise. b In CH2Cl2–CD2Cl2 solution 295 K. c Ligand→metal charge transfer bands. | ||||
| [NbF4{o-C6H4(PMe2)2}2][NbF6] | 102.3 (10 lines, 1JNbF = 335 Hz, [6F]), −7.8 (quintet, 2JPF = 47 Hz, [4F]) | 38.9 (br, m) | 94 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330, 32 330, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| [NbF4{o-C6H4(AsMe2)2}2][NbF6] | 103.6 (10 lines 1JNbF = 335 Hz, [6F]), +27.1 (s, [4F])b | — | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400(sh), 28 400(sh), 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| [NbF4{Me2P(CH2)2PMe2}2][NbF6] | 102.0 (10 lines 1JNbF = 335 Hz, [6F]), −10.9 (quintet 2JPF = 50 Hz, [4F]) | 36.9 (quintet) | 84 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300(sh), 33 300(sh), 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
| [NbF4{Et2P(CH2)2PEt2}2][NbF6] | 102.4 (10 lines 1JNbF = 335 Hz, [6F]), +3.3 (quintet, 2JPF = 45 Hz, [4F]) | 50.3 (s, br) | 69 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 32 000, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| [NbF4{o-C6H4(PPh2)2}2][NbF6] | 102.8 (10 lines, 1JNbF = 335 Hz, [6F]), 54.8 (br m, [4F]) | 40.3 (br) | 53 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, 33 300, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| [TaF4{o-C6H4(PMe2)2}2][TaF6] | 37.4 (s, [6F]), −39.8 (quintet 2JPF = 60 Hz, [4F]) | 35.4 (quintet) | 90 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000. |
| [TaF4{o-C6H4(AsMe2)2}2][TaF6] | 39.5 (s, [6F]), −28.0 (s, [4F])b | — | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400(sh), 27 400(sh), 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| [TaF4{Me2P(CH2)2PMe2}2][TaF6] | 38.3 (s, [6F]), −40.8 (quintet 2JPF = 60 Hz, [4F]) | 36.9 (quintet) | 84 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
| [TaF4{Et2P(CH2)2PEt2}2][TaF6] | 38.8 (s, [6F]), −25.7 (quintet 2JPF = 55 Hz, [4F]) | 45.3 (quintet) | 73 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| [TaF4{o-C6H4(PPh2)2}2][TaF6] | 38.6 (s, [6F]), 16.8 (quintet, 2JPF = 57 Hz, [4F]) | 38.6 (br) | 52 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300(sh) , 33 300(sh) , 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000. |
| [NbCl4{Me2P(CH2)2PMe2}2][NbCl6] | — | 56.3 (s)b | 103 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980, 32 330 980, 32 330 |
| [NbCl4{o-C6H4(PMe2)2}2]Cl | — | 58.5 (s)b | 113 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 34 000, 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| [TaCl4{o-C6H4(PMe2)2}2][TaCl6] | — | 44.6 (s)b | 100 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550, 31 550, 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
The 93Nb NMR spectra (93Nb: 100% abundance, I = 9/2, Ξ = 24.44 MHz, Q = −0.2 × 10−28 m2, Dc = 2740) of [NbF4(L–L)2][NbF6], show the characteristic binomial septet at δ ∼ –1550 ppm for the anion,5 but for L–L = o-C6H4(PMe2)2 or Me2P(CH2)2PMe2, very broad features at δ ∼ –1100 ppm (W1/2 ∼ 5000 Hz) were observed in the room temperature spectra, which are tentatively assigned to the dodecahedral cations; these resonances were lost on cooling the solutions. The complexes are very easily hydrolysed in solution, and trace water results first in the loss of the coupling patterns on the cation resonances, and then complete loss of the cation resonance, although the resonances of the water stable [MF6]− remain. The diffuse reflectance UV/Vis spectra of the [MF4(diphosphine)2][MF6] show several broad features in the range 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–33
000–33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, which for these d0 complexes can be assigned as P(σ)→M(d) charge transfer transitions since the F(π)→M(d) charge transfer bands are expected to occur in the far-UV.12 For those phosphines containing aromatic groups, there are also π→π* transitions in the near-UV region. Comparisons with the corresponding [MCl4(diphosphine)2][MCl6] (Table 2) show that the P(σ)→M(d) charge transfer bands occur at higher energy in the fluorides, an effect observed in other systems,7,10 and expected due to the strong M–F bonding which raises the energy of the metal d-orbitals. The Cl(π)→M(d) charge transfer bands are observed in the near ultraviolet region.13 From the data reported (Experimental section) it seems the Cl(π)→M(d) transitions in the dodecahedral cations occur at rather lower energy than in the octahedral anions.‡
000 cm−1, which for these d0 complexes can be assigned as P(σ)→M(d) charge transfer transitions since the F(π)→M(d) charge transfer bands are expected to occur in the far-UV.12 For those phosphines containing aromatic groups, there are also π→π* transitions in the near-UV region. Comparisons with the corresponding [MCl4(diphosphine)2][MCl6] (Table 2) show that the P(σ)→M(d) charge transfer bands occur at higher energy in the fluorides, an effect observed in other systems,7,10 and expected due to the strong M–F bonding which raises the energy of the metal d-orbitals. The Cl(π)→M(d) charge transfer bands are observed in the near ultraviolet region.13 From the data reported (Experimental section) it seems the Cl(π)→M(d) transitions in the dodecahedral cations occur at rather lower energy than in the octahedral anions.‡
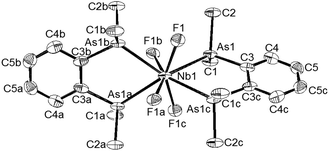
[MF4{o-C6H4(AsMe2)2}2][MF6]
The reaction of o-C6H4(AsMe2)2 with both MF5 reagents in anhydrous MeCN produced [MF4{o-C6H4(AsMe2)2}2][MF6] as cream powders. These are very rare examples of diarsine complexes with high valent fluorides.3 TiF4 does not complex with o-C6H4(AsMe2)2,10 and the complexes with SnF4 or GeF4 were too unstable to isolate.14,15 In contrast to the stability of the diphosphine complexes, the diarsines in [MF4{o-C6H4(AsMe2)2}2][MF6] are partially displaced on dissolution of the complexes in MeCN (NMR evidence), but they are stable for some hours in anhydrous CH2Cl2 solution, although slow reaction with the solvent occurs over several days. The successful isolation of the diarsine complexes from MeCN results from their deposition as the least soluble species from an exchanging mixture of complex, diarsine and nitrile adducts of MF5 in solution, whilst their relative stability to CH2Cl2, which contrasts with that of the phosphines, is due to the lower nucleophilicity of the arsenic centres. The [NbF4{o-C6H4(AsMe2)2}2][NbF6] is not isomorphous with the diphosphine analogue, but shows a similar eight-coordinate cation and six-coordinate anion geometry (Fig. 5).The d(Nb–F) in the diarsine complex is slightly longer than those in the diphosphine analogue by ∼0.04 Å. The complexes of this diarsine with NbCl5 and TaCl5 of type [MCl4{o-C6H4(AsMe2)2}2][MCl6]§, were reported many years ago,16 and the X-ray structures are available for [TaCl4{o-C6H4(AsMe2)2}2][TaCl5(OEt)]17 and for the [NbCl4{o-C6H4(AsMe2)2}2]+ with various anions – [NbOCl4]−, [NbO2Cl3]2− (ref. 17) and [NbCl5(OEt)]− (see ESI†). The d(Nb–As) distances in these various salts are not significantly different to those in [NbF4{o-C6H4(AsMe2)2}2][NbF6]. The 19F{1H} NMR resonances of the cations in [MF4{o-C6H4(AsMe2)2}2][MF6] are broad singlets to high frequency of the diphosphine analogues (Table 2). In the UV/Vis spectrum the As(σ)→M(d) in [MF4(diarsine)2][MF6] occur at lower energy (∼23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–28
000–28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1) than for the corresponding transitions in the diphosphines, as expected given the lower electronegativity of As.18
000 cm−1) than for the corresponding transitions in the diphosphines, as expected given the lower electronegativity of As.18
NbOF3 and NbOCl3 complexes
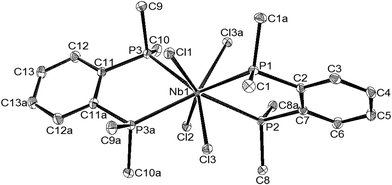
We recently reported that reaction of NbF5 with a range of N-(2,2′-bipy, 1,10-phen, tmeda) or O-donor (R3PO, Ph2P(O)CH2P(O)PPh2, dmso) ligands in MeCN–CH2Cl2 solution, followed by addition of the siloxane, HMDSO, produced white [NbOF3L2] (L = R3PO, dmso) or [NbOF3(L′–L′)] (L′–L′ = 2,2′-bipy, 1,10-phen, tmeda or Ph2P(O)CH2P(O)PPh2), although weaker donor ether, nitrile or thioether complexes did not form.5 In the present work attempts to use the same approach failed to yield NbOF3-diphosphine adducts, with [NbF4(L–L)2][NbF6] (L–L = Me2P(CH2)2PMe2 or o-C6H4(PMe2)2) solutions in MeCN being unchanged after 24 h. from addition of HMDSO, as shown by 19F and 31P NMR spectroscopy. Similarly, MeCN solutions of [NbOF3L2] (L = dmso or Ph3PO) did not react with o-C6H4(PMe2)2 or Me2P(CH2)2PMe2. We have reported elsewhere19 that alkyl diphosphines fail to displace the hard O-donor from [ZrF4L2] (L = dmso or dmf), and it is plausible that in the NbOF3 systems, the hard Nb centre prefers the O-donor to the softer phosphorus. However, the failure of the siloxane to perform O/F exchange was unexpected and the reason for this is not clear.Tertiary phosphine complexes of NbOCl3 include both six-coordinate [NbOCl3(PR3)2] (R = Me, Ph; R3 = MePh2) and seven-coordinate [NbOCl3(PMe3)3].20,21 In the present work, reaction of NbCl5 with HMDSO in MeCN, which forms [NbOCl3(MeCN)2] in situ, followed by addition of one mol. equivalent of Me2P(CH2)2PMe2, afforded white [(NbOCl3)2{Me2P(CH2)2PMe2}3]. The structure determined from colourless crystals grown from CH2Cl2 solution, showed this to be the symmetric dimer [{{Me2P(CH2)2PMe2}NbOCl3}2{μ-Me2P(CH2)2PMe2}] (Fig. 6), containing seven-coordinate niobium.
The bond lengths are similar to those in [NbOCl3(PMe3)3]20 but the geometry is quite different, due to the presence of the five-membered chelate ring, with P1–Nb1–P2 = 71.04(6)°, whereas in [NbOCl3(PMe3)3] the P–Nb–P angles are all >112°. The ν(Nb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of 939 cm−1 in [{{Me2P(CH2)2PMe2}NbOCl3}2{μ-Me2P(CH2)2PMe2}] contrasts with that of 882 cm−1 reported for [NbOCl3(PMe3)3].20,21 The dimer structure is retained in solution, shown most clearly by the 1H and 31P{1H} NMR spectra, which distinguish the bridging and chelating diphosphines. The phosphorus resonance [2P] of the bridging ligand has a coordination shift (Δ) of +47, whereas the more intense resonance [4P] has Δ = +80, indicative of a five-membered chelate ring.22
O) of 939 cm−1 in [{{Me2P(CH2)2PMe2}NbOCl3}2{μ-Me2P(CH2)2PMe2}] contrasts with that of 882 cm−1 reported for [NbOCl3(PMe3)3].20,21 The dimer structure is retained in solution, shown most clearly by the 1H and 31P{1H} NMR spectra, which distinguish the bridging and chelating diphosphines. The phosphorus resonance [2P] of the bridging ligand has a coordination shift (Δ) of +47, whereas the more intense resonance [4P] has Δ = +80, indicative of a five-membered chelate ring.22
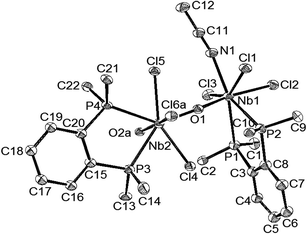
The reaction of NbCl5 and HMDSO in MeCN followed by addition of o-C6H4(PMe2)2, gave a white precipitate identified as [{o-C6H4(PMe2)2}NbOCl3(μ-O)NbCl3(CH3CN){o-C6H4(PMe2)2}], and red-orange [NbCl4{o-C6H4(PMe2)2}2][NbOCl4(CH3CN)] was isolated from the filtrate. The structure of the latter is described in ESI.†
The former contains seven-coordinate niobium centres, but in contrast to [(NbOCl3)2{Me2P(CH2)2PMe2}3], the two niobium centres have different ligand donor sets (P2O2Cl3 and P2NOCl3) and are linked by a near-linear (177°) oxido-bridge (Fig. 7). It may be that the rigid o-C6H4(PMe2)2, which is pre-organised for chelation, is disfavoured as a bridge in this case and the oxido-bridge is formed instead. The CSD contains only a single example of o-C6H4(PMe2)2 coordinated as a bridging ligand, in [(Cp*IrC12)2{μ-o-C6H4(PMe2)2}].23
Conclusions
A new series of complexes of niobium(V) and tantalum(V) fluoride with soft, neutral phosphorus or arsenic donor ligands has been prepared and characterised. All are of type [MF4(L–L)2][MF6] containing eight-coordinate cations, which seem to be the preferred structural units for many complexes of these two M(V) fluorides.3 Despite the extreme hard/soft Lewis acid/base combinations, they are robust complexes with no evidence for decomposition in the solid state if protected from moisture, and the NMR spectra show no significant dissociation of the ligands in inert solvents at ambient temperatures. These properties contrast with those of complexes containing other soft donors, e.g. the rapid decomposition of the selenoether complexes with fluorination at selenium, and the extensive dissociation of the thioether adducts in solution at ambient temperatures.6,7 The properties also contrast with the very unstable phosphine complexes of TiF4, and the absence of any arsine ligand adducts with TiF4, GeF4 or SnF4.10,14,15 Comparisons with the corresponding complexes of Nb or Ta pentachlorides show considerable similarities, both in composition and properties, which contrasts with the behaviour of Group 4 tetrahalides, where there is little in common between the chemistries of ZrF4 or HfF4 (which do not form complexes with soft donor ligands),19 and the six- or eight-coordinate complexes formed the heavier tetrahalides.24 The coordination chemistry of TiF4 is also very different from those of TiX4 (X = Cl, Br or I).10,25 The coordination chemistry of VF4 merits thorough study, and on the basis of the results reported herein, it is possible that even VF5 may form neutral ligand complexes under appropriate conditions.3Experimental
Infrared spectra were recorded as Nujol mulls between CsI plates using a Perkin Elmer Spectrum 100 over the range 4000–200 cm−1. 1H NMR spectra were recorded from CD2Cl2 or CD3CN solutions using a Bruker DPX400 spectrometer. 19F{1H}, 31P{1H} and 93Nb NMR spectra were recorded using a Bruker DPX400 spectrometer and are referenced to external CFCl3, external 85% H3PO4, and [Et4N][NbCl6] in MeCN respectively. UV/visible spectra were recorded from solid samples diluted with BaSO4 using the diffuse reflectance attachment of a Perkin-Elmer Lambda 19 spectrometer. Microanalyses on new complexes were undertaken by London Metropolitan University. Preparations used standard Schlenk and glove box techniques under a N2 atmosphere with rigorous exclusion of moisture. Solvents were dried by distillation from CaH2 (CH2Cl2 or CH3CN) or Na/benzophenone ketyl (diethyl ether). Hexamethyldisiloxane was dried over molecular sieves. Anhydrous NbX5 and TaX5 (X = F or Cl), PMe3, Me2P(CH2)2PMe2, Et2P(CH2)2PEt2 and o-C6H4(PPh2)2 were obtained from Aldrich, Apollo or Strem and used as received. o-C6H4(PMe2)2 and o-C6H4(AsMe2)2 were made by literature methods.26,27
[NbF4{o-C6H4(PMe2)2}2][NbF6]: NbF5 (0.18 g, 1.0 mmol) was dissolved in anhydrous MeCN (10 mL) and o-C6H4(PMe2)2 (0.20 g, 1.0 mmol) added. A clear solution formed which deposited a cream precipitate after ∼20 min. After stirring for 1 h the precipitate was filtered off, rinsed with MeCN (2 mL) and dried in vacuo. White powder. Yield: 0.30 g, 79%. Anal: required for C20H32F10Nb2P4 (772.2): C, 31.1; H, 4.2. Found: C, 31.0; H, 4.0%. 1H NMR (CD3CN, 295 K): δ = 1.70 (br s, [12H]), 7.82 (0 [2H]), 7.95 (s, [2H]). 19F{1H} NMR (CD3CN, 298 K): δ = 102.3 ([6F], 10 lines, 1JNbF = 335 Hz, [NbF6]−), −7.8 (quintet, 2JPF = 47 Hz, [4F], cation); (243 K) 102.3 ([6F], 10 lines, 1JNbF = 335 Hz), −10.8 (br s, [4F]). 31P{1H} NMR (CD3CN, 298 K): δ = 38.9 (br m); (233 K) 39.5 (br m). 93Nb NMR (CD3CN, 298 K): δ = ∼−1110 (vbr, cation), −1553 (septet, [NbF6]−); (233 K): −1554 (septet). IR (Nujol)/cm−1: 611 (vbr), 588 (sh) (NbF). UV/Vis (dr)/cm−1: 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330, 32
330, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900.
900.
[NbF4{o-C6H4(AsMe2)2}2][NbF6]: NbF5 (0.18 g, 1.0 mmol) was dissolved in anhydrous MeCN (15 mL) and o-C6H4(AsMe2)2 (0.29 g, 1.0 mmol) added. A clear yellow solution formed which deposited a pale yellow-cream precipitate after ∼20 min. After stirring for 1 h the solution was concentrated to ∼5 mL, the precipitate was filtered off, rinsed with MeCN (2 mL) and dried in vacuo. Yellow powder. Yield: 0.40 g, 85%. Anal: required for C20H32As4F10Nb2 (948.0): C, 25.3; H, 5.4. Found: C, 25.4; H, 5.3%. 1H NMR (CD2Cl2, 293 K): δ = 1.63 (s, [12H]), 7.58 (s, [2H]), 7.67 (s, [2H]); (243 K): 1.63 (s, [12H]), 7.56 (s, [2H]), 7.64 (s, [2H]). 19F{1H} NMR (CD2Cl2, 293 K): δ = 103.6 ([6F], 10 lines 1JNbF = 335 Hz, [NbF6]−), 27.1 (s, [4F], cation); (233 K): 103.6 (10 lines), 24.6 (s). 93Nb NMR (CD2Cl2, 293 K): δ = −1549 (septet, [NbF6]−). IR (Nujol)/cm−1: 620 (sh), 610 (sbr), 586 (sh) (NbF). UV/Vis (dr)/cm−1: 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (sh), 28
400 (sh), 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 32
000, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050.
050.
[NbF4{Me2P(CH2)2PMe2}2][NbF6]: NbF5 (0.18 g, 1.0 mmol) was dissolved in anhydrous MeCN (15 mL) and Me2P(CH2)2PMe2 (0.15 g, 1.0 mmol) added. A clear solution formed which deposited a white powder on stirring. After stirring for 1 h the solution was concentrated to ∼5 mL, and dry diethyl ether (5 mL) added. The precipitate was filtered off, rinsed with MeCN (2 mL) and dried in vacuo. White powder. Yield: 0.25 g, 76%. Anal: required for C12H32F10Nb2P4 (676.1): C, 21.3; H, 4.8. Found: C, 21.1; H, 4.9%. 1H NMR (CD3CN, 293 K): δ = 1.94 (s, [12H]), 2.33 (s, [4H]); (233 K): 1.96 (s, [12H]), 2.33 (s, [4H]). 19F{1H} NMR (CD3CN, 293 K): δ = 102.0 ([6F], 10 lines 1JNbF = 335 Hz, [NbF6]−), −10.9 (quintet 2JPF = 60 Hz, [4F], cation); (233 K): 102.7 ([6F], 10 lines, 1JNbF = 335 Hz,), −13.8 (quintet, [4F]). 31P{1H} NMR (CD3CN, 293 K): δ = 36.9 (quintet, 2JPF = 60 Hz); (233 K): 38.0 (quintet). 93Nb NMR (CD3CN, 293 K): δ = −1062 (vbr, s, cation), −1553 (septet, [NbF6]−); (233 K): −1555 (septet). IR (Nujol)/cm−1: 614 (sh), 573 (sbr), 554 (sh) (NbF). UV/Vis (dr)/cm−1: 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (sh), 33
300 (sh), 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550.
550.
[NbF4{Et2P(CH2)2PEt2}2][NbF6]: was made similarly. The complex is more soluble in MeCN and was isolated by removing the MeCN in vacuo and stirring the waxy white residue with dry diethyl ether (10 mL) for 2 h, after which the powder was filtered off and dried. White powder. Yield: 78%. Anal: required for C20H48F10Nb2P4 (788.3): C, 30.5; H, 6.1. Found: C, 30.4; H, 6.1%. 1H NMR (CD3CN, 293 K): δ = 1.13 (br s, [12H]), 1.89 (m, [8H]), 2.15 (m, [4H]); (233 K): 1.07 (m, [12H]), 1.85 (m, [8H]), 2.16 (m, [4H]). 19F{1H} NMR (CD3CN, 293 K): δ = 102.4 ([6F], 10 lines, 1JNbF = 335 Hz, [NbF6]−), +3.3 (quintet, 2JPF = 45 Hz, [4F], cation); (CD3CN, 243 K): 101.9 (10 lines), +0.04 (quintet, 2JPF = 45 Hz, [4F]). 31P{1H} NMR (CD3CN, 293 K): δ = 50.3 (br s); (233 K): 50.0 (br m). 93Nb NMR (CD3CN, 293 K): δ = −1553 (septet, [NbF6]−); (233 K): −1555 (septet). IR (Nujol)/cm−1: 630 (sh), 611 (sbr) (NbF). UV/Vis (dr)/cm−1: 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 32
000, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900.
900.
[NbF4{o-C6H4(PPh2)2}2][NbF6]: Powdered o-C6H4(PPh2)2 (0.40 g, 0.9 mmol) was suspended in dry MeCN (20 mL) and the mixture stirred vigorously. Powdered NbF5 (0.16 g, 0.9 mmol) was added and after ∼5 min a clear solution was produced. After a further 3 h the solution was concentrated to ∼5 mL in vacuo, when a pale cream solid deposited. This was filtered off, rinsed with MeCN (1 mL) and dried in vacuo. Yield: 0.39 g, 70%. Anal: required for C60H48F10Nb2P4 (1268.7): C, 56.8; H, 3.8. Found: C, 56.9; H, 3.6%. 1H NMR (CD2Cl2 293 K): δ = 7.65–7.23 (m). 19F{1H} NMR (CD2Cl2, 293 K): δ = 102.8 (10 lines, 1JNbF = 335 Hz, [6F], [NbF6]−) 54.8 (br m, [4F], cation); (CD2Cl2, 243 K): δ = 102.9 (10 lines. [6F]), 48.8 (br s, [4F]). 31P{1H} NMR (CD2Cl2, 293 K): δ = 40.3 (vbr); (233 K): 41.4 (quintet, 2JPF = 43 Hz). 93Nb NMR (CD2Cl2, 293 K): δ = −1549 (septet, [NbF6]−). IR (Nujol)/cm−1: 630 (s br), 602 (s br) (NbF). UV/Vis (dr)/cm−1: 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, 33
300, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300.
300.
[TaF4{o-C6H4(PMe2)2}2][TaF6]: TaF5 (0.28 g, 1.0 mmol) was dissolved in anhydrous MeCN (10 mL) and o-C6H4(PMe2)2 (0.20 g, 1.0 mmol) added. A clear solution formed which was stirred for 1 h, concentrated to ∼5 mL and the precipitate filtered off, rinsed with MeCN (2 mL) and dried in vacuo. White powder. Yield: 0.36 g, 75%. Anal: required for C20H32F10P4Ta2 (948.3): C, 25.3; H, 3.4. Found: C, 25.2; H, 3.3%. 1H NMR (CD3CN, 295 K): δ = 1.70 (br s, [12H]), 7.79 (s, [2H]), 7.92 (s, [2H]); (243 K): 1.68 (s, [12H]), 7.77 (s, [2H]), 7.92 (s, [2H]). 19F{1H} NMR (CD3CN, 298 K): δ = 37.4 ([6F], [TaF6]−), −39.8 (quintet, 2JPF = 60 Hz, [4F], cation); (243 K): 38.3 (s, [6F]), −42.3 (m, [4F]). 31P{1H} NMR (CD3CN, 298 K): δ = 35.4 (quintet, 2JPF = 60 Hz); (233 K): 36.1 (br s). IR (Nujol)/cm−1: 621 (sh), 596 (sh), 572 (vs) (TaF). UV/Vis (dr)/cm−1: 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
[TaF4{o-C6H4(AsMe2)2}2][TaF6]: TaF5 (0.28 g, 1.0 mmol) was dissolved in anhydrous MeCN (10 mL) and o-C6H4(AsMe2)2 (0.29 g, 1.0 mmol) added. A clear yellow solution formed which deposited a small amount of pale cream precipitate after ∼20 min. After stirring for 1 h the solution was concentrated to ∼5 mL, dry diethyl ether (5 mL) added, and the precipitate filtered off, rinsed with MeCN (2 mL) and dried in vacuo. Yellow powder. Yield: 0.40 g, 85%. Anal: required for C20H32As4F10Ta2 (1124.0): C, 21.4; H, 2.9. Found: C, 21.3; H, 2.8%. 1H NMR (CD2Cl2, 293 K): δ = 1.63 (s, [12H]), 7.59 (s, [2H]), 7.64 (s, [2H]); (243 K): 1.60 (s, [12H]), 7.55 (s, [2H]), 7.60 (s, [2H]). 19F{1H} NMR (CD2Cl2, 293 K): δ = 39.5 (s, [6F], [TaF6]−), −28.0 (s, [4F], cation); (243 K): 39.7 (s, [6F]), −29.0 (s, [4F]). IR (Nujol)/cm−1: 616 (sh), 578 (br) (TaF). UV/Vis (dr)/cm−1: 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (sh), 27
400 (sh), 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 30
000, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600.
600.
[TaF4{Me2P(CH2)2PMe2}2][TaF6]: was made similarly to the niobium analogue. White microcrystalline solid. Yield: 83%. Anal: required for C12H32F10P4Ta2 (852.2): C, 16.9; H, 3.8. Found: C, 16.8; H, 3.8%. 1H NMR (CD3CN, 293 K): δ = 1.81 (s, [12H]), 2.10 (s, [4H]); (233 K): 1.82 (s, [12H]), 2.10 (s, [4H]). 19F{1H} NMR (CD3CN, 293 K): δ = 38.3 (s, [6F], [TaF6]−), −40.8 (quintet, 2JPF = 60 Hz, cation); (CD3CN, 243 K): 38.8 (s, [6F]), −43.6 (quintet, [4F]). 31P{1H} NMR (CD3CN, 293 K): δ = 36.9 (quintet, 2JPF = 60 Hz); (233 K): 37.9 (quintet). IR (Nujol)/cm−1: 613 (sh), 575 (vbr, s) (TaF). UV/Vis (dr)/cm−1: 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400.
400.
[TaF4{Et2P(CH2)2PEt2}2][TaF6]: was made similarly to the niobium analogue. White waxy solid. Yield: 65%. 1H NMR (CD3CN, 293 K): δ = 1.17 (br s, [12H]), 1.80 (br s, [8H]), 2.15 (s, [4H]); (233 K): 1.08 (s, [12H]), 1.84 (s, [8H]), 2.12 (s [4H]). 19F{1H} NMR (CD3CN, 293 K): δ = 38.8 (s, [6F], [TaF6]−), −25.7 (quintet, 2JPF = 55 Hz, [4F], cation); (CD3CN, 243 K): 38.9 (s, [6F]), −28.8 (quintet, [4F]). 31P{1H} NMR (CD3CN, 293 K): δ = 45.0 (quintet, 2JPF = 55 Hz); (233 K): 45.3 (quintet). IR (Nujol)/cm−1: 615 (sh), 587 (vbr, s) (TaF). UV/Vis (dr)/cm−1: 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.
800.
[TaF4{o-C6H4(PPh2)2}2][TaF6]: was made similarly to the niobium analogue using o-C6H4(PPh2)2 (0.40 g, 0.9 mmol) and TaF5 (0.26 g, 0.9 mmol). Yield: 0.48 g, 73%. Anal: required for C60H48F10P4Ta2 (1444.8): C, 49.9; H, 3.6. Found: C, 49.8; H, 3.5%. 1H NMR (CD2Cl2, 293 K): δ = 7.77–7.23 (m). 19F{1H} NMR (CD2Cl2, 293 K): δ = 38.6 (s, [6F], [TaF6]−), 16.8 (quintet, 2JPF = 57 Hz, cation); (243 K): δ = 38.7 (s), 12.3 (br s). 31P{1H} NMR (CD2Cl2, 293 K): δ = 38.6 (br); (233 K): 39.0 (quintet, 2JPF = 57 Hz). IR (Nujol)/cm−1 583 (s, br), 545 (s, br) (TaF). UV/Vis (dr)/cm−1: 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, 33
300, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
[NbCl4{o-C6H4(AsMe2)2}2][NbCl6]: Made as described.16 Orange-red powder. 1H NMR (CD2Cl2, 293 K): δ = 1.92 (s, [12H]), 7.78 (s, [2H]), 7.90 (s, [2H]). 93Nb NMR (CD2Cl2, 293 K): δ = +6.2 (s, [NbCl6]−). IR (Nujol)/cm−1: 324 (vs) (NbCl). UV/Vis (dr)/cm−1: 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350, 29
350, 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760.
760.
[TaCl4{o-C6H4(AsMe2)2}2][TaCl6]: Made as described.16 Deep yellow powder. 1H NMR (CD2Cl2, 293 K): δ = 2.00 (s, [12H]), 7.80 (s, [2H]), 7.86 (s, [2H]). IR (Nujol)/cm−1 323 (vs), 303 (s) (TaCl). UV/Vis (dr)/cm−1: 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530, 31
530, 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250.
250.
[NbCl4{o-C6H4(PMe2)2}2]Cl: NbCl5 (0.135 g, 0.50 mmol) was dissolved in acetonitrile (5 mL) whilst stirring giving a bright yellow-green solution. o-C6H4(PMe2)2 (0.20 g, 1.0 mmol) was added slowly to the solution, which resulted in rapid formation of a red-brown precipitate. The reaction was left to stir another 10 min. The solution was filtered, the precipitate was washed with small amount of dichloromethane and dried in vacuo. Yield: 0.280 g, 83%. Red-orange single crystals of [NbCl4(o-C6H4(PMe2)2)2]Cl were grown from a saturated dichloromethane solution cooled in the freezer. Anal: required for C20H32Cl5NbP4 (666.5): C, 36.0; H, 4.8. Found: C, 35.9; H, 4.7%. 1H NMR (CD2Cl2, 295 K): δ = 1.96 (s, [12H]), 7.70–7.62 (m, [4H]). 31P{1H} NMR (CH2Cl2–CD2Cl2, 298 K): δ = 58.4 (s). IR (Nujol)/cm−1: 320 (s), 293 (s) (NbCl). UV/Vis (dr)/cm−1: 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 34
000, 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500.
500.
[NbCl4{Me2P(CH2)2PMe2}2][NbCl6]: NbCl5 (0.135 g, 0.50 mmol) was dissolved in acetonitrile (5 mL) whilst stirring giving a bright yellow-green solution. Me2P(CH2)2PMe2 (0.075 g, 0.50 mmol) in 3 mL acetonitrile was added to the solution which resulted in rapid formation of red-brown precipitate. The reaction was left to stir 10 min. The solution was filtered, the precipitate washed with small amount of dichloromethane and dried in vacuo. Yield: 0.130 g, 65%. Anal: required for C12H32Cl10Nb2P4 (840.6): C, 17.1; H, 3.8. Found: C, 17.3; H, 3.9%. 1H NMR (CD2Cl2, 293 K): δ = 1.73 (br s, [12H]), 2.01 (br, [4H]). 31P{1H} NMR (CH2Cl2–CD2Cl2, 298 K): δ = 56.3(s). 93Nb NMR (CH2Cl2–CD2Cl2, 298 K): δ = 6.1(s). IR (Nujol)/cm−1: 303 (s, vbr) (NbCl). UV/Vis (dr)/cm−1: 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980, 32
980, 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330.
330.
[TaCl4{o-C6H4(PMe2)2}2][TaCl6]: To a suspension of TaCl5 (0.090 g, 0.25 mmol) in acetonitrile (5 mL) was added o-C6H4(PMe2)2 (0.050 g, 0.25 mmol) with stirring which resulted in a formation of a white precipitate. The reaction was left to stir for 30 min, and then filtered. The white solid was washed with small amount of dichloromethane and dried. Yield: 0.10 g, 66%. Anal: required for C20H32Cl10P4Ta2 (1112.8): C, 21.6; H, 2.9. Found: C, 21.6; H, 3.0%. 1H NMR (CD2Cl2, 295 K): δ = 1.71 (s, [12H]), 7.75 (br, [4H]). 31P{1H} NMR CH2Cl2–CD2Cl2, 298 K): δ = 44.6 (br s). IR (Nujol/cm−1): 324 (s), 279 (s) (TaCl). UV/Vis (dr)/cm−1: 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550, 31
550, 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250.
250.
[NbCl4{o-C6H4(PMe2)2}2][NbOCl4(CH3CN)] and [{o-C6H4(PMe2)2}NbOCl3(μ-O)NbCl3(CH3CN){o-C6H4(PMe2)2}]: NbCl5 (0.135 g, 0.50 mmol) was dissolved in acetonitrile (5 mL) giving a bright yellow-green solution. HMDSO (0.10 g, 0.60 mmol) was added. The mixture was left to stir under nitrogen for 30 min during which time the solution turned very pale yellow. o-C6H4(PMe2)2 (0.10 g, 0.50 mmol) in 3 mL acetonitrile was added slowly to the solution which resulted in formation of white precipitate immediately with a solution colour change to red-orange. The reaction was left to stir for another 5 min and then the white precipitate filtered off. The filtrate was refrigerated for several days to give red-orange crystals of [NbCl4{o-C6H4(PMe2)2}2][NbOCl4(CH3CN)] Yield: 0.023 g, 10%. Anal: required for C22H35Cl8NNb2OP4 (922.8): C, 28.6; H, 3.8; N, 1.5. Found: C, 28.6; H, 3.8; N, 1.6. 1H NMR (CD2Cl2, 295 K): δ = 1.98 (s, [3H], MeCN), 2.11 (s, [24H]), 7.73–7.87 (m, [8H], aromatic H). 31P{1H} NMR (CH2Cl2–CDCl3, 298 K): δ = 55.2 (s). IR (Nujol/cm−1): 2305 (vw), 2279 (vw) (MeCN), 947 (s, NbO), 320 (vbr), 289 (s) (NbCl). The white precipitate was washed with small amount of dichloromethane and dried. Colourless single crystals of [{o-C6H4(PMe2)2}NbOCl3(μ-O)NbCl3(CH3CN){o-C6H4(PMe2)2}] were grown from a saturated dichloromethane solution in the freezer. Yield: 0.045 g, 12%. Anal: required for C22H35Cl6NNb2O3P4 (884.0): C, 29.9; H, 4.0, N, 1.6. Found: C, 30.5; H, 3.9; N, 1.6. 1H NMR (CD2Cl2, 295 K): δ = 1.58 (s, [6H], PMe2), 1.62 (s, [12H], PMe2), 1.77 (s, [6H], PMe2), 1.98 (s, [3H], MeCN), 7.57–7.68 (m, [8H]). 31P{1H} NMR (CH2Cl2–CDCl3, 298 K): δ = 42.9 (s, [P]), 38.3 (br, [2P]), 36.4 (s, [P]). IR (Nujol/cm−1): 943 (s, NbO), 824(s) (Nb–O–Nb), 355, 304 (s, NbCl).
[({Me2P(CH2)2PMe2}NbOCl3)2(μ-Me2P(CH2)2PMe2)]: NbCl5 (0.135 g, 0.50 mmol) was dissolved in acetonitrile (5 mL) whilst stirring giving a bright yellow-green solution. HMDSO (0.10 g, 0.60 mmol) was added. The mixture was left to stir under nitrogen for 30 min during which time the solution turned very pale yellow. Me2P(CH2)2PMe2 (0.075 g, 0.50 mmol) in 5 mL of dichloromethane was added to the solution which resulted in formation of white precipitate immediately. The reaction was left to stir another 10 min and then filtered. The precipitate was washed with a small amount of dichloromethane and dried in vacuo. Yield: 0.088 g, 76%. Colourless single crystals of [{Me2P(CH2)2PMe2}NbOCl3}2(μ-Me2P(CH2)2PMe2)] were grown from saturated dichloromethane solution in the freezer. Anal: required for C18H48Cl6Nb2O2P6 (880.9): C, 24.5; H, 5.5. Found: C, 24.5; H, 5.5%. 1H NMR (CD2Cl2, 295 K): δ = 1.52 (s, [12H], PMe2), 1.77 (s, [24H], PMe2), 2.14–2.38 (m, [12H], CH2). 31P{1H} NMR CH2Cl2–CD2Cl2, 298 K): δ = 33.3 (s, [4P]), 0.7 (s, [2P]). IR (Nujol)/cm−1: 939 (m, NbO), 321(w), 288(m) (NbCl).
X-ray experimental
Details of the crystallographic data collection and refinement parameters are given in Table 1. Crystals suitable for single crystal X-ray analysis were obtained as described above. Data collections used a Rigaku AFC12 goniometer equipped with an enhanced sensitivity (HG) Saturn724+ detector mounted at the window of an FR-E+ SuperBright molybdenum (λ = 0.71073 Å) rotating anode generator with VHF Varimax optics (70 μm focus) with the crystal held at 100 K (N2 cryostream). Structure solution and refinements were performed with either SHELX(S/L)97 or SHELX(S/L)201328 and were straightforward except as detailed below. H atoms bonded to C were placed in calculated positions using the default C–H distance and refined using a riding model. The anion in [NbF4{o-C6H4(AsMe2)2}2][NbF6] was disordered which was satisfactorily modeled with a number of partially occupied F positions and thermal parameter restraints. The [{o-C6H4(PMe2)2}NbOCl3-μ-O-NbCl3(CH3CN){o-C6H4(PMe2)2}] crystals were weakly diffracting with poorly defined reflection profiles and it was necessary to employ global restraints to the atomic displacement parameters. The apical chlorine and oxygen atoms coordinated to Nb2 are modeled as disordered (ca. 80/20) over the 2 positions using thermal parameter constraints and distance restraints (Nb–Cl = 2.47, Nb–O 1.8).Acknowledgements
This work was funded by EPSRC (through a Programme Grant EP/I033394/1 and also through EP/I010890/1). The SCFED Project (http://www.scfed.net) is a multidisciplinary collaboration of British universities investigating the fundamental and applied aspects of supercritical fluids.References
- A. J. Edwards, J. Chem. Soc., 1964, 3714 RSC.
- T. A. O'Donnell, in Advanced Inorganic Fluorides, ed. T. Nakajima, B. Zemva and A. Tressaud, Elsevier, Amsterdam, 2000, ch. 11 Search PubMed.
- S. L. Benjamin, W. Levason and G. Reid, Chem. Soc. Rev., 2013, 42, 1460 RSC.
- (a) F. Marchetti and G. Pampaloni, Chem. Commun., 2012, 48, 635 RSC; (b) R. Bini, C. Chiappe, F. Marchetti, G. Pampaloni and S. Zacchini, Inorg. Chem., 2010, 49, 339 CrossRef CAS PubMed; (c) F. Marchetti, G. Pampaloni and S. Zacchini, Inorg. Chem., 2008, 47, 365 CrossRef CAS PubMed; (d) F. Marchetti, G. Pampaloni and S. Zacchini, J. Fluorine Chem., 2010, 131, 21 CrossRef CAS PubMed; (e) F. Marchetti, G. Pampaloni and S. Zacchini, Dalton Trans., 2009, 8096 RSC; (f) F. Marchetti, G. Pampaloni and S. Zacchini, Dalton Trans., 2009, 6759 RSC; (g) M. Bortoluzzi, F. Marchetti, G. Pampaloni, M. Pucino and S. Zacchini, Dalton Trans., 2013, 42, 13054 RSC; (h) J. A. S. Howell and K. C. Moss, J. Chem. Soc. A, 1971, 2483 RSC.
- W. Levason, G. Reid, J. Trayer and W. Zhang, Dalton Trans., 2014, 43, 3649 RSC.
- (a) M. Jura, W. Levason, R. Ratnani, G. Reid and M. Webster, Dalton Trans., 2010, 39, 883 RSC; (b) M. Jura, W. Levason, G. Reid and M. Webster, Dalton Trans., 2009, 7610 RSC.
- S. L. Benjamin, A. Hyslop, W. Levason and G. Reid, J. Fluorine Chem., 2012, 137, 77 CrossRef CAS PubMed.
- L. G. Hubert-Pfalzgraf, M. Postel and J. G. Reiss, Comprehensive Coordination Chemistry, ed. G. Wilkinson, R. D. Gillard and J. A. McCleverty, Pergamon, Oxford, 1987, 3, 585 Search PubMed.
- S. L. Benjamin, A. Hyslop, W. Levason and M. Webster, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2011, 67, m221 CAS.
- M. Jura, W. Levason, E. Petts, G. Reid, M. Webster and W. Zhang, Dalton Trans., 2010, 39, 10264 RSC.
- F. A. Cotton, L. R. Falvello and R. C. Najar, Inorg. Chem., 1983, 32, 770 CrossRef.
- G. C. Allen and K. D. Warren, Struct. Bonding, 1974, 19, 105 CrossRef CAS.
- M. Valloton and A. E. Merbach, Helv. Chim. Acta, 1974, 57, 254 CrossRef.
- M. F. Davis, W. Levason, G. Reid and M. Webster, Dalton Trans., 2008, 2261 RSC.
- M. F. Davis, M. Clarke, W. Levason, G. Reid and M. Webster, Eur. J. Inorg. Chem., 2006, 2773 CrossRef CAS.
- R. J. H. Clark, D. L. Kepert and R. S. Nyholm, J. Chem. Soc., 1965, 2877 RSC.
- J. C. Dewan, D. L. Kepert, C. L. Raston and A. H. White, J. Chem. Soc., Dalton Trans., 1975, 2031 RSC.
- A. B. P. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 2nd edn, 1984 Search PubMed.
- S. L. Benjamin, W. Levason, D. Pugh, G. Reid and W. Zhang, Dalton Trans., 2012, 41, 12548 RSC.
- V. C. Gibson, T. P. Kee, R. M. Sorrell, A. P. Bashall and M. McPartlin, Polyhedron, 1988, 7, 2221 CrossRef CAS.
- V. C. Gibson and T. P. Kee, J. Chem. Soc., Dalton Trans., 1993, 1657 RSC.
- P. E. Garrou, Chem. Rev., 1985, 85, 171 CrossRef CAS.
- W. Keim, P. Kraneburg, G. Dahmen, G. Deckers, U. Englert, K. Linn, T. P. Spaniol, G. Raabe and C. Kriiger, Organometallics, 1994, 13, 3085 CrossRef CAS.
- (a) R. Hart, W. Levason, B. Patel and G. Reid, J. Chem. Soc., Dalton Trans., 2002, 3153 RSC; (b) W. Levason, B. Patel and G. Reid, Inorg. Chim. Acta, 2004, 357, 2115 CrossRef CAS PubMed; (c) W. Levason, M. L. Matthews, B. Patel, G. Reid and M. Webster, Dalton Trans., 2004, 3305 RSC.
- (a) W. Levason, B. Patel, G. Reid, V.-A. Tolhurst and M. Webster, J. Chem. Soc., Dalton Trans., 2000, 3001 RSCR. Hart, W. Levason, B. Patel and G. Reid, Eur. J. Inorg. Chem., 2001, 2927 CrossRef CAS.
- R. D. Feltham, A. Kasenally and R. S. Nyholm, J. Organomet. Chem., 1967, 7, 285 CrossRef CAS.
- E. P. Kyba, S. T. Liu and R. L. Harris, Organometallics, 1983, 2, 1877 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The crystallographic data and selected bond lengths and angles for [NbCl4{o-C6H4(PMe2)2}2][NbOCl4(CH3CN)] and [NbCl4{o-C6H4(AsMe2)2}2][NbCl5(OEt)]. CCDC 993845 [NbCl4{o-C6H4(PMe2)2}2]Cl, 993846 [NbCl4{o-C6H4(AsMe2)2}2][NbCl5(OEt)], 993847 [NbCl4{o-C6H4(PMe2)2}2][NbOCl4(MeCN)], 993848 [{{Me2P(CH2)2PMe2}NbOCl3}2{μ-Me2P(CH2)2PMe2}], 993849 [{o-C6H4(PMe2)2}NbOCl3(μ-O)NbCl3(CH3CN){o-C6H4(PMe2)2}], 993851 [NbF4{o-C6H4(AsMe2)2}2][NbF6], 993852 [NbF4{o-C6H4(PMe2)2}2][NbF6], 993853 [TaF4{o-C6H4(PMe2)2}2][TaF6]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4dt01029a |
| ‡ In D2d symmetry the metal d-orbitals split a1 + b1 + b2 + e, but a more detailed assignment is not possible on the limited data available. |
| § Originally formulated as seven-coordinate monomers. |
| This journal is © The Royal Society of Chemistry 2014 |