Chemical bond properties and charge transfer bands of O2−–Eu3+, O2−–Mo6+ and O2−–W6+ in Eu3+-doped garnet hosts Ln3M5O12 and ABO4 molybdate and tungstate phosphors
Abstract
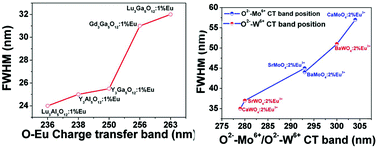
Charge transfer (CT) energy from the ligand to the central ions is an important factor in luminescence properties for rare earth doped inorganic phosphors. The dielectric theory of complex crystals was used to calculate chemical bond properties. Combining the photoluminescence and the dielectric theory of complex crystals, the CT bands of O2−–Eu3+, O2−–Mo6+ and O2−–W6+ for Eu3+-doped inorganic phosphors have been investigated experimentally and theoretically. Taking Eu3+-doped Ln3M5O12 (Ln = Y, Lu and M = Al, Ga), Gd3Ga5O12, MMoO4 (M = Ca, Sr, Ba) and MWO4 (M = Ca, Sr, Ba) as typical phosphors, we investigated the effects of the cation size on the CT bands and chemical bond properties including the bond length (d), the covalency (fc), the bond polarizability (αb) and the environmental factor (he) of O2−–Eu3+, O2−–Mo6+ and O2−–W6+, respectively. For systematic isostructural Ln3M5O12 (Ln = Y, Lu and M = Al, Ga) phosphors, with the increasing M ion radius, the bond length of Ln–O decreases, but fc and αb increase, which is the main reason that the environmental factor increased. For the isostructural MMoO4:Eu, with the increasing M ion radius, the Mo–O bond length increases, but fc and αb decrease, and thus he decreases. However, in the compound system MWO4:Eu (M = Ca, Ba) with the increasing M ion radius, the O–W bond length increases, but fc and αb increase, and thus he increases and the O–W CT energy decreases. Their O2−–Eu3+, O2−–Mo6+ and O2−–W6+ CT bands as well as their full width at half maximum (FWHM) were directly influenced by he. And with the increasing he, CT bands of O–Eu or O–Mo or O–W decrease and their FWHM increases. These results indicate a promising approach for changing the material properties, searching for new Eu3+ doped molybdate, tungstate or other oxide phosphors and analyzing the experimental result.

 Please wait while we load your content...
Please wait while we load your content...