Photocatalytic activity of transition-metal-ion-doped coordination polymer (CP): photoresponse region extension and quantum yields enhancement via doping of transition metal ions into the framework of CPs†
Abstract
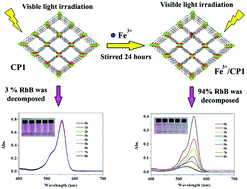
To improve photocatalytic activity of a coordination polymer (CP) in the visible light region, five different transition metal ions (Fe3+, Cr3+, Ru3+, Co2+ and Ni2+) were introduced into its framework through an ion-exchange process. Among all the resulting transition metal ion doped coordination polymers (TMI/CPs), the one doped with Fe3+ took on the most excellent photocatalytic activity and the highest quantum yields in the visible light region, decomposing 94% Rhodamine B (RhB) in 8 hours. It can be attributed to the doping of Fe3+, which reduced the band gap (Eg) of the original CP, facilitating photocatalysis of the obtained polymer. Compared with the coordination polymer with Fe3+ as a dopant, products doped with other metal ions presented weaker photocatalytic activities in the visible light region, while under the irradiation of ultraviolet light, they showed favorable photocatalytic properties. The results suggest that to dope transition metal ions into the framework of CPs would be an ideal option for enhancing the photocatalytic activity of coordination polymers.

 Please wait while we load your content...
Please wait while we load your content...